Abstract
Ischaemic heart disease, which often manifests clinically as myocardial infarction (MI), remains a major cause of mortality worldwide. Despite the development of effective pre-clinical cardioprotective therapies, clinical translation has been disappointing. Nevertheless, the ‘reperfusion injury salvage kinase’ (RISK) pathway appears to be a promising target for cardioprotection. This pathway is crucial for the induction of cardioprotection by numerous pharmacological and non-pharmacological interventions, such as ischaemic conditioning. An important component of the cardioprotective effects of the RISK pathway involves the prevention of mitochondrial permeability transition pore (MPTP) opening and subsequent cardiac cell death. Here, we will review the historical perspective of the RISK pathway and focus on its interaction with mitochondria in the setting of cardioprotection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of myocardial infarction (MI) is in decline in developing countries. However, the morbidity and mortality resulting from MI remain high and may even start to increase as a consequence of the increasing prevalence of metabolic disease [18]. Hence, there remains an unmet need for the development of effective cardioprotective therapies to salvage the myocardium following an acute MI. Mitochondria are crucial organelles for the provision of ATP to maintain the viability and function of cardiomyocytes [40]. However, during myocardial ischaemia, mitochondrial respiration ceases. Subsequent reperfusion of the blocked coronary arteries contributes to mitochondrial-related oxidative insult, cell death, and inflammation [8, 82]. Hence, mitochondria have been extensively studied during the past few decades in the setting of ischaemia reperfusion injury (IRI) [22, 93]. In this context, cardioprotective signalling cascades are suggested to activate protein kinase C which subsequently regulates mitochondrial reactive oxygen species (ROS) and Ca2+ to prevent cell death following IRI [14, 15, 25, 41]. Relevantly, mitochondrial potassium ATP channels can mediate or initiate cell death by modulating mitochondrial ROS, and they are fundamental for the action of ischaemic preconditioning (IPC) [32, 65]. Therefore, mitochondria are crucial for the execution of various cardioprotective strategies.
In the early 2000s, Yellon and colleagues investigated the potential for growth factors such as transforming growth factor-beta1 (TGF-β1) and insulin to protect the heart from IRI via anti-apoptotic signalling pathways. In these initial studies, they demonstrated anti-apoptotic effects in both rat cardiomyocytes and isolated Langendorff perfused rat hearts when insulin or TGF-β1 administration was given at reoxygenation/reperfusion, following a lethal period of hypoxia/ischaemia, respectively. They further demonstrated that these agents mediated their effect via a number of pathways including the p70S6 kinase and phosphoinositide 3-kinase (PI3K) pathway as well as the ERK1/ERK2 mitogen-activated protein kinases (MAPK) signalling pathway, respectively. In these studies, they concluded that manipulation of growth factor “survival” signalling mechanisms may provide a promising route to attenuate lethal reperfusion injury [7, 42, 43].
Investigating this growth-factor signalling further using Urocortin, a growth factor which upregulates the ERK1/ERK2 (then known as p44 and p42 respectively, official names: MAPK3/MAPK1) MAPK signalling pathway, they demonstrated its ability to protect the heart against reperfusion-induced injury in isolated perfused and in vivo rat hearts [71]. Urocortin was given at the time of reperfusion following 35 min of ischaemia. The significant protection observed was associated with the upregulation of the ERK1/ERK2 MAPK-dependent signalling pathway [71]. As a result of these studies, they proposed that the heart possesses “prosurvival reperfusion injury salvage kinase” pathways that may be exploited when developing agents that can be used to protect the myocardium against the consequences of lethal reperfusion injury—the so-called RISK pathway. Importantly, the RISK pathway was defined as the kinases activated during early reperfusion [40]. The fact that intervention at or immediately prior to reperfusion was capable of limiting infarct size was important as it demonstrated that reperfusion injury exists.
Further studies used bradykinin as a cardioprotective agent to demonstrate that PI3K activity subsequently resulted in rapid phosphorylation of AKT/Protein kinase B (PKB) and endothelial NO synthase (eNOS) within the first 5 min of reperfusion, and that this was involved in attenuating reperfusion injury. This led to the proposal that salvaging the myocardium following IRI must involve the recruitment of the PI3K/AKT cell survival signalling cascade and an increase in eNOS activity to attenuate cell death during reperfusion [11].
Following these initial studies using growth factors, it was confirmed that activation of PI3K/AKT and ERK1/ERK2 MAPK pathways at reperfusion was essential for IPC [39]. Interestingly, ‘cross-talk’ was observed between these two pathways, whereby inhibiting one cascade activates the other and vice versa [38].
There is evidence that the extent to which the RISK pathway is recruited during cardioprotection varies between species. For example, the RISK pathway was shown to be required for the induction of cardioprotection by ischaemic post-conditioning (IPost) in rodents [46, 84] and small mammals [91], whereas it was dispensable in pigs [72, 77]. Interestingly, this lack of conservation between species does not appear to be unique to the RISK pathway, since PKC, whilst required for protection in small rodents and rabbits, appears to be dispensable for protection in pigs. [85]. Furthermore, the ability of IPC to activate the SAFE pathway to protect the hearts of pigs from IRI may be affected by genetic background [47]. Regardless of which kinases are required for ischaemic conditioning strategies in animals, the RISK pathway is required to protect human myocardium from simulated IRI by the use of either pharmacological cardioprotective agents or hypoxia/reoxygenation strategies [62, 75, 76].
Furthermore, cardiovascular comorbidities can directly or indirectly influence cardioprotection strategies, and in some instances, this has been shown to be via suppression of RISK pathway activation [9, 26]. For instance, chronic type 2 diabetes is known to abolish the cardioprotective effects of IPC by reducing AKT phosphorylation [70, 75, 83] and this is exacerbated by age [88]. Similarly, IPost has been shown to be ineffective in limiting IRI in spontaneously hypertensive rats [86].
As the RISK pathway kinases have several different isoforms and led to the phosphorylation and activation of a number of downstream kinases, it was important to ascertain whether any specific isoform was directly associated with transmitting the cardioprotective signal. There exist three different isoforms of PI3K, including PI3Kα, PI3Kβ, and PI3Kγ, which are expressed in cardiomyocytes [29]. In 2008, a study by Tsushima and his team showed that the genetic deletion of PI3Kγ can interfere with IPC signalling and exacerbate cardiac dysfunction following IRI. This was attributed to the inhibition of AKT and glycogen synthase kinase-3β (GSK-3β) phosphorylation in the mutant mice [5]. Similarly, later work by our group revealed that selective inhibition of PI3Kα by GDC–G326 can abrogate the cardioprotective effects of IPC confirming the essential role of PI3Kα in cardioprotective signal transduction [69]. In comparison to these two isoforms, the role of PI3Kβ in the RISK pathway is less understood. However, hearts carrying cardiomyocytes with specific deletion of the PI3Kβ isoform have been shown to have worse systolic function and exhibit higher levels of cell death following IRI versus their wild-type littermate [19]. Interestingly, endothelial deletion of PI3Kβ induced opposite effects in the hearts following IRI, which indicates the cell-specific function of this isoform in the heart [19].
The protein sequences of ERK1 and ERK2 are ~ 84% identical to each other in mammals and appear to have almost identical functions [17]. Early work by Fryer et al. showed that both ERK1 and ERK2 isoforms are activated by IPC within 5 min of reperfusion [27]. They also showed the phosphorylation of the ERK1 (P44) MAPK at reperfusion was greater than ERK2 (P42) MAPK [27]. However, a later study showed that infarct size increased in response to heterozygous deletion of ERK2 and homozygous deletion of ERK1 did not have any effects on infarct size [53].
AKT is activated in response to various physiological and stress stimuli, such as growth factors, hormones, hypoxia, and oxidative stress. We investigated which of the isoforms of AKT may be important downstream to PI3K in IRI [48]. This study showed for the first time that AKT1 but not AKT2 is an essential mediator of myocardial protection following IPC, i.e., only AKT1 plays the key role in transmitting the cardioprotective signal resulting in amelioration of myocardial injury as a result of severe IRI [48]. Collectively, these studies revealed the isoform-specific effects in the induction of cardioprotection mediated by different kinases of the RISK pathway.
The RISK pathway and the MPTP
The role of mitochondria in cardiomyocyte death following IRI did not become evident until a series of experiments by Crompton and colleagues showed that isolated rat cardiac mitochondria can become lethally permeabilised due to the opening of a mitochondrial pore in the presence of Ca2+ and inorganic phosphate or in response to oxidative stress [20, 21]. Following these studies, in the early 1990s, Halestrap and colleagues documented that the mitochondrial permeability transition pore (MPTP) opens in the intact heart, in response to IRI [31]. They reported that, following the opening of non-specific pores, cardiac mitochondria undergo swelling and depolarisation. The pore opening was the result of reperfusion, and low pH during ischaemia limited its occurrence [31, 35]. Subsequent research showed that reperfusion causes calcium overload and oxidative stress, that together initiate MPTP opening [24]. These were the defining points that highlighted the importance of mitochondria in the pathogenesis of IRI and placed an emphasis on the detrimental role of reperfusion injury, rather than the ischaemia alone, following MI. Further research by Halestrap and others investigated the molecular composition of the MPTP and its mechanism of activation [3, 34]. They showed that the translocation of cyclophilin D (CYPD) from the mitochondrial matrix to the mitochondrial inner membrane was necessary for the induction of MPTP opening. This could be inhibited by cyclosporine A administered at the onset of reperfusion [34]. Most cardioprotective strategies that act via the MPTP also require the presence of CYPD. Although transgenic mice deficient in CYPD are protected against IRI, different studies have shown that IPC and IPost are unable to augment this protection due to the lack of the CYPD protein [37, 51]. To prove the potential clinical significance of the MPTP in the human heart, sanglifehrin-A (SfA), a related compound able to inhibit MPTP opening, was administered to surgically isolated human atrial trabeculae subjected to simulated IRI. Once given at the start of reoxygenation, SfA blocked the MPTP opening and enhanced functional recovery of the human atrial trabeculae following simulated IRI [73]. However, the direct translation of therapeutic benefits of MPTP inhibition in human patients has proven to be challenging, with two major clinical trials of MPT inhibitors conducted on patients with ST-segment elevation MI (CIRCUS and CYCLE) both returning neutral results [60, 68].
Although numerous studies shed light on the benefits of MPTP inhibition in the setting of cardioprotection, the link between cardioprotective signalling pathways such as RISK and the MPTP remained elusive. To demonstrate the downstream effects of the RISK pathway and its relation to the MPTP, Davidson et al. examined the hypothesis that the activation of the prosurvival kinase pathway could protect cardiomyocytes by reducing the probability of MPTP opening [23]. They showed that the overexpression of constitutively active AKT was sufficient to significantly delay MPTP opening, indicating that activation of the PI3K–AKT prosurvival kinase pathway inhibits the opening of the MPTP. These studies demonstrated an important link between the survival kinases and the MPTP [23].
The nature of the putative effector protein(s) that modulate the MPTP following the signal transduction via the RISK pathway remains a subject of debate. Juhaszova et al. were amongst the first groups to discover a common signalling route linking various cardioprotective treatments to the MPTP [44]. They showed that insulin can protect isolated cardiomyocytes subjected to hypoxia and reoxygenation (HR) by reducing MPTP opening, and this effect was abolished in the presence of an inhibitor of PI3K. The underlying mechanism behind this protection was attributed to the increased phosphorylation of GSK-3β at Serine 9 (Ser9), thereby inhibiting its activity. Insulin’s effects were similar to the protection achieved in cardiomyocytes by eliminating GSK-3β (but not GSK-3α), thereby emphasising the importance of GSK-3β inactivation in the induction of cardioprotection by the RISK pathway [44]. In contrast to this report, Marber et al. verified the role of GSK-3β in the same setting and developed transgenic GSK-3β mice lacking both the critical Ser9 and Ser21 phosphorylation sites [63]. They showed that GSK-3β knock-in heart could be still protected against MPTP opening by insulin, IPC, and IPost treatments, thereby raising questions about the importance of GSK-3β function in the induction of the MPTP. Instead, they proposed that these cardioprotective treatments may block the MPTP by reducing oxidative stress at the onset of reperfusion [63]. A more recent study utilised both genetic and pharmacological approaches to document that GSK-3β inhibition additionally incorporates mechanisms independent of the MPTP such as autophagy to elicit cardioprotection [96]. Therefore, the cardioprotective effects of GSK-3β inhibition may go beyond the blockade of the MPTP. Several other candidate proteins such as those that participate in mitochondrial dynamics have been suggested to bridge the gap between the RISK pathway and MPTP induction. These proteins have been shown to be post translationally modified by the kinases of the RISK pathway which allows them to confer protection by reducing ROS, MPTP opening, and infarct size following IRI [45, 56, 66, 67]. Moreover, accumulating evidence during the past decade has documented the role of FOF1-ATPase in modulating MPTP opening during ischaemia and reperfusion [1, 13, 61]. However, the interplay between the RISK pathway and subunits of FOF1-ATPase remains elusive.
PI3K activation and cardioprotection
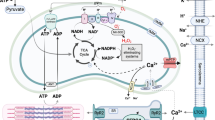
PI3K is central to the induction of the RISK pathway and major cardioprotective signalling molecules, as summarised in Table 1, utilise its function to induce their effects (Fig. 1). Since little was known about the roles of the individual isoforms of PI3K in cardioprotection, studies were designed to elucidate which isoform played a significant role in the protection observed following IRI. The main focus was placed on PI3Kα, since it was shown to play a central role in cardioprotection by participating in cardiac physiology, improving contractility, and promoting physiological exercise-induced growth but not pathological hypertrophy [59]. A study by Tsushima and his team showed that mice with PI3Kα dominant negative hearts, lacking 77% of PI3Kα activity, were resistant to IRI and this protection was lost in PI3Kγ−/− mice [5]. The protective effects in PI3Kα-dominant negative hearts were therefore attributed to the compensatory upregulation of PI3Kγ. However, the same group showed that heterozygous PI3Kγ+/− mice were also resistant to myocardial IRI. In addition, it was not shown whether complete deletion of PI3Kα was beneficial to the heart [5]. Conversely, double-mutant mice carrying PI3Kγ−/− and cardiac-specific PI3KαDN were later shown to have enhanced recovery following the IRI [57]. Contrary to these findings, constitutively active PI3Kα was demonstrated to improve left-ventricular function in heart failure, and indeed, the PI3Kα activator, insulin, was proven to be cardioprotective via PI3K [58, 92].
To address these controversial findings and based on the notion that insulin has been shown to be a canonical activator of PI3Kα, we took a pharmacological approach to investigate the role of PI3Kα isoform in cardioprotection. Using insulin to activate PI3Kα, in combination with highly selective inhibitors of PI3Kα, Rossello et al. undertook a series of experiments using the isolated perfused mouse heart subjected to IRI as well as immortalised mouse cardiac endothelial cells, cardiomyocytes, and human tissue to ascertain the role of pharmacological activation of PI3Kα activation in cells and tissues [69]. In summary, these studies demonstrated that activity of the PI3Kα isoform activity was required, during the early reperfusion phase, to reduce myocardial infarct size. This led to the conclusion that the development of drugs, specifically enhancing PI3Kα activity at reperfusion, could potentially promote myocardial salvage in patients undergoing acute MI [69].
If the above is correct, then harnessing the potential beneficial effects of kinase signalling through the generation of an isoform-specific PI3Kα activator could be of direct benefit to patients presenting with an acute MI. To develop such highly specific PI3Kα activators, we undertook a series of experiments with a number of collaborators at UCL (Gong et al. Nature 2023 in press, https://doi.org/10.1038/s41586-023-05972-2) in which we conducted an unbiased high throughput screen on 450,000 compounds from the AstraZeneca screening library with the aim of identifying small molecules that could activate the in vitro lipid kinase activity of recombinant human p110α/p85α, namely PI3Kα. Medicinal chemistry (at UCL) was then used to increase in vitro potency (as measured by in vitro activity on recombinant PI3Kα) and cellular potency (as measured by AKT phosphorylation in human A549 cells), all of which led to the generation of UCL-TRO-1938—referred to as 1938. We demonstrated that 1938 was able to induce the PI3Kα pathway in mouse primary cells as well as demonstrate infarct size limitation using both isolated ex vivo and in vivo rat heart models subjected to IRI. Further studies are now underway to assess the exact impact of UCL-TRO-1938 on the signalling cascade of the RISK pathway and MPTP.
Conclusions and perspective
The results discussed above represent the culmination of more than 3 decades of research in the field of cardioprotection that have highlighted the significance of the RISK pathway in the treatment of IRI. There is now extensive pre-clinical evidence that the activation of the RISK pathway, by a range of pharmacologic agents or by mechanical interventions such as IPC or IPost, reduces MI size by up to 50% via modulation of the MPTP. In accordance with the step-by-step criteria for IMproving Preclinical Assessment of Cardioprotective Therapies (‘IMPACT’) [49], the next steps are now to investigate the efficacy of RISK pathway activation in small animal models in the presence of potentially confounding comorbidities, such as age or diabetes [26], and the large animals of IRI.
Data availability
No data was generated for this article.
References
Antoniel M, Jones K, Antonucci S, Spolaore B, Fogolari F, Petronilli V, Giorgio V, Carraro M, Di Lisa F, Forte M, Szabo I, Lippe G, Bernardi P (2018) The unique histidine in OSCP subunit of F-ATP synthase mediates inhibition of the permeability transition pore by acidic pH. EMBO Rep 19:257–268. https://doi.org/10.15252/embr.201744705
Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP (2013) Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res 10:301–312. https://doi.org/10.1016/j.scr.2013.01.002
Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD (2005) Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434:658–662. https://doi.org/10.1038/nature03434
Baines CP, Wang L, Cohen MV, Downey JM (1999) Myocardial protection by insulin is dependent on phospatidylinositol 3-kinase but not protein kinase C or KATP channels in the isolated rabbit heart. Basic Res Cardiol 94:188–198. https://doi.org/10.1007/s003950050142
Ban K, Cooper AJ, Samuel S, Bhatti A, Patel M, Izumo S, Penninger JM, Backx PH, Oudit GY, Tsushima RG (2008) Phosphatidylinositol 3-kinase gamma is a critical mediator of myocardial ischemic and adenosine-mediated preconditioning. Circ Res 103:643–653. https://doi.org/10.1161/CIRCRESAHA.108.175018
Basalay MV, Mastitskaya S, Mrochek A, Ackland GL, Del Arroyo AG, Sanchez J, Sjoquist PO, Pernow J, Gourine AV, Gourine A (2016) Glucagon-like peptide-1 (GLP-1) mediates cardioprotection by remote ischaemic conditioning. Cardiovasc Res 112:669–676. https://doi.org/10.1093/cvr/cvw216
Baxter GF, Mocanu MM, Brar BK, Latchman DS, Yellon DM (2001) Cardioprotective effects of transforming growth factor-beta1 during early reoxygenation or reperfusion are mediated by p42/p44 MAPK. J Cardiovasc Pharmacol 38:930–939. https://doi.org/10.1097/00005344-200112000-00015
Beikoghli Kalkhoran S, Kararigas G (2022) Oestrogenic regulation of mitochondrial dynamics. Int J Mol Sci. https://doi.org/10.3390/ijms23031118
Bell RM, Basalay M, Botker HE, Beikoghli Kalkhoran S, Carr RD, Cunningham J, Davidson SM, England TJ, Giesz S, Ghosh AK, Golforoush P, Gourine AV, Hausenloy DJ, Heusch G, Ibanez B, Kleinbongard P, Lecour S, Lukhna K, Ntsekhe M, Ovize M, Salama AD, Vilahur G, Walker JM, Yellon DM (2022) Remote ischaemic conditioning: defining critical criteria for success-report from the 11th Hatter Cardiovascular Workshop. Basic Res Cardiol 117:39. https://doi.org/10.1007/s00395-022-00947-2
Bell RM, Yellon DM (2003) Atorvastatin, administered at the onset of reperfusion, and independent of lipid lowering, protects the myocardium by up-regulating a pro-survival pathway. J Am Coll Cardiol 41:508–515. https://doi.org/10.1016/s0735-1097(02)02816-4
Bell RM, Yellon DM (2003) Bradykinin limits infarction when administered as an adjunct to reperfusion in mouse heart: the role of PI3K, Akt and eNOS. J Mol Cell Cardiol 35:185–193. https://doi.org/10.1016/s0022-2828(02)00310-3
Bhamra GS, Hausenloy DJ, Davidson SM, Carr RD, Paiva M, Wynne AM, Mocanu MM, Yellon DM (2008) Metformin protects the ischemic heart by the Akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic Res Cardiol 103:274–284. https://doi.org/10.1007/s00395-007-0691-y
Bonora M, Giorgi C, Pinton P (2022) Molecular mechanisms and consequences of mitochondrial permeability transition. Nat Rev Mol Cell Biol 23:266–285. https://doi.org/10.1038/s41580-021-00433-y
Botker HE, Cabrera-Fuentes HA, Ruiz-Meana M, Heusch G, Ovize M (2020) Translational issues for mitoprotective agents as adjunct to reperfusion therapy in patients with ST-segment elevation myocardial infarction. J Cell Mol Med 24:2717–2729. https://doi.org/10.1111/jcmm.14953
Bouwman RA, Salic K, Padding FG, Eringa EC, van Beek-Harmsen BJ, Matsuda T, Baba A, Musters RJ, Paulus WJ, de Lange JJ, Boer C (2006) Cardioprotection via activation of protein kinase C-delta depends on modulation of the reverse mode of the Na+/Ca2+ exchanger. Circulation 114:I226-232. https://doi.org/10.1161/CIRCULATIONAHA.105.000570
Bromage DI, Taferner S, He Z, Ziff OJ, Yellon DM, Davidson SM (2019) Stromal cell-derived factor-1alpha signals via the endothelium to protect the heart against ischaemia-reperfusion injury. J Mol Cell Cardiol 128:187–197. https://doi.org/10.1016/j.yjmcc.2019.02.002
Busca R, Pouyssegur J, Lenormand P (2016) ERK1 and ERK2 Map Kinases: Specific Roles or Functional Redundancy? Front Cell Dev Biol 4:53. https://doi.org/10.3389/fcell.2016.00053
Camacho X, Nedkoff L, Wright FL, Nghiem N, Buajitti E, Goldacre R, Rosella LC, Seminog O, Tan EJ, Hayes A, Hayen A, Wilson N, Blakely T, Clarke P (2022) Relative contribution of trends in myocardial infarction event rates and case fatality to declines in mortality: an international comparative study of 1.95 million events in 80.4 million people in four countries. Lancet Public Health 7:e229–e239. https://doi.org/10.1016/S2468-2667(22)00006-8
Chen X, Zhabyeyev P, Azad AK, Wang W, Minerath RA, DesAulniers J, Grueter CE, Murray AG, Kassiri Z, Vanhaesebroeck B, Oudit GY (2019) Endothelial and cardiomyocyte PI3Kbeta divergently regulate cardiac remodelling in response to ischaemic injury. Cardiovasc Res 115:1343–1356. https://doi.org/10.1093/cvr/cvy298
Crompton M, Costi A (1990) A heart mitochondrial Ca2(+)-dependent pore of possible relevance to re-perfusion-induced injury. Evidence that ADP facilitates pore interconversion between the closed and open states. Biochem J 266:33–39. https://doi.org/10.1042/bj2660033
Crompton M, Costi A, Hayat L (1987) Evidence for the presence of a reversible Ca2+-dependent pore activated by oxidative stress in heart mitochondria. Biochem J 245:915–918. https://doi.org/10.1042/bj2450915
Davidson SM, Adameova A, Barile L, Cabrera-Fuentes HA, Lazou A, Pagliaro P, Stenslokken KO, Garcia-Dorado D, Action E-CC (2020) Mitochondrial and mitochondrial-independent pathways of myocardial cell death during ischaemia and reperfusion injury. J Cell Mol Med 24:3795–3806. https://doi.org/10.1111/jcmm.15127
Davidson SM, Hausenloy D, Duchen MR, Yellon DM (2006) Signalling via the reperfusion injury signalling kinase (RISK) pathway links closure of the mitochondrial permeability transition pore to cardioprotection. Int J Biochem Cell Biol 38:414–419. https://doi.org/10.1016/j.biocel.2005.09.017
Davidson SM, Yellon DM, Murphy MP, Duchen MR (2012) Slow calcium waves and redox changes precede mitochondrial permeability transition pore opening in the intact heart during hypoxia and reoxygenation. Cardiovasc Res 93:445–453. https://doi.org/10.1093/cvr/cvr349
Diaz-Ruiz JL, Macias-Lopez A, Alcala-Vargas F, Guevara-Chavez JG, Mejia-Uribe A, Silva-Palacios A, Zuniga-Munoz A, Zazueta C, Buelna-Chontal M (2019) Redox signaling in ischemic postconditioning protection involves PKCepsilon and Erk1/2 pathways and converges indirectly in Nrf2 activation. Cell Signal 64:109417. https://doi.org/10.1016/j.cellsig.2019.109417
Ferdinandy P, Andreadou I, Baxter GF, Botker HE, Davidson SM, Dobrev D, Gersh BJ, Heusch G, Lecour S, Ruiz-Meana M, Zuurbier CJ, Hausenloy DJ, Schulz R (2023) Interaction of cardiovascular nonmodifiable risk factors, comorbidities and comedications with ischemia/reperfusion injury and cardioprotection by pharmacological treatments and ischemic conditioning. Pharmacol Rev 75:159–216. https://doi.org/10.1124/pharmrev.121.000348
Fryer RM, Pratt PF, Hsu AK, Gross GJ (2001) Differential activation of extracellular signal regulated kinase isoforms in preconditioning and opioid-induced cardioprotection. J Pharmacol Exp Ther 296:642–649
Gao J, Chang Chua C, Chen Z, Wang H, Xu X, Hamdy RC, McMullen JR, Shioi T, Izumo S, Chua BH (2007) Resistin, an adipocytokine, offers protection against acute myocardial infarction. J Mol Cell Cardiol 43:601–609. https://doi.org/10.1016/j.yjmcc.2007.08.009
Ghigo A, Laffargue M, Li M, Hirsch E (2017) PI3K and calcium signaling in cardiovascular disease. Circ Res 121:282–292. https://doi.org/10.1161/CIRCRESAHA.117.310183
Granata R, Trovato L, Gallo MP, Destefanis S, Settanni F, Scarlatti F, Brero A, Ramella R, Volante M, Isgaard J, Levi R, Papotti M, Alloatti G, Ghigo E (2009) Growth hormone-releasing hormone promotes survival of cardiac myocytes in vitro and protects against ischaemia-reperfusion injury in rat heart. Cardiovasc Res 83:303–312. https://doi.org/10.1093/cvr/cvp090
Griffiths EJ, Halestrap AP (1995) Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J 307(Pt 1):93–98. https://doi.org/10.1042/bj3070093
Gross GJ, Auchampach JA (1992) Blockade of ATP-sensitive potassium channels prevents myocardial preconditioning in dogs. Circ Res 70:223–233. https://doi.org/10.1161/01.res.70.2.223
Guo J, Bian Y, Bai R, Li H, Fu M, Xiao C (2013) Globular adiponectin attenuates myocardial ischemia/reperfusion injury by upregulating endoplasmic reticulum Ca(2)(+)-ATPase activity and inhibiting endoplasmic reticulum stress. J Cardiovasc Pharmacol 62:143–153. https://doi.org/10.1097/FJC.0b013e31829521af
Halestrap AP, Connern CP, Griffiths EJ, Kerr PM (1997) Cyclosporin A binding to mitochondrial cyclophilin inhibits the permeability transition pore and protects hearts from ischaemia/reperfusion injury. Mol Cell Biochem 174:167–172
Halestrap AP, Griffiths EJ, Connern CP (1993) Mitochondrial calcium handling and oxidative stress. Biochem Soc Trans 21:353–358. https://doi.org/10.1042/bst0210353
Hausenloy DJ, Iliodromitis EK, Andreadou I, Papalois A, Gritsopoulos G, Anastasiou-Nana M, Kremastinos DT, Yellon DM (2012) Investigating the signal transduction pathways underlying remote ischemic conditioning in the porcine heart. Cardiovasc Drugs Ther 26:87–93. https://doi.org/10.1007/s10557-011-6364-y
Hausenloy DJ, Lim SY, Ong SG, Davidson SM, Yellon DM (2010) Mitochondrial cyclophilin-D as a critical mediator of ischaemic preconditioning. Cardiovasc Res 88:67–74. https://doi.org/10.1093/cvr/cvq113
Hausenloy DJ, Mocanu MM, Yellon DM (2004) Cross-talk between the survival kinases during early reperfusion: its contribution to ischemic preconditioning. Cardiovasc Res 63:305–312. https://doi.org/10.1016/j.cardiores.2004.04.011
Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM (2005) Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol 288:H971-976. https://doi.org/10.1152/ajpheart.00374.2004
Hausenloy DJ, Yellon DM (2004) New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res 61:448–460. https://doi.org/10.1016/j.cardiores.2003.09.024
Heusch G (2020) Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol 17:773–789. https://doi.org/10.1038/s41569-020-0403-y
Jonassen AK, Brar BK, Mjos OD, Sack MN, Latchman DS, Yellon DM (2000) Insulin administered at reoxygenation exerts a cardioprotective effect in myocytes by a possible anti-apoptotic mechanism. J Mol Cell Cardiol 32:757–764. https://doi.org/10.1006/jmcc.2000.1118
Jonassen AK, Sack MN, Mjos OD, Yellon DM (2001) Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circ Res 89:1191–1198. https://doi.org/10.1161/hh2401.101385
Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ (2004) Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest 113:1535–1549. https://doi.org/10.1172/JCI19906
Kalkhoran SB, Kriston-Vizi J, Hernandez-Resendiz S, Crespo-Avilan GE, Rosdah AA, Lees JG, Costa J, Ling NXY, Holien JK, Samangouei P, Chinda K, Yap EP, Riquelme JA, Ketteler R, Yellon DM, Lim SY, Hausenloy DJ (2022) Hydralazine protects the heart against acute ischaemia/reperfusion injury by inhibiting Drp1-mediated mitochondrial fission. Cardiovasc Res 118:282–294. https://doi.org/10.1093/cvr/cvaa343
Kin H, Zatta AJ, Lofye MT, Amerson BS, Halkos ME, Kerendi F, Zhao ZQ, Guyton RA, Headrick JP, Vinten-Johansen J (2005) Postconditioning reduces infarct size via adenosine receptor activation by endogenous adenosine. Cardiovasc Res 67:124–133. https://doi.org/10.1016/j.cardiores.2005.02.015
Kleinbongard P, Lieder HR, Skyschally A, Alloosh M, Godecke A, Rahmann S, Sturek M, Heusch G (2022) Non-responsiveness to cardioprotection by ischaemic preconditioning in Ossabaw minipigs with genetic predisposition to, but without the phenotype of the metabolic syndrome. Basic Res Cardiol 117:58. https://doi.org/10.1007/s00395-022-00965-0
Kunuthur SP, Mocanu MM, Hemmings BA, Hausenloy DJ, Yellon DM (2012) The Akt1 isoform is an essential mediator of ischaemic preconditioning. J Cell Mol Med 16:1739–1749. https://doi.org/10.1111/j.1582-4934.2011.01491.x
Lecour S, Andreadou I, Botker HE, Davidson SM, Heusch G, Ruiz-Meana M, Schulz R, Zuurbier CJ, Ferdinandy P, Hausenloy DJ, on behalf of the European Union CCAC (2021) IMproving preclinical assessment of cardioprotective therapies (IMPACT) criteria: guidelines of the EU-CARDIOPROTECTION COST Action. Basic Res Cardiol 116:52. https://doi.org/10.1007/s00395-021-00893-5
Liao Y, Li H, Pi Y, Li Z, Jin S (2019) Cardioprotective effect of IGF-1 against myocardial ischemia/reperfusion injury through activation of PI3K/Akt pathway in rats in vivo. J Int Med Res 47:3886–3897. https://doi.org/10.1177/0300060519857839
Lim SY, Davidson SM, Hausenloy DJ, Yellon DM (2007) Preconditioning and postconditioning: the essential role of the mitochondrial permeability transition pore. Cardiovasc Res 75:530–535. https://doi.org/10.1016/j.cardiores.2007.04.022
Lim SY, Davidson SM, Paramanathan AJ, Smith CC, Yellon DM, Hausenloy DJ (2008) The novel adipocytokine visfatin exerts direct cardioprotective effects. J Cell Mol Med 12:1395–1403. https://doi.org/10.1111/j.1582-4934.2008.00332.x
Lips DJ, Bueno OF, Wilkins BJ, Purcell NH, Kaiser RA, Lorenz JN, Voisin L, Saba-El-Leil MK, Meloche S, Pouyssegur J, Pages G, De Windt LJ, Doevendans PA, Molkentin JD (2004) MEK1-ERK2 signaling pathway protects myocardium from ischemic injury in vivo. Circulation 109:1938–1941. https://doi.org/10.1161/01.CIR.0000127126.73759.23
Liu SQ, Roberts D, Kharitonenkov A, Zhang B, Hanson SM, Li YC, Zhang LQ, Wu YH (2013) Endocrine protection of ischemic myocardium by FGF21 from the liver and adipose tissue. Sci Rep 3:2767. https://doi.org/10.1038/srep02767
Maciel L, de Oliveira DF, Mesquita F, Souza H, Oliveira L, Christie MLA, Palhano FL, de Campos Carvalho AC, Nascimento JHM, Foguel D (2021) New cardiomyokine reduces myocardial ischemia/reperfusion injury by PI3K-AKT pathway via a putative KDEL-receptor binding. J Am Heart Assoc 10:e019685. https://doi.org/10.1161/JAHA.120.019685
Maneechote C, Palee S, Kerdphoo S, Jaiwongkam T, Chattipakorn SC, Chattipakorn N (2022) Modulating mitochondrial dynamics attenuates cardiac ischemia-reperfusion injury in prediabetic rats. Acta Pharmacol Sin 43:26–38. https://doi.org/10.1038/s41401-021-00626-3
McLean BA, Kienesberger PC, Wang W, Masson G, Zhabyeyev P, Dyck JR, Oudit GY (2013) Enhanced recovery from ischemia-reperfusion injury in PI3Kalpha dominant negative hearts: investigating the role of alternate PI3K isoforms, increased glucose oxidation and MAPK signaling. J Mol Cell Cardiol 54:9–18. https://doi.org/10.1016/j.yjmcc.2012.10.015
McMullen JR, Amirahmadi F, Woodcock EA, Schinke-Braun M, Bouwman RD, Hewitt KA, Mollica JP, Zhang L, Zhang Y, Shioi T, Buerger A, Izumo S, Jay PY, Jennings GL (2007) Protective effects of exercise and phosphoinositide 3-kinase(p110alpha) signaling in dilated and hypertrophic cardiomyopathy. Proc Natl Acad Sci USA 104:612–617. https://doi.org/10.1073/pnas.0606663104
McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, Izumo S (2003) Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci USA 100:12355–12360. https://doi.org/10.1073/pnas.1934654100
Mewton N, Bergerot C, Ovize M (2016) Cyclosporine before PCI in acute myocardial infarction. N Engl J Med 374:90. https://doi.org/10.1056/NEJMc1514192
Morciano G, Preti D, Pedriali G, Aquila G, Missiroli S, Fantinati A, Caroccia N, Pacifico S, Bonora M, Talarico A, Morganti C, Rizzo P, Ferrari R, Wieckowski MR, Campo G, Giorgi C, Trapella C, Pinton P (2018) Discovery of novel 1,3,8-triazaspiro[4.5]decane derivatives that target the c subunit of F(1)/F(O)-adenosine triphosphate (ATP) synthase for the treatment of reperfusion damage in myocardial infarction. J Med Chem 61:7131–7143. https://doi.org/10.1021/acs.jmedchem.8b00278
Mudalagiri NR, Mocanu MM, Di Salvo C, Kolvekar S, Hayward M, Yap J, Keogh B, Yellon DM (2008) Erythropoietin protects the human myocardium against hypoxia/reoxygenation injury via phosphatidylinositol-3 kinase and ERK1/2 activation. Br J Pharmacol 153:50–56. https://doi.org/10.1038/sj.bjp.0707461
Nishino Y, Webb IG, Davidson SM, Ahmed AI, Clark JE, Jacquet S, Shah AM, Miura T, Yellon DM, Avkiran M, Marber MS (2008) Glycogen synthase kinase-3 inactivation is not required for ischemic preconditioning or postconditioning in the mouse. Circ Res 103:307–314. https://doi.org/10.1161/CIRCRESAHA.107.169953
Okumura H, Nagaya N, Itoh T, Okano I, Hino J, Mori K, Tsukamoto Y, Ishibashi-Ueda H, Miwa S, Tambara K, Toyokuni S, Yutani C, Kangawa K (2004) Adrenomedullin infusion attenuates myocardial ischemia/reperfusion injury through the phosphatidylinositol 3-kinase/Akt-dependent pathway. Circulation 109:242–248. https://doi.org/10.1161/01.CIR.0000109214.30211.7C
Oldenburg O, Cohen MV, Yellon DM, Downey JM (2002) Mitochondrial K(ATP) channels: role in cardioprotection. Cardiovasc Res 55:429–437. https://doi.org/10.1016/s0008-6363(02)00439-x
Ong SB, Hall AR, Dongworth RK, Kalkhoran S, Pyakurel A, Scorrano L, Hausenloy DJ (2015) Akt protects the heart against ischaemia-reperfusion injury by modulating mitochondrial morphology. Thromb Haemost 113:513–521. https://doi.org/10.1160/TH14-07-0592
Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ (2010) Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 121:2012–2022. https://doi.org/10.1161/CIRCULATIONAHA.109.906610
Ottani F, Latini R, Staszewsky L, La Vecchia L, Locuratolo N, Sicuro M, Masson S, Barlera S, Milani V, Lombardi M, Costalunga A, Mollichelli N, Santarelli A, De Cesare N, Sganzerla P, Boi A, Maggioni AP, Limbruno U, Investigators C (2016) Cyclosporine A in reperfused myocardial infarction: the multicenter, controlled, open-label CYCLE trial. J Am Coll Cardiol 67:365–374. https://doi.org/10.1016/j.jacc.2015.10.081
Rossello X, Riquelme JA, He Z, Taferner S, Vanhaesebroeck B, Davidson SM, Yellon DM (2017) The role of PI3Kalpha isoform in cardioprotection. Basic Res Cardiol 112:66. https://doi.org/10.1007/s00395-017-0657-7
Russell JS, Griffith TA, Helman T, Du Toit EF, Peart JN, Headrick JP (2019) Chronic type 2 but not type 1 diabetes impairs myocardial ischaemic tolerance and preconditioning in C57Bl/6 mice. Exp Physiol 104:1868–1880. https://doi.org/10.1113/EP088024
Schulman D, Latchman DS, Yellon DM (2002) Urocortin protects the heart from reperfusion injury via upregulation of p42/p44 MAPK signaling pathway. Am J Physiol Heart Circ Physiol 283:H1481-1488. https://doi.org/10.1152/ajpheart.01089.2001
Schwartz LM, Lagranha CJ (2006) Ischemic postconditioning during reperfusion activates Akt and ERK without protecting against lethal myocardial ischemia-reperfusion injury in pigs. Am J Physiol Heart Circ Physiol 290:H1011-1018. https://doi.org/10.1152/ajpheart.00864.2005
Shanmuganathan S, Hausenloy DJ, Duchen MR, Yellon DM (2005) Mitochondrial permeability transition pore as a target for cardioprotection in the human heart. Am J Physiol Heart Circ Physiol 289:H237-242. https://doi.org/10.1152/ajpheart.01192.2004
Simpkin JC, Yellon DM, Davidson SM, Lim SY, Wynne AM, Smith CC (2007) Apelin-13 and apelin-36 exhibit direct cardioprotective activity against ischemia-reperfusion injury. Basic Res Cardiol 102:518–528. https://doi.org/10.1007/s00395-007-0671-2
Sivaraman V, Hausenloy DJ, Wynne AM, Yellon DM (2010) Preconditioning the diabetic human myocardium. J Cell Mol Med 14:1740–1746. https://doi.org/10.1111/j.1582-4934.2009.00796.x
Sivaraman V, Mudalagiri NR, Di Salvo C, Kolvekar S, Hayward M, Yap J, Keogh B, Hausenloy DJ, Yellon DM (2007) Postconditioning protects human atrial muscle through the activation of the RISK pathway. Basic Res Cardiol 102:453–459. https://doi.org/10.1007/s00395-007-0664-1
Skyschally A, van Caster P, Boengler K, Gres P, Musiolik J, Schilawa D, Schulz R, Heusch G (2009) Ischemic postconditioning in pigs: no causal role for RISK activation. Circ Res 104:15–18. https://doi.org/10.1161/CIRCRESAHA.108.186429
Smith CC, Mocanu MM, Davidson SM, Wynne AM, Simpkin JC, Yellon DM (2006) Leptin, the obesity-associated hormone, exhibits direct cardioprotective effects. Br J Pharmacol 149:5–13. https://doi.org/10.1038/sj.bjp.0706834
Sovershaev MA, Egorina EM, Andreasen TV, Jonassen AK, Ytrehus K (2006) Preconditioning by 17beta-estradiol in isolated rat heart depends on PI3-K/PKB pathway, PKC, and ROS. Am J Physiol Heart Circ Physiol 291:H1554-1562. https://doi.org/10.1152/ajpheart.01171.2005
Takahama H, Minamino T, Hirata A, Ogai A, Asanuma H, Fujita M, Wakeno M, Tsukamoto O, Okada K, Komamura K, Takashima S, Shinozaki Y, Mori H, Mochizuki N, Kitakaze M (2006) Granulocyte colony-stimulating factor mediates cardioprotection against ischemia/reperfusion injury via phosphatidylinositol-3-kinase/Akt pathway in canine hearts. Cardiovasc Drugs Ther 20:159–165. https://doi.org/10.1007/s10557-006-8285-8
Takov K, He Z, Johnston HE, Timms JF, Guillot PV, Yellon DM, Davidson SM (2020) Small extracellular vesicles secreted from human amniotic fluid mesenchymal stromal cells possess cardioprotective and promigratory potential. Basic Res Cardiol 115:26. https://doi.org/10.1007/s00395-020-0785-3
Torp MK, Vaage J, Stenslokken KO (2023) Mitochondria-derived damage-associated molecular patterns and inflammation in the ischemic-reperfused heart. Acta Physiol (Oxf) 237:e13920. https://doi.org/10.1111/apha.13920
Tsang A, Hausenloy DJ, Mocanu MM, Carr RD, Yellon DM (2005) Preconditioning the diabetic heart: the importance of Akt phosphorylation. Diabetes 54:2360–2364. https://doi.org/10.2337/diabetes.54.8.2360
Tsang A, Hausenloy DJ, Mocanu MM, Yellon DM (2004) Postconditioning: a form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ Res 95:230–232. https://doi.org/10.1161/01.RES.0000138303.76488.fe
Vahlhaus C, Schulz R, Post H, Onallah R, Heusch G (1996) No prevention of ischemic preconditioning by the protein kinase C inhibitor staurosporine in swine. Circ Res 79:407–414. https://doi.org/10.1161/01.res.79.3.407
Wagner C, Ebner B, Tillack D, Strasser RH, Weinbrenner C (2013) Cardioprotection by ischemic postconditioning is abrogated in hypertrophied myocardium of spontaneously hypertensive rats. J Cardiovasc Pharmacol 61:35–41. https://doi.org/10.1097/FJC.0b013e3182760c4d
Wang F, Wang H, Liu X, Yu H, Zuo B, Song Z, Wang N, Huang W, Wang G (2018) Pharmacological postconditioning with Neuregulin-1 mimics the cardioprotective effects of ischaemic postconditioning via ErbB4-dependent activation of reperfusion injury salvage kinase pathway. Mol Med 24:39. https://doi.org/10.1186/s10020-018-0040-7
Whittington HJ, Harding I, Stephenson CI, Bell R, Hausenloy DJ, Mocanu MM, Yellon DM (2013) Cardioprotection in the aging, diabetic heart: the loss of protective Akt signalling. Cardiovasc Res 99:694–704. https://doi.org/10.1093/cvr/cvt140
Wynne AM, Mocanu MM, Yellon DM (2005) Pioglitazone mimics preconditioning in the isolated perfused rat heart: a role for the prosurvival kinases PI3K and P42/44MAPK. J Cardiovasc Pharmacol 46:817–822. https://doi.org/10.1097/01.fjc.0000188365.07635.57
Yan L, Zhu TB, Wang LS, Pan SY, Tao ZX, Yang Z, Cao K, Huang J (2011) Inhibitory effect of hepatocyte growth factor on cardiomyocytes apoptosis is partly related to reduced calcium sensing receptor expression during a model of simulated ischemia/reperfusion. Mol Biol Rep 38:2695–2701. https://doi.org/10.1007/s11033-010-0412-8
Yang XM, Philipp S, Downey JM, Cohen MV (2005) Postconditioning’s protection is not dependent on circulating blood factors or cells but involves adenosine receptors and requires PI3-kinase and guanylyl cyclase activation. Basic Res Cardiol 100:57–63. https://doi.org/10.1007/s00395-004-0498-4
Yao H, Han X, Han X (2014) The cardioprotection of the insulin-mediated PI3K/Akt/mTOR signaling pathway. Am J Cardiovasc Drugs 14:433–442. https://doi.org/10.1007/s40256-014-0089-9
Ye X, Zhang P, Zhang Y, Luan J, Xu C, Wu Z, Ju D, Hu W (2022) GSDMD contributes to myocardial reperfusion injury by regulating pyroptosis. Front Immunol 13:893914. https://doi.org/10.3389/fimmu.2022.893914
Yu L, Li B, Zhang M, Jin Z, Duan W, Zhao G, Yang Y, Liu Z, Chen W, Wang S, Yang J, Yi D, Liu J, Yu S (2016) Melatonin reduces PERK-eIF2alpha-ATF4-mediated endoplasmic reticulum stress during myocardial ischemia-reperfusion injury: role of RISK and SAFE pathways interaction. Apoptosis 21:809–824. https://doi.org/10.1007/s10495-016-1246-1
Zeng B, Liu L, Liao X, Zhang C (2021) Cardiomyocyte protective effects of thyroid hormone during hypoxia/reoxygenation injury through activating of IGF-1-mediated PI3K/Akt signalling. J Cell Mol Med 25:3205–3215. https://doi.org/10.1111/jcmm.16389
Zhai P, Sciarretta S, Galeotti J, Volpe M, Sadoshima J (2011) Differential roles of GSK-3beta during myocardial ischemia and ischemia/reperfusion. Circ Res 109:502–511. https://doi.org/10.1161/CIRCRESAHA.111.249532
Acknowledgements
The authors acknowledge the support of the British Heart Foundation (BHF PG/16/85/32471, PG/18/44/33790, PG/19/51/34493, and PG/21/10798).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
This article is part of the topical collection “Mitochondria at the heart of cardioprotection”.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yellon, D.M., Beikoghli Kalkhoran, S. & Davidson, S.M. The RISK pathway leading to mitochondria and cardioprotection: how everything started. Basic Res Cardiol 118, 22 (2023). https://doi.org/10.1007/s00395-023-00992-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-023-00992-5