Abstract
Purpose
Diet quality is a critical modifiable factor related to health, including the risk of cardiometabolic complications. Rather than assessing the intake of individual food items, it is more meaningful to examine overall dietary patterns. This study investigated the adherence to common dietary indices and their association with serum/metabolic parameters of disease risk.
Methods
Dietary intakes of the general adult population (n = 1404, 25–79 years) were assessed by a validated food-frequency questionnaire (174 items). The French ANSES-Ciqual food composition database was used to compute nutrient intakes. Seven indicators were calculated to investigate participants’ diet quality: the Alternative Healthy Eating Index (AHEI), Dietary Approaches to Stop Hypertension Score (DASH-S), Mediterranean Diet Score (MDS), Diet Quality Index-International (DQI-I), Dietary Inflammatory Index (DII), Dietary Antioxidant Index (DAI), and Naturally Nutrient-Rich Score (NNRS). Various serum/metabolic parameters were used in the validity and association analyses, including markers of inflammation, blood glucose, and blood lipid status.
Results
Following linear regression models adjusted for confounders, the DASH-S was significantly associated with most metabolic parameters (14, e.g., inversely with blood pressure, triglycerides, urinary sodium, uric acid, and positively with serum vitamin D), followed by the DQI-I (13, e.g., total cholesterol, apo-A/B, uric acid, and blood pressure) and the AHEI (11, e.g., apo-A, uric acid, serum vitamin D, diastolic blood pressure and vascular age).
Conclusion
Food-group-based indices, including DASH-S, DQI-I, and AHEI, were good predictors for serum/metabolic parameters, while nutrient-based indices, such as the DAI or NNRS, were less related to biological markers and, thus, less suitable to reflect diet quality in a general population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The quality of the diet, along with other lifestyle factors, such as physical activity and smoking, which are regarded as being among the most critical modifiable factors related to the incidence of several non-communicable diseases (NCDs) and health issues, including cardiovascular disease (CVD) [1], type 2 diabetes (T2D), obesity/overweight [2, 3], some types of cancer [4] and nonalcoholic fatty liver disease (NAFLD) [5]. Many of these NCDs are surging, e.g., the age-standardized prevalence of T2D in adults has almost doubled, from 4.7% in 1980 to 8.5% in 2014, though heterogeneity in the global distribution is considerable [6].
Despite that much emphasis has been placed on individual dietary constituents, such as limiting sugar and salt, as well as saturated fat intake or increasing dietary fiber consumption, it has been highlighted that the overall dietary patterns constitute a better marker for the healthiness of a diet [7]. It is possibly better suited to be related to the risk of certain chronic diseases than individual food items alone [8]. Indeed, studies have shown that diet quality can be considered as an independent factor for predicting the risk of various diseases [2,3,4,5].
However, it is paramount to define the best scheme or method to evaluate the overall quality of the diet in different populations. For this purpose, several indices have been developed that capture various aspects of the diet. Though these dietary indices partly focus on different aspects of the diet, almost all of them strive to provide a comprehensive and complete perspective of dietary quality regarding a specific target, such as the intake of antioxidants. In contrast to nutritional surveys investigating only macro-micronutrient intake, the indices aim to examine various aspects of a person's diet, such as variety, balance, adequacy, and health-related aspects [9], considering the intake of certain nutrients (nutrient-based indices) and/or food items (food-group-based indices). For example, the Healthy Eating Index (HEI), a food-group-based index, has been designed to examine the overall adherence of individuals to the 2015–2020 Dietary Guidelines for Americans [10]. A recent study showed that the HEI-2015 and its constituents was associated with inflammatory biomarkers, e.g., lower circulating c-reactive protein (CRP) and interleukin-6 (IL-6) concentrations, as well as white blood cell (WBC) counts [11]. Likewise, an exploratory analysis concluded that there existed a significant association between the HEI and total serum antioxidant capacity and inflammatory markers, including tumour necrosis factor α (TFN-α) and Il-6 [12]. As another example, the Diet Quality Index-International (DQI-I), a nutrient and food-group-based index, is one of the indices designed to assess the overall quality of an individual's diet [13]. The DQI-I includes four scoring subgroups that examine complementary aspects of diet, i.e., variety, moderation, adequacy, and overall diet balance (Supplementary Tables 1 and 4) [13, 14]. Studies have shown that the DQI-I correlated with several biomarkers associated with cardiometabolic risk factors, including inversely with total serum cholesterol, body mass index (BMI), and positively with high-density lipoprotein cholesterol (HDL-c), among others [14,15,16].
Some indices focus on more specific aspects of diet-related disease risk. For example, the Dietary Inflammatory Index (DII) focuses mainly on the pro- vs. anti-inflammatory properties of the diet, as many NCDs have been related to chronic inflammation, such as CVD [1], diabetes [17], cancer [18], NAFLD [19], and obesity [20]. The positive association of the DII with inflammation-related biomarkers such as TNF-α, IL-6, and CRP and more indirect biomarkers such as the serum levels of insulin and the erythrocyte sedimentation rate (ESR) has been shown in several studies [1, 17,18,19]. The Dietary Antioxidant Index (DAI) is another example where the main focus rests on the antioxidant properties of the diet [21], as increased oxidative stress levels also characterize many NCDs.
Nonetheless, each of these indices has inherent strengths and limitations, and some inevitably overlap, despite showing complementarity. For instance, strong correlations have been found between the DQI-I and Dietary Approaches to Stop Hypertension Score (DASH-S) [22]. Moreover, a systematic review and meta-analysis of cohort studies concluded that dietary indices such as DASH-S were associated with health status, including all-cause mortality, CVD and cancer incidence or mortality, T2D, and neurodegenerative disease and related (bio)markers, including inflammatory indicators and body composition, among others [23]. However, due to the large number of published dietary indices and, in part, their large diversity, choosing an index that can thoroughly analyze dietary quality and correlate with the targeted health outcome, such as specific biological endpoints, is challenging. Systematic and narrative reviews have attempted to identify/introduce the most suitable and effective index to capture total dietary patterns; however, this is impossible due to the complexity of the diet and its many food components and eating habits/patterns. According to conclusions stated in several reviews, rather than pursuing a “one size fits all”, the best strategy may be to choose the most appropriate index or indices depending on the research question, taking into account the strengths and limitations of that index/those indices.
In the present study, we selected a range of frequently used indices (nutrient-based, food-group-based, as well as food-group and nutrient-based indices) to examine the association between the quality of the diet and selected biomarkers of disease risk in a general adult population residing in Luxembourg, taking part in the second wave of the ORISCAV-LUX study. The indices were calculated based on valid food-frequency questionnaires (FFQs) and were associated with selected serum and metabolic parameters.
Materials and methods
Study population and design
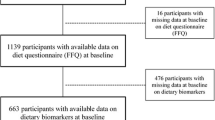
The full study protocol and method description have been published previously [24, 25]. Briefly, the findings are based on the second wave of the Observation of Cardiovascular Risk Factors in Luxembourg (ORISCAV-LUX 2; 2016–2017), the second nationwide study on CVD prevalence and related risk factors in the Luxembourgish adult population. This is a follow-up to the ORISCAV-LUX 1 study, which was implemented on adults residing in Luxembourg aged 18–69 years in 2007–2008 [25]. Luxembourgish residents aged 25–81 years, with a total of 1558 persons, were enrolled in the second wave, ORISCAV-LUX 2. As the research protocol stipulated that people until 79 years of age can participate, one participant (81 years old) was excluded from the analyses. In addition, participants without anthropometric and energy intake data (n = 7), FFQ (n = 120), and extreme values (top 1 and bottom percentile) in dietary energy intakes (n = 26) were excluded from the final analyses. Therefore, 1404 individuals were retained, i.e., they delivered a complete dataset including the nutritional aspects.
General data collection
Data from questionnaires related to lifestyle, sociodemographic aspects, and self-reported health conditions were included. Clinical measurements and anthropometrics were also assessed, as well as scheduled appointments at a private accredited laboratory (Ketterthill, Esch-sur-Alzette, Luxembourg) for blood and urine sample collections and analyses. All participants were informed about the objectives of the study orally and in written and consented to participate in the survey (written consent was obtained from all participants). The study was approved by the National Research Ethics Committee (CNER, No. 201-505/12) and the National Commission for Data Protection (CNPD).
Data on age, gender, education, job, income, and marital status were collected using a general information questionnaire. A trained nurse carried out the anthropometric measures, including weight, height, and waist circumference (WC). The body weight (kg), height (cm), and WC (cm) were measured in a light dress without shoes using a digital scale. The participants’ BMI was assessed as weight (kg) divided by the square of height in meters (kg/m2).
Assessment of dietary intakes and indices scoring algorithms
The individuals completed a validated quantitative food-frequency questionnaires (FFQ) [26] under the supervision of a nurse. The frequency and quantity of 174 food and beverage items were documented to assess dietary intakes. A frequency ranging from ‘never/rarely’, ‘one–three times/month’, ‘one–two times/week’, ‘three–five times/week’ ‘once a day’, to ‘twice or more a day’, and portion size images were used to estimate macro-and micronutrient intakes. The daily food and nutrient intakes were calculated by multiplying the frequency of consumption by the portion sizes of all food items and considering the content of macro-or micronutrients as listed in the French ANSES-Ciqual food composition database (indexing the nutritional composition of > 3100 food items) [27]. The results were employed to determine the selected dietary indices (Table 1). The full description of the calculation of the indices and their scoring algorithm is provided in the supplementary file (Supplementary Tables 1–5); however, we briefly describe them here:
Alternate Healthy Eating Index (AHEI)
The AHEI was developed as an alternative to the Healthy Eating Index (HEI). It is based on foods that may prevent chronic disease risk and comprises 13 components that entail different food groups and recommendations [28]. The AHEI-2010 constitutes an updated version and shows more advantages than the HEI for predicting major chronic disease and CVD risks [10, 29]. All individual component scores were summed up for a total AHEI score ranging from 0 (worst) to 75 (best) (Supplementary Table 2).
Mediterranean Diet Score (MDS)
Another frequently applied index is the Mediterranean Diet Score (MDS), which measures adherence to the Mediterranean diet (MD) [30, 31]. The MD is one of the most well-known diets related to reducing the risk of CVD and other related diseases. Using the population-specific medians among the participants as cut-off values, points of 0 or 1 were assigned to each of the 9 indicated items. This MDS can, thus, take a score from 0 points (minimal adherence) to 9 (maximal adherence).
Dietary Approaches to Stop Hypertension Score (DASH-S)
DASH-S [32] measures how people adhere to a diet that is related to a lower risk of hypertension (DASH), though associated outcomes such as CVD and diabetes have also been examined [33]. This index's main feature is considering individuals' sodium intake, which generally remains above recommendations in Western cultures. We classified participants into quintiles for each component according to their intake ranking. We then summed up the component scores to attain an overall DASH-S ranging from 8 to 40 (Supplementary Table 3).
Diet Quality Index-International (DQI-I)
Based on dietary guidelines, the DQI-I is designed and developed based on international recommendations by the FAO/WHO [13, 14]. This index comprehensively integrates different aspects of the diet and examines public health nutrition in various communities [13, 14]. The four major categories (Supplementary Table 4) are variety, adequacy, moderation, and overall balance of the diet—with total scores ranging from 0 (poorest diet) to 100 (highest possible score, excellent diet).
Dietary Inflammatory Index (DII®)
The DII aims to study diet-induced inflammation [34] and includes 45 food items (anti-inflammatory ones such as dietary fiber and pro-inflammatory ones such as red meat). The DII has been validated in several human studies by CRP, TNF-α, IL-6, and other inflammatory biomarkers, and thus can predict, to some extent, the serum levels of these biomarkers in relation to diet and has been correlated with a large number of NCDs [18, 34]. The computation of the DII is based on dietary intake data linked to the regionally representative world database that provides an accurate and robust assessment of each parameter's mean and standard deviation [34]. These then become the multipliers to represent an individual's exposure relative to the 'standard global mean' as a Z-score. This is attained by subtracting the 'standard mean' from the reported amount and dividing this value by its standard deviation (means and standard deviations for all 45 parameters are shown in Supplementary Table 5). From those 45 parameters, in our study, there were 32 available items to calculate the DII. According to validation reports, using even only 21 out of 45 items can correctly predict serum inflammatory biomarkers [35].
Dietary Antioxidant Index (DAI)
The DAI focuses on antioxidant diets. Since the Western diet (a high-fat, refined-carbohydrate diet) has often been associated with a pro-oxidant/antioxidant imbalance [36], a diet fostering antioxidant reactions that counteract the effects of reactive oxygen species (ROS) can contribute to the prevention or treatment of oxidative stress-related diseases [37, 38]. Of note, there is a close relationship between oxidative stress and inflammation [39]. By standardizing the intake of six major dietary antioxidants, including vitamins A, E, and C, and magnesium, zinc, and selenium (the minerals participating in enzymatic antioxidant reactions), the DAI can predict the antioxidant properties of the diet and thus, the risk of various disease outcomes such as cancer [21], obesity [38] and CVD [37]. The DAI has been validated using biological measures, including total antioxidant capacity (TAC) and malondialdehyde (MDA) in plasma/serum [21].
n = the number of antioxidants included in the formula; i = this formula is calculated separately for each antioxidant and finally divided by n; Global means and SDs = are extracted from the reference database.
Naturally Nutrient-Rich Score (NNRS)
The Naturally Nutrient-Rich Score (NNRS) is based on a nutrient-to-calorie ratio [40]. This index is one of the few indices that examines the quantity of micronutrients based on guidelines, e.g., a report of a Joint FAO/WHO consultation, and its primary purpose is to ensure adequate intake of micronutrients to improve the quality of diet. Fourteen essential key nutrients and recommended daily values (DVs) for each 2000 kcal energy intake based on the USA dietary reference intakes (DRI) were used to calculate the NNRS (Table 1 and Supplementary Table 1).
Assessment of physical activity
A short form of the International Physical Activity Questionnaire (IPAQ) was used to estimate physical activity [41]. This IPAQ is a self-reported validated 7-item measure of physical activity over the past week. The amount of time that each individual spent on an activity was multiplied by the corresponding metabolic equivalent of task (METs) while considering the frequency of engagement during the past seven days. The continuous score of physical activity, expressed as METs-min per week, was then obtained by summing up the scores for the different activities (walking, moderate-intensity, and vigorous-intensity activities).
Measurement of blood/urine parameters
After overnight fasting, venous blood samples were drawn, and urine samples were collected as early morning midstream urine specimens. All blood and urine samples were stored in the Integrated BioBank of Luxembourg (IBBL), and a commercial accredited company (Ketterthill) later performed the analyses. From the blood samples, we obtained fasting blood glucose (FBG), high sensitive C-reactive protein (hs-CRP), apo-A and B, triglycerides, total cholesterol, low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), free triiodothyronine (FT3) and free thyroxine (FT4) hormones, thyroid-stimulating hormone (TSH), insulin, and glycated hemoglobin (HbA1C), hematocrit, and hemoglobin, as well as serum levels of sodium, uric acid, creatinine, magnesium, potassium, calcium, magnesium, ferritin, and 25(OH) vitamin D. From the spot urine samples, we measured microalbuminuria, creatinine, and urinary sodium concentration. In addition, using FBG and insulin levels, we estimated the Homeostatic Model Assessment for Insulin Resistance (HOMA-IR).
Systemic Immune-Inflammation Index (SII)
The SII is a promising prognostic indicator for systemic immune-inflammation-related conditions [42]. In fact, the SII assesses three of the homeostatic system markers that play a role in the inflammatory procedure: platelets, lymphocytes, and neutrophils. This index correlated with low-grade inflammation, characterized by a mildly elevated CRP [43]. Similar to increased serum levels of CRP, the evidence indicates that platelet/lymphocytes/neutrophils parameters are biomarkers that reflect a systemic inflammatory response [43].
The SII was estimated as total platelet count (P) × neutrophil-to-lymphocyte ratio (N/L) [44].
Vascular and kidney function
A trained and experienced nurse measured systolic and diastolic blood pressure (SBP and DBP) several times (a standardized method was applied). The average (mean) of the measurements was used as the final variable. In addition, arterial age was determined as the average age for a given carotid-femoral pulse wave velocity (PWV). PWV, central systolic and diastolic blood pressure, arterial age, and blood pressure in a lying position were measured with Complior™. The PWV was estimated by dividing the carotid-femoral distance by the transit time of the forward-traveling pulse between the carotid and femoral arteries.
In addition, glomerular filtration rate (GFR) as a vascular function-related measurement was estimated by the Modification of Diet in Renal Disease (MDRD) method. The MDRD was evaluated using an equation based on six variables: age, gender, ethnicity, serum creatinine, urea, and albumin [45].
Statistical analysis
The normality of the data distribution and homogeneity of variance was assessed using Q–Q normality plots and the Kolmogorov–Smirnov test (KS test), and a box plot. For the non-normally distributed data, a log-transformation was performed. For a first explorative purpose, bivariate correlation analyses with Spearman-rank correlation coefficients were calculated.
To study the association between the dietary indices and serum and all metabolic parameters, linear regression modeling in SPSS was carried out. This included a set of confounders that were chosen due to physiological plausibility and based on literature. For models, two-sided p-values above 0.1 were selected as means for elimination. This step resulted in acquiring a model (saturated model) from the thorough batch of variables by automatically (step-down procedure) dismissing those that did not contribute significantly to the model. The respective dietary quality indices were the explanatory, independent variable, while the measured metabolic parameters were the observed, dependent outcome variable.
Results
General characteristics of the population
The distribution of participants' characteristics in the quartiles of dietary indices is presented in Table 2. Table 2 shows the level of adherence of the participants in the sociodemographic groups to different indicators. For example, older people (> 65 years) were more adherent to the AHEI or MDS (being in a higher index score quartile) than younger people (≤ 34.99 years). In addition, the distribution (median, interquartile range) of participants' biomarkers and diet quality indices according to age and gender groups are presented in Table 3.
Correlations
Spearman correlation (ρ (rho)) between the investigated dietary quality indices and respective linear trendlines are shown in Fig. 1. According to Spearman correlation analyses, all dietary indices significantly correlated with one another (Fig. 1).
Spearman correlation (ρ) between the investigated dietary quality indices and respective linear trendlines. AHEI Alternative Healthy Eating Index, MDS Mediterranean Diet Score, DASH-S Dietary Approaches to Stop Hypertension Score, DQI-I Diet Quality Index-International, DII Dietary Inflammatory Index, DAI Dietary Antioxidant Index, NNRS Naturally Nutrient-Rich Score
In addition, the Spearman correlation between the investigated dietary indices and daily nutrient intakes is presented in Table 4. The largest number of significant correlations (ρ > 0.50) between dietary indices and nutrient intakes (36 nutrients in total) was found for the NNRS (34 nutrients), followed by the DAI (33 nutrients), DII (31 nutrients), AHEI (13 nutrients), DQI-I (4 nutrients), DASH-S (2 nutrients), and MDS (1 nutrient). Strongest correlations between dietary indices and nutrients were AHEI with total dietary fiber (ρ = 0.827); MDS with soluble dietary fiber (ρ = 0.524); DASH-S with total dietary fiber (ρ = 0.540); DQI-I with vitamin C (ρ = 0.633); DII with folate (ρ = − 0.853); DAI (ρ = 0.900) and NNRS (ρ = 0.923) with phosphorus (Table 4). Also, the Spearman correlation between the investigated dietary indices and daily food group intakes (14 groups in total) is shown in Table 5. The indices correlating (ρ > 0.50) significantly with most food groups were DII (3 groups), DAI (3 groups), and NNRS (3 groups), followed by AHEI (2 groups) and DASH-S (2 groups) (Table 5). The highest Spearman correlations between dietary indices and food groups included AHEI (ρ = 0.705), MDS (ρ = 0.554), and DII (ρ = -0.649) with vegetables; DASH-S (ρ = 0.515) and DQI-I (ρ = 0.613) with fruits; DAI (ρ = 0.683) and NNRS (ρ = 0.719) with protein-rich foods (Table 5). In addition, significant correlations (p value < 0.001) were found between protein-rich foods, fast foods, red meat group, fish group, lipids, sugary products with NNRS; grains, and starchy vegetables with DAI; fruits and vegetables with AHEI; dairy group, and sugar-sweetened beverages with DASH-S; non-caloric beverages with DII: and alcoholic beverages with DQI-I (Table 5).
Regression models
Associations between diet quality indices and serum and metabolic biomarkers
Multivariable general linear regression models (adjusted for age, gender, birth country, marital status, education, job, income, IPAQ scoring, and current smoking) of the associations between diet quality indices as continuous variables revealed high significant associations between dietary indices and metabolic biomarkers: between NNRS (Beta = 0.077, 95% CI 0.011, 0.144) and urinary sodium, and DASH-S (Beta = − 2.001, 95% CI − 3.572, − 0.430) with triglycerides (Table 6). According to Table 6, the largest number of significant associations between a Diet Quality Index and a measured metabolic parameter was found for DASH-S (with 14 parameters), followed by the DQI-I (n = 13), AHEI (n = 11), MDS (n = 8), DAI (n = 5), NNRS (n = 6) and the lowest number for DII, with two parameters. Similar results were obtained, with slightly higher beta-coefficients, when we used quality indices as a categorical variable (i.e., quartiles, Supplementary Table 8).
When looking for combinations of 2 dietary indices that explain the largest number of measured serum and metabolic parameters, the combinations of DASH-S with AHEI (together significantly associated with a total of 18 serum/metabolic parameters), as well as DASH-S with DAI (17) and DASH-S with NNRS (17) were most promising, with both the DAI and the NNRS being nutrient-based indices, compared to the DASH-S (Supplementary Table 9).
In addition, unadjusted multivariable linear regression models of the associations between diet quality indices as continuous and categorical variables and metabolic biomarkers are shown in Supplementary Tables 6 and 7, respectively. As expected, when using dietary indices as quartiles, the results were similar to analyses based on indices as continuous variables, except that the beta-coefficients increased (Supplementary Tables 6 and 7).
Discussion
In this study, we investigated the association of frequently employed diet quality indices, covering complementary dietary aspects, including nutrient- and food-group-based ones, appertaining to their association with a number of parameters, including biomarkers related to disease risk and/or nutrient status in a rather general adult population. As there are major differences between diet quality indices that are food-group-based (which do not require linking them with food composition databases, introducing another source of variability) and indices that are based on nutrients, we tried to choose indices of both types. Some indices included both aspects (and even three aspects when considering non-nutrients). Our final regression models highlighted that these diet quality indices were associated with different serum and metabolic parameters, such as anthropometry, inflammation, blood glucose, blood lipids, kidney-related parameters, nutritional and hormonal status, and vascular function, with the highest number of significant associations found for the DASH-S, followed by DQI-I, AHEI, MDS, DAI, NNRS and finally the DII.
Nutrition-related diseases are predominantly multifactorial, influenced by the entire array of macro-, micro- and non-nutrients ingested and their interactions [46]. For this purpose, various indices, such as the DASH-S, AHEI, DQI-I, and DAI have been developed and used in research and public health, considering several aspects of the diet. Some of these indices have been validated, such as by measuring their association with serum biomarkers, and their construction criteria and reliability have been examined, with their clinical diagnostic power having been tested for certain populations and certain disease endpoints [9, 47].
In our study, the DASH-S was associated with the largest number of selected parameters (Table 6). A systematic review and meta-analysis of randomized controlled trials reported that adherence to DASH could reduce SBP and DBP [33]. Phillips et al. [48] also examined the association between DASH-S and a large number of cardiometabolic relevant biomarkers, concluding that DASH-S was associated with improved adiposity measures such as BMI and WC and a less insulin-resistant, less pro-thrombotic, less pro-inflammatory, and less pro-atherogenic cardiometabolic profile. In the present study, the significant association of the DASH-S with blood pressure-related biomarkers and sodium excretion shows the validity of this index, as the DASH diet was designed for this purpose. Indeed, dietary sodium intake remains almost 2 times above WHO recommendations of 5 g/d for most Westernized countries [49], being a major cause of elevated blood pressure and cardiovascular-related deaths. According to a recent report [50], over-consumption of dietary sodium is related to 3 million annual deaths globally and 60 million DALYs. In the present study, median sodium excretion was 97 mg/dL, which is likely to represent a higher-than-needed salt intake.
In addition, in line with our results, a recent study that examined the relationship between dietary quality, assessed by DASH-S, and cardiometabolic health biomarkers, concluded that a higher DASH-S was significantly associated with lower BMI, WC, TNF-α, IL-6, white blood count (WBC) and plasminogen activator inhibitor-1 (PAI-1) concentrations, and reduced insulin resistance [48]. In addition, fewer small LDL-c, HDL-c, and VLDL-c particles were observed among those with better DASH-S [48]. Participants in the top DASH-S quartile had a 48% and 54% lower likelihood of metabolic syndrome and central obesity, respectively, than those in the lowest DASH-S quartile [48]. The authors suggested that a high-quality diet assessed with DASH-S was associated with less insulin resistance, improved adiposity measures and favourable pro-inflammatory and pro-atherogenic cardiometabolic profile, and less pro-thrombotic properties and might affect metabolic syndrome and central obesity risk [48]. These findings could have public health and clinical significance regarding dietary approaches to promote cardiometabolic health and warrant further investigations.
Similar to the DASH-S, the highly correlated DQI-I was associated with also most anthropometric markers, urinary sodium, and blood pressure as well as certain blood lipids. The DQI-I score of 64% suggested a rather limited dietary diversity. Similar as for the DQI, Vandevijvere et al. also investigated various aspects of the diet, such as within-food group and overall diversity, and some dimensions of diet quality similar to the DQI-I, such as moderation, adequacy, and balance, derived from the food-based dietary guidelines (FBDG) in Belgium [51], concluding that overall diet diversity derived from the FBDG is a practical benchmark of dietary quality. Another advantage of the DQI-I may be its compromise of being both a food group and nutrient-based index, and such a combination may constitute a more sophisticated manner to assess the overall quality of the diet [52, 53].
Similar findings as for the DQI-I were encountered for the AHEI, which also correlated highly with the DQI-I, though its association with blood pressure markers and sodium intake was less pronounced. Other studies, such as the one by Kim et al., also showed a significant correlation between (among others) DQI-I and AHEI and glycemic status (including HbA1c, FBG, and postprandial 2-h glucose) in Korean patients with T2D [54], which was not found in the present study. It is possible that the different populations or the dietary assessment method influenced the results. Their study estimated dietary intake based on a single 24-h recall method. While such a method may reflect a more current diet than FFQ, the guidelines recommend several (repeated) 24 recalls [55].
However, AHEI was significantly associated with cardiometabolic risk factors, including anthropometric measurements (BMI, WC, and WHR), apo-A, and vessel-related functions (DBP, CDBP, and vascular age). Lavigne-Robichaud et al. [56] compared AHEI with Food Quality Score (FQS) and index, examining the contribution of ultra-processed products (UPP) to total daily dietary energy intake. While all three indices were related to cardiometabolic risk, only the UPP was significantly associated with metabolic syndrome risk [56]. Such rather novel indices could be of interest, as indeed processing techniques and especially ultra-processed food items have been associated with high a intake of sodium, saturated fats, and simple sugars, all of which have been associated with cardiometabolic risk factors [57]. AHEI would also capture similar aspects, as it includes the consumption of fruits and the quality of the consumed fats, though less specifically focusing on sodium and simple sugar intake. In the present study, median AHEI results of 37 (ideal score 75) suggested rather a deviation from the recommended dietary guidelines.
The NNRS, focusing on essential nutrient requirements, showed, as with other nutrient-focused indices, a relatively low association with the observed serum and metabolic parameters. Besides anthropometric markers, fasting blood glucose and sodium (in urine and serum) were significantly associated, which is interesting as neither sodium nor sugars are incorporated into the index. It is possible that factors such as potassium intake played a role (as a high potassium status could reduce sodium re-uptake by the kidneys [58] or that the consumption of proteins was related to lower simple sugar intake [59]. In a study by Kramer et al. on the European elderly, linear regression models analyzed the association between an adapted NNRS and the micronutrient status of folate, vitamin D, vitamin B12, homocysteine, and CRP [60]; a one-unit increase in the adapted NNRS score was associated with a 1.6%/2.2% increase in serum folate for Polish/Dutch participants. The authors also reported a significant inverse association between their NNRS and circulating homocysteine levels, a marker often associated with CVD, in both populations [60]. However, they failed to find a significant association between their NNRS and CRP and serum vitamin D levels [60]. These results are in line with ours, as we also could not find a significant association between NNRS and hs-CRP and serum vitamin D, despite vitamin D intake being part of the NNRS. Results of the NNRS index (median 129%) proposed that the population in Luxembourg did not have any significant deficits in the captured nutrients.
The MDS is another prevalent food-group-based index, which resulted in an intermediate number of significant associations with serum and metabolic parameters. It has been reported [22] that the MDS has a high aptness to predict changes in risk biomarkers and is significantly associated with lower levels of blood pressure, apo-B, renal function indicators (creatinine), and liver enzymes (serum glutamate-pyruvate transaminase and γ-glutamyl-transpeptidase) [22]. These results were similar to our study, revealing significant associations between MDS and BMI, waist circumference, apo-A, urinary creatinine, TSH, and several blood pressure measures. Our study's findings confirm earlier ones [22] and highlight the possibility that a Mediterranean diet can reduce some nutrition-related disease risks. Compared to these earlier reported values [22], also derived from Luxembourg, our present values (median 4) indicate a deviation from the recommendations (ideal score 9), in line with a more Western-type diet, as also emphasized recently [25].
The DAI is a rather recently developed index focusing on a few antioxidants, including vitamins and minerals, as part of antioxidant enzymes. Dietary compounds that could influence antioxidant status via, e.g., transcription factors, e.g., carotenoids or polyphenols, acting on Nrf2 or NF-kB, are not included, even though these factors may play a more significant role in oxidative stress status than direct quenching effects [61]. We reported previously [62], in the Iranian population, a significant association between the DAI and some inflammatory and stress oxidative biomarkers, such as Il-6, MDA, serum insulin, and HOMA-IR [62]. Similarly, in the present study, we also found a significant association between the DAI and some biomarkers, such as hs-CRP, HbA1c, and FBG. However, one of the limitations of the present study is that we did not measure oxidative stress-related biomarkers.
An index that has recently attracted much attention is the DII, due to its relation to a large number of chronic diseases, from cardiometabolic ones [1, 17] to cancer [18], NAFLD [19], and obesity [20]. However, in the present study, this index produced the lowest number of significant associations – only 25-hydroxyvitamin D and urinary creatinine. Several studies have addressed the validation of the DII (a (non-)nutrient-based index) by correlating it with inflammatory markers such as hs-CRP, TNF-a, and IL-6 [18, 63]. However, the only inflammatory marker measured in our study was hs-CRP, and we could not find a significant association between it and the DII. We also examined the association of DII with the SII (previously associated with chronic inflammation [43]), but we failed to find a significant association. However, and interestingly, when we applied an energy-adjusted DII (E-DII, data not shown), a robust and significant association between it and SII was seen in crude and adjusted models (Supplementary Table 6); although the association between E-DII and the hs-CRP still was not significant. It is possible that further adjustment for energy, which is often not included in the indices, would improve the strengths of associations, as, e.g., higher intakes of unhealthy items may merely signal higher energy needs and larger intake, and not necessarily an unhealthier diet.
Correlating indices with each other, we observed that the highest correlations (strong and significant) were between the DAI and NNRS and between the DII and DAI. Given that all of these rather nutrient-based indices, with some overlaps in their considered nutrients, this may not be too surprising. DII and DAI comprise a number of antioxidants, and the NNRS likewise includes several of the same nutrients, emphasizing some redundancy between these. On the other hand, low correlations were obtained for other indices, perhaps pointing to a rather complementarity of these indices, such as between the NNRS (a nutrient-based score) and the DASH-S, MDS, and DQI-I, being rather food-group-based indices. NNRS, for instance, was the single indicator being significantly associated with serum sodium and fasting blood glucose; thus, measuring more than 1 established index may yield further insights into dietary patterns. When investigating meaningful combinations of 2 indices (Supplementary Table 9), the DASH-S diet with either the NNRS or the DAI (both nutrient based or with the AHEI produced the most significant associations with the analyzed parameters (17), highlighting the usefulness of to study combinations of certain, possibly somewhat complementary, indices.
Finally, a critical evaluation of current scoring systems/algorithms for using a priori diet quality scores for CVD risk summarized strengths and limitations of these dietary indices/scores and described index components, calculation methods, and the application of these indices to different population groups [64]. Similar as to our conclusions, the authors emphasized that future applications and interpretations of dietary indices/scores in nutritional epidemiologic studies assessing diet quality should consider food items as well as nutrients when interpreting a score. For instance, scores/indices relying solely on food groups may overlook the importance of the intercorrelation of nutrients with outcomes [64]. It was further suggested that future investigations should consider cross-cultural and other differences between population groups, address the limitations, and identify translational challenges inherent to attempt creating a relevant Diet Quality Index for application in disease prevention at a population level [64].
Our study has several strengths and weaknesses. Examining seven indicators (nutrient, food, and nutrient-food-based indices) for assessing diet quality and associating them with various serum and metabolic biomarkers, and considering typical confounders, was one of the strengths of our study. The measured dietary intake was further derived from an extensive and validated FFQ applied by a trained nurse. This comprehensive contemplation of diet quality using different indicators allowed us to examine multiple aspects of the diet and to emphasize the usefulness of the indices with regard to the observed parameters. However, a limited number of markers for stress oxidative and inflammation were one of the limitations of our study, and though alternatives were investigated, such as the SII as a marker of inflammation, the original DII was not validated against this marker. Another limitation of our study was that it was a cross-sectional survey, so we could not assess the reliability of the indices. Cohort studies with prospective designs would be more suitable to determine the causal relationship between indices and biomarkers and examine their reliability.
Conclusion
In this study, we examined the association between seven dietary quality indices and serum and metabolic biomarkers in a general adult population. In line with the literature, e.g., in a meta-analysis [65], as opposed to food-based indicators, nutrient-based indices such as the DII and the DAI were less potent than food-group-based indicators such as the DASH-S, DQI-I, or AHEI to predict more general serum indicators and metabolic biomarkers in general populations. Though nutrient-based indicators such as the DII and the DAI have their importance when focusing on more specific populations, due to their higher disease-specificity, for a more general population to reflect less specific cardiometabolic markers and markers of nutrient status such as the ones employed, a rather food-group-based indicator may be considered a more suitable approach. Nevertheless, a combination of complementary indices, such as a general, rather food-group-based one and a more specific, nutrient-based one, is expected to yield more insightful information into a dietary pattern than only a single index would allow. Hence, depending on the targeted health/research question, a combination of carefully selected and complementary indices is advised.
Data availability statement
Available on request from the corresponding author. Due to our institute's rules and laws, the data are not publicly available.
References
Vahid F, Goodarzi R, Shivappa N, Hébert JR, Fazeli Moghadam E (2020) Dietary Inflammatory Index (DII®): a significant association between coronary heart disease and DII® in Armenian adults. Eur J Prev Cardiol 27(19):2235–2237
Mehrdad M, Vahid F, Eftekhari MH (2020) Nutritional quality’s key role in the odds of overweight in adults with rs9939609 polymorphism of FTO gene- the role of manganese and vitamin D. Am J Med Sci 360(6):678–685. https://doi.org/10.1016/j.amjms.2020.06.027
Gholamalizadeh M, Rastgoo S, Doaei S, Vahid F, Malmir H, Ashoori N, Jarrahi AM (2021) Index of Nutritional Quality (INQ) and the risk of obesity in male adolescents: a case-control study. Biol Trace Elem Res 199(5):1701–1706. https://doi.org/10.1007/s12011-020-02297-3
Vahid F, Rahmani G, Jafari Naeini A, Falahnejad H, Davoodi SH (2018) The association between index of nutritional quality (INQ) and gastric cancer and evaluation of nutrient intakes of gastric cancer patients: a case-control study. Int J Cancer Manag 11(1)
Vahid F, Hekmatdoost A, Mirmajidi S, Doaei S, Rahmani D, Faghfoori Z (2019) Association between index of nutritional quality and nonalcoholic fatty liver disease: the role of vitamin D and B group. Am J Med Sci 358(3):212–218
Roglic G (2016) WHO Global report on diabetes: a summary. Int J Noncommun Dis 1(1):3
Ocké MC (2013) Evaluation of methodologies for assessing the overall diet: dietary quality scores and dietary pattern analysis. Proc Nutr Soc 72(2):191–199. https://doi.org/10.1017/S0029665113000013
Schulze MB, Martínez-González MA, Fung TT, Lichtenstein AH, Forouhi NG (2018) Food based dietary patterns and chronic disease prevention. BMJ 361:k2396. https://doi.org/10.1136/bmj.k2396
Kourlaba G, Panagiotakos DB (2009) Dietary quality indices and human health: a review. Maturitas 62(1):1–8. https://doi.org/10.1016/j.maturitas.2008.11.021
Krebs-Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, Wilson MM, Reedy J (2018) Update of the healthy eating index: HEI-2015. J Acad Nutr Diet 118(9):1591–1602. https://doi.org/10.1016/j.jand.2018.05.021
Millar SR, Navarro P, Harrington JM, Perry IJ, Phillips CM (2021) Dietary quality determined by the healthy eating index-2015 and biomarkers of chronic low-grade inflammation: a cross-sectional analysis in middle-to-older aged adults. Nutrients 13(1):222
Crowe-White KM, Ellis AC, Mehta T, Locher JL, Ard JD (2019) Dietary quality assessed by the HEI-2010 and biomarkers of cardiometabolic disease: an exploratory analysis. J Am Coll Nutr 38(7):640–647. https://doi.org/10.1080/07315724.2019.1580168
Kim S, Haines PS, Siega-Riz AM, Popkin BM (2003) The Diet Quality Index-International (DQI-I) provides an effective tool for cross-national comparison of diet quality as illustrated by China and the United States. J Nutr 133(11):3476–3484. https://doi.org/10.1093/jn/133.11.3476
Cho IY, Lee KM, Lee Y, Paek CM, Kim HJ, Kim JY, Lee K, Han JS, Bae WK (2021) Assessment of Dietary Habits Using the Diet Quality Index-international in cerebrovascular and cardiovascular disease patients. Nutrients. https://doi.org/10.3390/nu13020542
Cheung LTF, Chan RSM, Ko GTC, Lau ESH, Chow FCC, Kong APS (2018) Diet quality is inversely associated with obesity in Chinese adults with type 2 diabetes. Nutr J 17(1):63. https://doi.org/10.1186/s12937-018-0374-6
Zamani B, Daneshzad E, Mofrad MD, Namazi N, Larijani B, Bellissimo N, Azadbakht L (2021) Dietary Quality Index and cardiometabolic risk factors among adult women. Iran J Public Health 50(8):1713–1721. https://doi.org/10.18502/ijph.v50i8.6819
Vahid F, Shivappa N, Karamati M, Naeini AJ, Hebert JR, Davoodi SH (2017) Association between Dietary Inflammatory Index (DII) and risk of prediabetes: a case-control study. Appl Physiol Nutr Metab 42(4):399–404
Vahid F, Shivappa N, Faghfoori Z, Khodabakhshi A, Zayeri F, Hebert JR, Davoodi SH (2018) Validation of a Dietary Inflammatory Index (DII) and association with risk of gastric cancer: a case-control study. Asian Pac J Cancer Prev 19(6):1471–1477. https://doi.org/10.22034/APJCP.2018.19.6.1471
Vahid F, Shivappa N, Hekmatdoost A, Hebert JR, Poustchi H, Shamsipour A, Eslamparast T, Meibodi M, Rahmani D (2018) Association of Pro-inflammatory dietary intake and non-alcoholic fatty liver disease: findings from Iranian case-control study. Int J Vitam Nutr Res 88(3–4):144–150. https://doi.org/10.1024/0300-9831/a000571
Mehrdad M, Vahid F, Shivappa N, Hébert JR, Fardaei M, Hassan Eftekhari M (2021) High dietary inflammatory index (DII) scores increase odds of overweight in adults with rs9939609 polymorphism of FTO gene. Clin Nutr ESPEN 42:221–226. https://doi.org/10.1016/j.clnesp.2021.01.034
Vahid F, Rahmani D, Davoodi SH (2020) Validation of Dietary Antioxidant Index (DAI) and investigating the relationship between DAI and the odds of gastric cancer. Nutr Metab 17(1):102. https://doi.org/10.1186/s12986-020-00529-w
Aa A, Vernier C, Crichton GE, Sauvageot N, Shivappa N, Hébert JR (2015) Cross-comparison of diet quality indices for predicting chronic disease risk: findings from the Observation of Cardiovascular Risk Factors in Luxembourg (ORISCAV-LUX) study. Br J Nutr 113(2):259–269. https://doi.org/10.1017/S0007114514003456
Morze J, Danielewicz A, Hoffmann G, Schwingshackl L (2020) Diet quality as assessed by the healthy eating index, alternate healthy eating index, dietary approaches to stop hypertension score, and health outcomes: a second update of a systematic review and meta-analysis of cohort studies. J Acad Nutr Diet 120(12):1998-2031.e1915. https://doi.org/10.1016/j.jand.2020.08.076
Rollet M, Bohn T, Vahid F, Group obotOW (2022) Association between dietary factors and constipation in adults living in Luxembourg and taking part in the ORISCAV-LUX 2 survey. Nutrients 14(1):122
Vahid F, Brito A, Le Coroller G, Vaillant M, Samouda H, Bohn T, Group obotOW (2021) Dietary intake of adult residents in Luxembourg taking part in two cross-sectional studies—ORISCAV-LUX (2007–2008) and ORISCAV-LUX 2 (2016–2017). Nutrients 13(12):4382
Sauvageot N, Alkerwi A, Albert A, Guillaume M (2013) Use of food frequency questionnaire to assess relationships between dietary habits and cardiovascular risk factors in NESCAV study: validation with biomarkers. Nutr J 12(1):1–11
French Agency for Food EaOHS ANSES-CIQUAL French Food Composition Table for Nutritional Intakes Calculation CALNUT. Available online: https://ciqual.anses.fr/
McCullough ML, Willett WC (2006) Evaluating adherence to recommended diets in adults: the Alternate Healthy Eating Index. Public Health Nutr 9(1a):152–157
McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Hunter DJ, Colditz GA, Willett WC (2002) Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr 76(6):1261–1271. https://doi.org/10.1093/ajcn/76.6.1261
Trichopoulou A, Kouris-Blazos A, Wahlqvist ML, Gnardellis C, Lagiou P, Polychronopoulos E, Vassilakou T, Lipworth L, Trichopoulos D (1995) Diet and overall survival in elderly people. BMJ 311(7018):1457–1460. https://doi.org/10.1136/bmj.311.7018.1457
Hodge AM, Bassett JK, Dugué PA, Shivappa N, Hébert JR, Milne RL, English DR, Giles GG (2018) Dietary Inflammatory Index or Mediterranean Diet Score as risk factors for total and cardiovascular mortality. Nutr Metab Cardiovasc Dis 28(5):461–469. https://doi.org/10.1016/j.numecd.2018.01.010
Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB (2008) Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 168(7):713–720. https://doi.org/10.1001/archinte.168.7.713
Siervo M, Lara J, Chowdhury S, Ashor A, Oggioni C, Mathers JC (2015) Effects of the dietary approach to stop hypertension (DASH) diet on cardiovascular risk factors: a systematic review and meta-analysis. Br J Nutr 113(1):1–15
Cavicchia PP, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Hébert JR (2009) A New Dietary Inflammatory Index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr 139(12):2365–2372. https://doi.org/10.3945/jn.109.114025
Wright ME, Mayne ST, Stolzenberg-Solomon RZ, Li Z, Pietinen P, Taylor PR, Virtamo J, Albanes D (2004) Development of a comprehensive dietary antioxidant index and application to lung cancer risk in a cohort of male smokers. Am J Epidemiol 160(1):68–76. https://doi.org/10.1093/aje/kwh173
Roberts CK, Barnard RJ, Sindhu RK, Jurczak M, Ehdaie A, Vaziri ND (2005) A high-fat, refined-carbohydrate diet induces endothelial dysfunction and oxidant/antioxidant imbalance and depresses NOS protein expression. J Appl Physiol 98(1):203–210
Vahid F, Nasiri Z, Abbasnezhad A, Moghadam EF (2021) Antioxidant potential of diet: association between dietary antioxidant index and odds of coronary heart disease: a case-control study. Mediterr J Nutr Metab Preprint. https://doi.org/10.3233/MNM-211503
Vahid F, Rahmani D, Davoodi SH (2021) The correlation between serum inflammatory, antioxidant, glucose handling biomarkers, and Dietary Antioxidant Index (DAI) and the role of DAI in obesity/overweight causation: population-based case–control study. Int J Obes. https://doi.org/10.1038/s41366-021-00944-w
Bondia-Pons I, Ryan L, Martinez JA (2012) Oxidative stress and inflammation interactions in human obesity. J Physiol Biochem 68(4):701–711
Drewnowski A (2005) Concept of a nutritious food: toward a nutrient density score. Am J Clin Nutr 82(4):721–732. https://doi.org/10.1093/ajcn/82.4.721
Lee PH, Macfarlane DJ, Lam TH, Stewart SM (2011) Validity of the international physical activity questionnaire short form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act 8(1):1–11
Yan Q, Ertao Z, Zhimei Z, Weigang D, Jianjun P, Jianhui C, Chuangqi C (2020) Systemic Immune-Inflammation Index (SII): a more promising inflammation-based prognostic marker for patients with synchronic colorectal peritoneal carcinomatosis. J Cancer 11(18):5264–5272. https://doi.org/10.7150/jca.46446
Ustundag Y, Huysal K, Gecgel SK, Unal D (2018) Relationship between C-reactive protein, systemic immune-inflammation index, and routine hemogram-related inflammatory markers in low-grade inflammation. Int J Med Biochem 1(1):24–28
Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, Zhang X, Wang WM, Qiu SJ, Zhou J, Fan J (2014) Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res 20(23):6212–6222. https://doi.org/10.1158/1078-0432.Ccr-14-0442
Levey AS, Coresh J, Greene T, Stevens LA, Zhang Y, Hendriksen S, Kusek JW, Van Lente F, Collaboration* CKDE (2006) Using staication of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145(4):247–254
Geissler C, Powers HJ (2017) Human nutrition. Oxford University Press
Burggraf C, Teuber R, Brosig S, Meier T (2018) Review of a priori dietary quality indices in relation to their construction criteria. Nutr Rev 76(10):747–764. https://doi.org/10.1093/nutrit/nuy027
Phillips CM, Harrington JM, Perry IJ (2019) Relationship between dietary quality, determined by DASH score, and cardiometabolic health biomarkers: a cross-sectional analysis in adults. Clin Nutr 38(4):1620–1628
Kloss L, Meyer JD, Graeve L, Vetter W (2015) Sodium intake and its reduction by food reformulation in the European Union—a review. NFS J 1:9–19. https://doi.org/10.1016/j.nfs.2015.03.001
Afshin A, Sur PJ, Fay KA, Cornaby L, Ferrara G, Salama JS, Mullany EC, Abate KH, Abbafati C, Abebe Z, Afarideh M, Aggarwal A, Agrawal S, Akinyemiju T, Alahdab F, Bacha U, Bachman VF, Badali H, Badawi A, Bensenor IM, Bernabe E, Biadgilign SKK, Biryukov SH, Cahill LE, Carrero JJ, Cercy KM, Dandona L, Dandona R, Dang AK, Degefa MG, El Sayed ZM, Esteghamati A, Esteghamati S, Fanzo J, Farinha CSES, Farvid MS, Farzadfar F, Feigin VL, Fernandes JC, Flor LS, Foigt NA, Forouzanfar MH, Ganji M, Geleijnse JM, Gillum RF, Goulart AC, Grosso G, Guessous I, Hamidi S, Hankey GJ, Harikrishnan S, Hassen HY, Hay SI, Hoang CL, Horino M, Ikeda N, Islami F, Jackson MD, James SL, Johansson L, Jonas JB, Kasaeian A, Khader YS, Khalil IA, Khang Y-H, Kimokoti RW, Kokubo Y, Kumar GA, Lallukka T, Lopez AD, Lorkowski S, Lotufo PA, Lozano R, Malekzadeh R, März W, Meier T, Melaku YA, Mendoza W, Mensink GBM, Micha R, Miller TR, Mirarefin M, Mohan V, Mokdad AH, Mozaffarian D, Nagel G, Naghavi M, Nguyen CT, Nixon MR, Ong KL, Pereira DM, Poustchi H, Qorbani M, Rai RK, Razo-García C, Rehm CD, Rivera JA, Rodríguez-Ramírez S, Roshandel G, Roth GA, Sanabria J, Sánchez-Pimienta TG, Sartorius B, Schmidhuber J, Schutte AE, Sepanlou SG, Shin M-J, Sorensen RJD, Springmann M, Szponar L, Thorne-Lyman AL, Thrift AG, Touvier M, Tran BX, Tyrovolas S, Ukwaja KN, Ullah I, Uthman OA, Vaezghasemi M, Vasankari TJ, Vollset SE, Vos T, Vu GT, Vu LG, Weiderpass E, Werdecker A, Wijeratne T, Willett WC, Wu JH, Xu G, Yonemoto N, Yu C, Murray CJL (2019) Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 393(10184):1958–1972. https://doi.org/10.1016/S0140-6736(19)30041-8
Vandevijvere S, De Vriese S, Huybrechts I, Moreau M, Van Oyen H (2010) Overall and within-food group diversity are associated with dietary quality in Belgium. Public Health Nutr 13(12):1965–1973. https://doi.org/10.1017/S1368980010001606
Gil Á, de Victoria EM, Olza J (2015) Indicators for the evaluation of diet quality. Nutr Hosp 31(3):128–144
EFSA Panel on Dietetic Products N, Allergies (2010) Scientific opinion on establishing food-based dietary guidelines. EFSA J 8(3):1460
Kim J, Cho Y, Park Y, Sohn C, Rha M, Lee M-K, Jang HC (2013) Association of dietary quality indices with glycemic status in Korean patients with type 2 diabetes. CNR 2(2):100–106. https://doi.org/10.7762/cnr.2013.2.2.100
Knüppel S, Norman K, Boeing H (2019) Is a Single 24-hour dietary recall per person sufficient to estimate the population distribution of usual dietary intake? J Nutr 149(9):1491–1492. https://doi.org/10.1093/jn/nxz118
Lavigne-Robichaud M, Moubarac J-C, Lantagne-Lopez S, Johnson-Down L, Batal M, Laouan Sidi EA, Lucas M (2018) Diet quality indices in relation to metabolic syndrome in an Indigenous Cree (Eeyouch) population in northern Québec Canada. Public Health Nutr 21(1):172–180. https://doi.org/10.1017/S136898001700115X
Adams J, Hofman K, Moubarac J-C, Thow AM (2020) Public health response to ultra-processed food and drinks. BMJ 369:m2391. https://doi.org/10.1136/bmj.m2391
Clausen MJV, Poulsen H (2013) Sodium/potassium homeostasis in the cell. In: Banci L (ed) Metallomics and the cell. Springer, Netherlands, Dordrecht, pp 41–67. https://doi.org/10.1007/978-94-007-5561-1_3
Meng H, Matthan NR, Ausman LM, Lichtenstein AH (2017) Effect of macronutrients and fiber on postprandial glycemic responses and meal glycemic index and glycemic load value determinations. Am J Clin Nutr 105(4):842–853. https://doi.org/10.3945/ajcn.116.144162
Kramer CS, Szmidt MK, Sicinska E, Brzozowska A, Santoro A, Franceschi C, de Groot L, Berendsen AAM (2019) The elderly-nutrient rich food score is associated with biochemical markers of nutritional status in European older adults. Front Nutr 6:150. https://doi.org/10.3389/fnut.2019.00150
Bohn T (2019) Carotenoids and markers of oxidative stress in human observational studies and intervention trials: implications for chronic diseases. Antioxidants 8(6):179
Vahid F, Rahmani D, Davoodi SH (2021) The correlation between serum inflammatory, antioxidant, glucose handling biomarkers, and Dietary Antioxidant Index (DAI) and the role of DAI in obesity/overweight causation: population-based case–control study. Int J Obes 45(12):2591–2599
Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR (2014) Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr 17(8):1689–1696
Aljuraiban GS, Gibson R, Oude Griep LM, Okuda N, Steffen LM, Van Horn L, Chan Q (2019) Perspective: the application of a priori diet quality scores to cardiovascular disease risk—a critical evaluation of current scoring systems. Adv Nutr 11(1):10–24. https://doi.org/10.1093/advances/nmz059
Waijers PM, Feskens EJ, Ocké MC (2007) A critical review of predefined diet quality scores. Br J Nutr 97(2):219–231. https://doi.org/10.1017/s0007114507250421
Acknowledgements
We appreciate the dedication of all participants who take part in the ORISCAV-LUX and ORISCAV-LUX 2 studies. We thank the research nurses involved in the ORISCAV-LUX 2 study. We are finally very much indebted to all members of the ORISCAV-Working Group who contributed to the planning and conducting of the ORISCAV-LUX studies: Ala’a Alkerwi, Stephanie Noppe, Charles Delagardelle, Jean Beissel, Anna Chioti, Saverio Stranges, Jean-Claude Schmit, Marie-Lise Lair, Marylène D’Incau, Jessica Pastore, Gloria Aguayo, Gwenaëlle Le Coroller, Michel Vaillant, Hanen Samouda, Brice Appenzeller, Laurent Malisoux, Sophie Couffignal, Manon Gantenbein, Yvan Devaux, Laetitia Huiart, Dritan Bejko, Guy Fagherazzi, Magali Perquin, Maria Ruiz-Castell and Isabelle Ernens.
Funding
The ORISCAV-LUX 2 data collection was funded by the LIH (Ministry of Higher Education and internal research funding).
Author information
Authors and Affiliations
Consortia
Contributions
FV and TB performed the statistical analyses, interpreted the data, and drafted the manuscript. FV and TB provided expertise and oversight on the intellectual content. AH provided data on food and nutrient consumption. JRH was involved in analyzing the DII and critically reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Informed consent statement
All participants were informed and consented to take part in the study.
Additional information
* The ORISCAV working group is listed in the acknowledgments.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vahid, F., Hoge, A., Hébert, J.R. et al. Association of diet quality indices with serum and metabolic biomarkers in participants of the ORISCAV-LUX-2 study. Eur J Nutr 62, 2063–2085 (2023). https://doi.org/10.1007/s00394-023-03095-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-023-03095-y