Abstract
Chitosan is a natural elicitor, used for stimulating plant growth and inducing plant defense. However, due to difficulty in monitoring root growth and activity, the effects of chitosan treatment on plant root systems have been less studied as compared to plant shoot parts that include leaves, seeds, and fruits. This results in an indefinite outcome of the benefits of chitosan on plant roots. Therefore, this review aims to evaluate the effects of chitosan treatment on root growth and defense responses based on current evidence. Interestingly, many studies have demonstrated that chitosan can induce plant root defense systems, yet conversely inhibiting root growth. The effects were most clearly observed from studies using liquid or solid media as substrates, while the results from the studies using soil were inconclusive and require additional investigation to observe the effects of environmental factors. In addition, root chitosan treatment showed variable effects on shoot growth, where low chitosan concentrations tend to promote shoot growth, but high chitosan concentrations may affect shoot development. Additionally, this review discusses the potential methods of chitosan application onto plant roots. Water insolubility of chitosan is likely a major issue for root treatment. Chitosan can be dissolved in acids, but this could induce acidity stress in plant roots. Modified versions of chitosan, such as chitosan nanoparticles, carboxylated chitosan, and graft chitosan copolymers have been developed to improve solubility and functionality. Chitosan nanoparticles can also be used to encapsulate other biocontrol agents to augment biological effects on plant defense. In conclusion, root chitosan treatment could help to promote plant defense and prevent root infections, abating the uses of chemical fungicides in agriculture. However, further research is required to monitor the impact of root chitosan treatment on long-term plant growth in order to gain multifaceted information to maximize the effectiveness of root chitosan application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chitosan is a natural polymer, composed of β-1,4-linked N-acetyl-d-glucosamine (N-GlcNAc) and d-glucosamine subunits. It is used in a range of applications to benefit humanity. In biomedical and pharmaceutical applications, it is used as a drug carrier, vaccine adjuvant, wound dressing material, and cartilage and bone tissue engineering scaffold (Muxika et al. 2017). Chitosan is also utilized in the food and agricultural industries owing to its antimicrobial, antifungal, and plant defense-eliciting properties (Pongprayoon et al. 2022; Shahbaz et al. 2022).
Chitin is another natural polysaccharide made of β-1,4-linked N-GlcNAc subunits. It is the primary source of chitosan for both natural production and industrial synthesis (Fig. 1). Chitin is one of the most abundant polymers in the world and predominantly found in fungal cell walls and exoskeletons of insects and crustaceans, such as scorpions, spiders, beetles, crayfishes, shrimps, and crabs. In contrast, chitosan is only found in the cell walls of certain fungal species and to a lower degree than chitin. Naturally, chitosan is synthesized from chitin by deacetylation using specific chitin deacetylase enzymes (Fig. 1). Chitosan can be extracted from fungal cell walls of Mucor spp. and Rhizopus spp. in the Mucoromycota phylum, Candida spp. and Saccharomyces spp. in the Ascomycota, or Pleurotus spp. and Lentinus spp. in the Basidiomycota (Ghormade et al. 2017). For mass production, chitosan is converted from chitin via chemical reactions using a strong base and high heat (Younes and Rinaudo 2015). Since shrimp and crab shells are common by-products from the seafood industry, chitin and chitosan are considered as readily accessible and affordable natural resources (Younes and Rinaudo 2015).
Different techniques of chitosan preparation affect physicochemical properties of chitosan end-products, such as size, viscosity, crystallinity, and degree of deacetylation, which could result in different biological activities and suit different applications (Brasselet et al. 2019; Román-Doval et al. 2023). Sources of chitin and chitosan materials, types of chemicals used in demineralization and deacetylation steps along with chemical concentrations, and temperatures and times are important controlling factors in chitosan preparation (Román-Doval et al. 2023; Younes and Rinaudo 2015). Sizes of chitosan can be divided into low- (< 150 kDa), medium- (150–700 kDa), and high molecular weight (> 700 kDa) (Boamah et al. 2023). Viscosity and solubility of chitosan are related to the size of chitosan—the larger or longer polymers, the higher viscosity, and the lower solubility (Aranaz et al. 2021). Crystallinity determines other physiological properties, such as porosity, water-absorption, and moisture-retention properties (Román-Doval et al. 2023). Degree of deacetylation is a key qualification of chitosan end-products. The high degree of deacetylation such as 80% deacetylation means that 80% of acetyl groups in N-GlcNAc monomers of long-chain chitosan are deacetylated. Higher degrees of deacetylation associate with increasing chitosan-like properties, including advancement of some physicochemical properties of chitosan, such as increased solubility in acidic environment (Brasselet et al. 2019). In the market, chitosan products come in a wide range of sizes, crystallinity, and degree of deacetylation. These parameters have a significant impact on chitosan biological properties and its applications. For example, low-molecular weight chitosan can be used as a plant elicitor, due to its enhanced solubility and higher antimicrobial activities but high-molecular weight chitosan is usually developed into film and used as external protectants, such as for seed or fruit coating (Boamah et al. 2023; Román-Doval et al. 2023). Nonetheless, chitosan either derived from fungal cell walls or processed from marine organisms could yield similar end products if they have identical physicochemical properties. The details of chitosan preparations, formulations, chemical and biological properties, and potential applications are well articulated in many review articles (Aranaz et al. 2021; Elieh-Ali-Komi and Hamblin 2016; Jimenez-Gomez and Cecilia 2020; Khan and Alamry 2021; Morin-Crini et al. 2019; Román-Doval et al. 2023; Younes and Rinaudo 2015; Yu et al. 2021).
In agricultural application, chitosan has been studied and used to promote plant growth and induce plant defense. Under biotic stress conditions, chitosan treatment has been shown to enhance plant resistance against pest infestations and pathogen infections (Fan et al. 2023). Several methods can be applied to perform chitosan treatment: for example, seed soaking before sowing, foliar spraying during plant growth, or fruit coating after harvesting (Riseh et al. 2022). The evidence supporting the protective roles of chitosan on aboveground plant tissues is well established (Chakraborty et al. 2020; Divya and Jisha 2017; Malerba and Cerana 2016, 2018; Pichyangkura and Chadchawan 2015; Riseh et al. 2022; Sharif et al. 2018; Stasińska-Jakubas and Hawrylak-Nowak 2022; Yu et al. 2021). However, the protective effects of chitosan have been investigated less on plant root systems. This is potentially due to the challenges of working with belowground materials, which are hard to observe and sample from (Lopez-Moya et al. 2019; Suwanchaikasem et al. 2022). As a consequence, studying plant roots requires effort, strategic planning and specialized tools to facilitate root visualization, treatment, and analysis.
The root system is an indispensable part of plants. It functions to anchor plants in place and absorb nutrients and water. Roots also form a complex biosystem with a myriad of surrounding microorganisms and organic substances underground, called the rhizosphere, making roots subject to regular fluctuations of environmental factors (York et al. 2016). Any changes of soil microbes, nutrients, moisture, pH, and temperature could affect plant growth (de la Fuente Cantó et al. 2020). Hence, understanding root activities and interactions with surrounding environments is fundamental knowledge, especially relevant in agriculture to support plant growth and improve crop yield. Common practices including supplying fertilizers, plowing soil, removing weeds, adding beneficial microbes and applying chemical pesticides, herbicides, and fungicides are routinely carried out to support root functions and to promote overall plant growth (Hakim et al. 2021; Watt et al. 2006). However, overusing pesticides and fungicides can be hazardous to humans, plants, and environments (Sharma et al. 2019; Tsalidis 2022). To limit or avoid the use of chemicals, natural elicitors such as chitosan could be an alternative platform for pest and disease management. Therefore, understanding plant root responses to chitosan treatment is essential to underpin the practical implementation of substituting chemical pesticides and fungicides with natural elicitors in agriculture. Furthermore, although the effects of chitosan have been well demonstrated on plant shoot tissues, the effects of chitosan on plant root systems might be different to what appears to the shoots due to a variety of environmental factors attributed to plant shoot and root tissue exposures.
This review aimed to assess the current knowledge from research that applied chitosan treatment on plant roots and monitored overall plant growth and defense responses. A literature search was conducted using three online databases, ScienceDirect, Web of Science and Wiley Library, using the basic search function most recently in April 2023. Search terms included “chitosan,” “root,” “growth,” and “defense,” where “chitosan” and “root” were fixed terms, while “growth” and “defense” were variable terms. The search was performed multiple times using AND as a Boolean operator between each term for all combinations. Date and year of publication were not restricted. The search results were filtered by article type, where research article was selected, while other forms, such as review article, book, book chapter, and proceeding abstract, were excluded. The number of returned hits varied from 90 to 4,263 publications (Table S1).
Subsequently, the results were sorted by “relevance” mode, where the most relevant articles were first listed. Starting from the first article, the method section of each article was checked to ensure that chitosan was applied onto plant roots, and overall plant growth and defense were monitored. The studies that applied chitosan on shoot tissues, such as foliar spray and seed soaking, were excluded, even though root responses were monitored. Likewise, the studies that applied chitosan on plant roots but did not examine plant physiological or biological responses were excluded. For returned lists with over 200 articles, once twenty articles in a row were found to be unrelated to the search criteria, the checks were stopped, and the rest of the articles were omitted. Duplicate articles across different searches were also removed. Finally, the shortlist was cross-checked with any review articles that contained sections summarizing the effects of chitosan on plant roots. Research publications related to the topic but absent from the shortlist were additionally included. In total, the number of research articles identified and included in this review was 24. Fifteen articles performed root chitosan treatment in in vitro or tissue culture settings. Nine articles reported the effects of root chitosan treatment under soil conditions. Most of the articles reported either growth parameters or defense mechanisms. Only five articles monitored both growth and defense responses concurrently in the same study.
This review is organized into eight sections. In "Impact of Root Chitosan Treatment on Shoot Growth Varies Upon Chitosan Dose and Treatment Factors" and "Root Development is Inhibited by Chitosan Treatment" sections summarize the effects of root chitosan treatment on shoot and root growth, respectively. In "Root Defense is Induced by Chitosan Treatment" section discusses the effects of chitosan on biological and biochemical plant defense. In "Growth-Defense Tradeoff is a Result of Root Chitosan Treatment" section integrates information from sections "Root Development is Inhibited by Chitosan Treatment" and "Root Defense is Induced by Chitosan Treatment" to surmise that root growth-defense tradeoff could be a consequence of root chitosan treatment. In "Methods to Apply Chitosan Onto Plant Root Systems" section articulates possible pathways of chitosan application onto plant roots. In "Combination of Chitosan Treatment with Other Methods for Fungal Disease Control" section describes the potential of chitosan when used in combination with other techniques to manage diseases. In "Conclusion and Perspective" section concludes the key messages and suggests further studies to advance the effectiveness of chitosan application. This review provides inclusive information regarding the effects of chitosan on plant root systems, which could encourage the use of chitosan in plant disease management schemes.
Impact of Root Chitosan Treatment on Shoot Growth Varies Upon Chitosan Dose and Treatment Factors
Chitosan is well demonstrated to promote plant growth when applied as a foliar spray. For example, periodically spraying chitosan (0.0125–0.1% w/v) on strawberry leaves for two months prior to flowering significantly increased plant height, leaf size, individual, and total fruit weights. Upon harvest, fruit biomass was increased by 29–42% (Rahman et al. 2018). Spraying 0.1% chitosan on one-month-old tomato leaves significantly promoted the number of flowers and fruits per plant by 14% and 77%, respectively. Total fruit fresh weights per plant increased by 2.45 times (Sathiyabama et al. 2014). The results from other studies showing shoot growth promotion according to chitosan foliar spray are well collated and discussed in review articles (Chakraborty et al. 2020; Divya and Jisha 2017; Malerba and Cerana 2018; Pichyangkura and Chadchawan 2015; Sharif et al. 2018; Stasińska-Jakubas and Hawrylak-Nowak 2022). In contrast, root chitosan treatment has not been consistently shown to promote shoot growth. As a biostimulant, chitosan was also expected to stimulate overall plant growth and could be one way of improving crop yield when applying to plant roots by mixing with soil or adding into hydroponic solution (Asghari-Zakaria et al. 2009; Ohta et al. 2001). However, the results in the literature show variable outcomes of root chitosan treatment on shoot growth, which is different from the results of the direct application of chitosan on shoot tissues.
Studies have demonstrated the positive effect of root chitosan treatment toward shoot growth (Fig. 2). For example, in chili (Capsicum annuum), after 30 days grown in soil amended with 1% w/w high-molecular weight chitosan, plant height, and leaf area were increased by 2.5–3 times and the number of fruit and fruit weight per plant were approximately 10 times increased. Treatments with low- and medium-molecular weight chitosan also conferred similar shoot growth promoting results (Chookhongkha et al. 2012). In prairie gentian (Eustoma grandiflorum), after 10 weeks grown in soil supplied with 1% chitosan, leaf size was increased by approximately 40–60% and shoot fresh and dry weight were promoted by approximately 5–6 times (Ohta et al. 2001). Moreover, supplying soil with both fertilizer and 1% chitosan showed a synergistic effect, where all shoot growth parameters were significantly higher than the single treatments of either chitosan or fertilizer alone (Ohta et al. 2000). The shoot growth-promoting effect of root chitosan treatment was also observed in other ornamental plants, where total shoot fresh weights of lobelia (Lobelia erinus), gloxinia (Sinningia speciosa), and Persian violet (Exacum affine) were increased by 52, 26, and 9 times, respectively, after approximately 6–13 weeks of soil amendment with 1% chitosan as compared to the normal condition of untreated fertilized soil (Ohta et al. 2004). In tomato (Solanum lycopersicum), plants were grown in soil drenched with 1% w/v chitosan solution (dissolved in 1% acetic acid) and compared to the control plants, grown in soil drenched with pure water or neutralized acetic acid. After 1–2 months, plant height, shoot fresh and dry weight of chitosan-treated plants were 1.5–2 higher than the controls (Algam et al. 2010). These results suggest that root chitosan treatment has potential to promote shoot growth upon soil cultivation.
Effects of chitosan on shoot and root growth among various plants and experimental settings. Based on experimental settings, all studies are classified into two groups; in vitro culture using growth media and potting system using soil. Bar graphs show approximate percentages of shoot and root growth inhibition (in red) or promotion (in green) upon the highest concentration of chitosan treatment within the particular study. Box plots (in teal), comprising interquartile range box and whiskers, show summary of each group. “ND” refers to not determined
However, several studies have shown conflicting results (Fig. 2). For example, in lettuce (Lactuca sativa), after 35 days grown in the soil amended with chitosan, the number of leaves per plant, leaf area, shoot fresh, and dry weight were significantly increased in the low concentrations of chitosan treatment (0.05–0.2%) but all reduced in 0.3% chitosan treatment. The fresh weights were increased by 26–39% for 0.05–0.2% chitosan treatments but 26% decreased in 0.3% concentration (Xu and Mou 2018). In tomato (S. lycopersicum), after 30 days grown in sand irrigated daily with nutrient solution supplied with chitosan, shoot dry weights were increased by 31% in 0.005% chitosan treatment but reduced by 19% in 0.03% chitosan treatment. The reduction was more obvious in the condition containing beneficial nematode parasite, Pochonia chlamydosporia, where a 58% decrease of shoot dry weight was detected in the highest concentration (0.03%) of chitosan treatment (Escudero et al. 2017). In chili (C. annuum), after grown in soil drenched with chitosan, size and weight of individual fruit were not different from control but the number of ripen fruit and total fruit yield were significantly reduced by 5–7% (Moon et al. 2012). In tomato (S. lycopersicum) and barley (Hordeum vulgare), after 21 days grown in sand with daily irrigation of nutrient solution supplemented with chitosan, shoot fresh weights were reduced by approximately 30% in 0.05% chitosan and more than 50% in 0.1–0.2% chitosan conditions, but the reduction was not observed in the low concentrations of chitosan treatment (0.001–0.01%) (Lopez-Moya et al. 2017). In milk thistle (Silybum marianum), after 72 days grown in soil mixed with 0.01–0.1% chitosan, plant height, shoot dry weight, and total biomass were comparable to control. However, improvements were detected in chitosan treatments under salinity conditions, for example, shoot dry weight was increased by 20–40% in chitosan treatment under mild salt stress (4 dS m−1) (Safikhan et al. 2018). In cucumber (Cucumis sativus), after 2–6 days of growth in hydroponic solution, shoot developments of the chitosan-treated plants (0.01–0.04% chitosan) were described as more vigorous than the untreated plants (El Ghaouth et al. 1994). In industrial hemp (Cannabis sativa), after eight days grown in hydroponic solution with 0.1–0.5% chitosan, total shoot fresh weight was not different to control (Suwanchaikasem et al. 2023a).
In tissue culture or in vitro experiments, the results of root chitosan treatment on shoot growth were also ambiguous. In Arabidopsis thaliana, after 21 days grown in the nutrient agar amended with chitosan, shoot fresh weights were approximately 40% lower than control in the lower doses of chitosan treatment (0.001–0.01%) and more than 80% decreased in the higher doses of the treatment (0.05–0.2%). The number of leaves per plant was also reduced by 30–50% in 0.05–0.2% chitosan treatments (Lopez-Moya et al. 2017). In protocorm culture of aloe-leafed cymbidium orchid (Cymbidium aloifolium), after 10 weeks cultured in the solid media supplied with chitosan, shoot height and total fresh weight were not different from control in the lower doses (0.05–0.1 ppm) but decreased by approximately 40% in the highest dose (1 ppm) of chitosan treatment. Number of leaves per protocorm was promoted in the lowest dose (0.05 ppm) but reduced in the highest doses (1 ppm) by approximately 25% (Noor Rohmah and Taratima 2021). In protocorm culture of long-lipped tongue orchid (Serapias vomeracea), the protocorm was grown in solid media supplemented with two types of chitosan, long-chain polymer (containing 70 subunits) and short-chain oligomer (with 2–15 subunits). The measurement was carried out 180 days after incubation. Among four doses of long-chain chitosan treatments, shoot length was significantly increased by approximately 1.8–2 times in the highest doses of 15–20 ppm concentrations but significantly decreased in the lowest dose of 5 ppm. In contrast, shoot length was increased in the lowest dose (5 ppm) of chitosan oligomer but significantly decreased in the higher doses of 15–20-ppm oligomer (Acemi 2020). In grapevine (Vitis vinifera), dipping cuttings into 0.01–0.02% chitosan solution for 24 h before planting improved number of internodes and new canes by approximately 10–30% and length of canes by approximately 30–40%, but the increments were not observed in the highest concentration (0.04%) of chitosan treatment (Górnik et al. 2008). In chili (C. annuum) grown in nutrient media supplemented with crude chitosan and chitosan nanoparticles, low concentrations (10–20 ppm) of crude chitosan improved total leaf area, leaf biomass, and shoot dry weight by 15–60%, but the highest concentration (100 ppm) inhibited shoot development by approximately 90% in all growth parameters. The similar outcome was also observed from chitosan nanoparticles, where all shoot growth parameters were significantly increased in the lowest dose of 1 ppm but 70–90% decreased in the higher doses of 5–20-ppm chitosan treatments (Asgari-Targhi et al. 2018). In potato (Solanum tuberosum) plantlets, after 21 days grown in solid media supplemented with chitosan, shoot fresh weight was significantly increased by approximately 40% in the highest concentration (500 ppm) of chitosan treatment. However, the lower doses of chitosan concentrations (5–150 ppm) did not affect shoot biomass (Asghari-Zakaria et al. 2009).
Based on current evidence, it could be summarized that unlike shoot treatment, root treatment with chitosan does not always promote shoot growth. The effect is likely to rely on several factors, including chitosan concentration, timing, duration and frequency of application, plant species, and growth conditions. Treating roots with relatively low to moderate chitosan concentrations tends to promote shoot growth, but once the concentration goes beyond certain limits, root chitosan treatment is likely to have no effect or, in some cases, contribute a negative impact on shoot growth. The threshold of chitosan concentration in soil-based systems seems to be higher than that in hydroponic system or micropropagation. Chitosan treatment on root tissues is likely to show greater benefits toward shoot growth when plants are under stress conditions. However, more research is required for appraisal before drawing definite conclusions for the effects of root chitosan treatment on shoot growth.
Root Development is Inhibited by Chitosan Treatment
Several studies have consistently demonstrated that chitosan has a negative impact on root development (Lopez-Moya et al. 2019). This inhibitory effect was not apparent in soil or field experiments. However, it was clearly recognized from the studies using hydroponic or nutrient-based systems (Fig. 2 and Table 1). This could be because in soil, roots are hidden underground and difficult to monitor, whereas in nutrient-based settings, roots can be readily observed through transparent liquid or solid media.
After 21-day cultivation in solid media, root growth of Arabidopsis seedlings was interrupted by the higher doses (0.01–0.2%) of chitosan treatment. The inhibitory effect was likely to be dose dependent since low doses of chitosan (0.001–0.005%) did not affect root growth, but 0.01% chitosan treatment reduced total root length by approximately 15%. The effect was strongest in the highest doses of 0.1% and 0.2% chitosan, where total root lengths of those conditions were more than 80% shorter than control (Lopez-Moya et al. 2017). A similar outcome was observed in tomato (S. lycopersicum) and barley (H. vulgare), where the low doses of 0.005–0.01% chitosan slightly arrested root growth by approximately 25%, but the higher doses of 0.1–0.2% chitosan showed more than 50% and 70% reductions of total root length and root fresh weights, respectively (Lopez-Moya et al. 2017). Another Arabidopsis study showed a similar result, where primary root lengths of Arabidopsis seedlings grown in solid media supplemented with the lower doses of 0.1–1 ppm chitosan were comparable to control. However, the plants grown in the media supplemented with the higher doses of 10–100-ppm chitosan showed more than 70% shorter primary root length than control after 3 days of treatment. The lateral root length and root number of the highest dose of 100-ppm chitosan were also significantly lower than those of control (Iglesias et al. 2019). In industrial hemp (C. sativa) grown in hydroponic solution, root growth was inhibited by 0.1–0.5% colloidal chitosan treatments. After eight days of treatment, total root length and surface area of chitosan-treated plants were more than 50% shorter and smaller than control (Suwanchaikasem et al. 2023a). In cymbidium orchid (C. aloifolium), the protocorms cultivated in the media supplied with the low doses (0.05 and 0.1 ppm) of chitosan for 10 weeks showed comparable total root number and root length to control, whereas those parameters were significantly decreased by approximately 20–50% in the highest dose (1 ppm) of chitosan treatment (Noor Rohmah and Taratima 2021). In St. John’s wort (Hypericum perforatum), plants cultivated in liquid media supplemented with 0.02% chitosan showed significantly lower total root biomass than control since day three after treatment. At 15 days after treatment, total root biomass was decreased by approximately 50% (Tocci et al. 2011).
In some studies, root growth was promoted upon the treatments with low concentrations of chitosan but inhibited upon high doses of chitosan treatment (Fig. 2). In chili (C. annuum), seedlings were grown in solid media supplemented with bulk chitosan and chitosan nanoparticles. Total root fresh weight was increased by approximately 40–70% in the conditions of low chitosan concentrations, including 10–20-ppm bulk chitosan and 1-ppm chitosan nanoparticles. However, root growth was strongly inhibited by high chitosan concentrations of 100-ppm bulk chitosan and 5–20-ppm chitosan nanoparticles. Total root length and fresh weight were approximately 80–90% lower than control (Asgari-Targhi et al. 2018). In grapevine (V. vinifera), cane cuttings were dipped in chitosan solutions for 24 h before cultivated in sand mix. After 75 days of cultivation, the number of primary roots originating from the cuttings was approximately 20% more than that of the control in the low concentrations of 0.01% and 0.02% chitosan solutions. Nonetheless, the number of primary roots was not different from control in the highest dose of 0.04% chitosan solution (Górnik et al. 2008). In protocorm culture of serapias orchid (S. vomeracea) treated with chitosan long-chain polymer and short-chain oligomer, root length was significantly enhanced by both types of chitosan. In chitosan long-chain polymer, the maximum increase of total root length was observed in the highest dose (20 ppm) of chitosan treatment. However, in the presence of chitosan oligomer, the maximum increase was observed in the lowest dose (5 ppm) of chitosan with approximately a four-time increase. The highest concentration of chitosan oligomer (20 ppm) showed approximately 2.5 times increase of root growth (Acemi 2020). In potato (S. tuberosum) plantlet, root fresh weight was significantly increased by approximately 13–43% in the lowest doses (5–15 ppm) of chitosan treatment but significantly decreased by approximately 18–32% in the highest doses of 50–500-ppm chitosan after 21 days grown in solid media (Asghari-Zakaria et al. 2009).
In soil-based experiments, the impact of chitosan treatment toward root growth is variable (Fig. 2). In cucumber (C. sativus), secondary root length of the plants grown in soil amended with 0.04% chitosan was described as shorter but thicker than control after two weeks of treatment (El Ghaouth et al. 1994). In tomato (S. lycopersicum), plantlets were grown in sand with and without beneficial fungus, P. chlamydosporia, and irrigated daily with the nutrient solution supplemented with chitosan. After 30 days of treatment, the root fresh weight and maximum root length were increased by approximately 30–154% in the low doses of 50 and 100 ppm of chitosan concentrations. However, the highest dose of chitosan treatment (300 ppm) significantly reduced root fresh weight by 53% in the condition containing the fungus and by 9% in the condition without the fungus (Escudero et al. 2017). In milk thistle (S. marianum), root dry weight was increased by approximately 6–11% in the soils supplemented with 0.01–0.1% chitosan after 72 days grown in pot (Safikhan et al. 2018). In prairie gentian (E. grandiflorum), root dry weight of the plant grown in soil amended with 1% chitosan was increased by 3.9 times as compared to the plants grown in untreated soil after 10 weeks (Ohta et al. 2001). The increases of root growth were also observed in other ornamental plants upon 1% chitosan treatment, such as 3–4 times increased in total root length of Rieger begonia (Begonia hiemalis) and Italian bellflower (Campanula fragilis) as compared to the control plants grown in soil added with normal fertilizer (Ohta et al. 2004).
To summarize these observations, chitosan treatment is prone to inhibit root growth (Fig. 2 and Table 1). The inhibitory effect tends to be dose dependent as the higher doses of chitosan have shown a stronger effect than the treatment with the lower doses. In terms of the molecular mechanism explaining these observations, the expressions of genes related to root elongation, including WOX5 and AQC1, were suppressed upon chitosan treatment in Arabidopsis seedlings. Whereas, genes involved in the auxin biosynthesis pathway, including YUC2, AAO1, and AMI1, were upregulated, but PIN1, an auxin efflux-related gene, was downregulated, resulting in an accumulation of auxins at the root meristem, causing discontinuation of root elongation (Lopez-Moya et al. 2017, 2019). This could be one of the reasons for root growth arrest following chitosan treatment. Apart from auxin accumulation, further exploration is required to identify other factors that could contribute to this phenomenon. Investigation of the molecular interplay between the root surface membrane and chitosan binding would provide essential information on the critical steps involved in plant recognition of chitosan. In addition, expanding the research of chitosan effects toward root growth in agricultural fields would contribute significant impact for chitosan application. The knowledge gained would be an essential asset for further study to characterize downstream signaling molecules and cascades, contributing to root growth inhibition caused by chitosan.
Root Defense is Induced by Chitosan Treatment
Many reviews have addressed the research on the potential of chitosan to induce plant defense (Katiyar et al. 2015; Malerba and Cerana 2016; Pichyangkura and Chadchawan 2015; Riseh et al. 2022; Sharif et al. 2018; Stasińska-Jakubas and Hawrylak-Nowak 2022; Xing et al. 2014). The reviews show that any method of chitosan application, including foliar spraying, pregerminated seed priming, post-harvest coating, soil amendment, and regular irrigation, all effectively promote plant defense. The reviews also collectively illustrate that different types of chitosan, i.e., low, medium or high molecular weights, short or long chains, normal-sized or nano-sized particles, and soluble or powder forms, all are capable of eliciting plant defense (Faqir et al. 2021; Sravani et al. 2023). The eliciting effect of chitosan has also been widely detected across different crops, grown in different settings, with plant responses analyzed using an array of different techniques (Malerba and Cerana 2018; Pichyangkura and Chadchawan 2015).
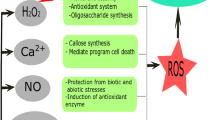
In terms of molecular mechanisms, the first step of chitosan triggering plant defense is likely the binding of chitosan with plant cell membrane receptors (Fig. 3). To date, the receptors that can bind specifically with a chitosan molecule have not been identified (Cord-Landwehr and Moerschbacher 2021). The only plant surface membrane protein found to interact with chitosan is a chitin elicitor receptor kinase (CERK), a receptor-like kinase that is activated by chitin oligosaccharides (Gubaeva et al. 2018; Yin et al. 2016). The receptor binding either by chitosan or chitin oligosaccharides, in association with activation of calcium influx via adjacent calcium channels, triggers downstream signaling processes, in which the levels of secondary messengers, i.e., nitric oxide (NO), hydrogen peroxide (H2O2) and cytosolic calcium ion (Ca2+), and defense hormones including salicylic acid (SA), jasmonic acid (JA), ethylene (ET) and abscisic acid (ABA) are increased in plant cells (Chandra et al. 2017; Gong et al. 2020; Katiyar et al. 2015; Lin et al. 2005; Lopez-Moya et al. 2017). Intermediate proteins and transcription factors, such as proteins in WRKY and AP2/ERF families, are involved in the signaling processes of chitosan induction (Coqueiro et al. 2015; Povero et al. 2011; Sripinyowanich et al. 2023). Finally, the signal activates the expressions of defense genes in the nucleus, leading to increased production of defense proteins, enzymes, and metabolites (De Bona et al. 2021; Katiyar et al. 2015). Defense proteins and enzymes upregulated upon chitosan treatment are, for example, pathogenesis-related (PR) proteins, catalase, chitinase, peroxidase, polyphenol oxidase (PPO), phenylalanine ammonia lyase (PAL), and superoxide dismutase (SOD). Activated defense metabolites are, for instance, phytoalexins, flavonoids, and phenolic compounds (Katiyar et al. 2015; Pichyangkura and Chadchawan 2015; Sravani et al. 2023). Chitosan was a stronger elicitor than chitin to induce production and secretion of plant defense hormones and proteins in root tissues of industrial hemp (C. sativa) (Suwanchaikasem et al. 2023a). Chitosan treatment can also manipulate plant physical barriers by increasing callose deposition and lignification of the plant cell wall (Sravani et al. 2023). Consequentially, chitosan-induced defense mechanisms promote plant resistance against both biotic and abiotic stresses (Hidangmayum et al. 2019; Mukarram et al. 2023).
Overview of possible molecular mechanisms underlying chitosan-manipulated plant defense and growth responses as modified from Das et al. (2015), García et al. (2021), Wang et al. (2020). Chitosan potentially binds to plant receptors, such as CERK1-containing receptor, to activate MAPK cascade and transcription factors to induce gene regulation in nucleus. Additionally, the binding of chitosan to the receptor is likely to increase reactive oxygen species (ROS) production via the activation of plant respiratory burst oxidase homolog (RBOH). Chitosan may promote calcium influx and activate the CDPK pathway to mediate gene regulation. Upon alteration of gene regulation, phytohormone biosynthesis may be affected, resulting in changes to cellular hormone levels and hormonal pathway crosstalk. Phytohormones, for example, auxins, salicylic acid (SA), jasmonic acid (JA) and abscisic acid (ABA) have intracellular roles that activate self-responses and intercellular functions signaling neighboring cells. Other phytohormones, such as brassinosteroids and gibberellins, may also play a part in the process. In the end, the defense-related genes may be preferentially upregulated, resulting in promotion of plant defense responses at the expense of plant root growth. Plants may strengthen their cell wall with callose deposition. Plants may increase production of defense metabolites and proteins, such as phytoalexin, peroxidase (PER), catalase (CAT), superoxide dismutase (SOD) and pathogenesis-related (PR) proteins in response to chitosan. CERK1 chitin elicitor receptor kinase 1, MAPK mitogen-activated protein kinase, CDPK calcium-dependent protein kinase, ROS reactive oxygen species. This figure was created with Biorender.com
The research demonstrating plant defense-eliciting properties of chitosan specifically on root defense systems is summarized below and in Table 1. In Arabidopsis, the levels of phytohormones including SA, JA, and indole-3-acetic acid (IAA) were 2.5–3.6 times increased, and expressions of genes involved in SA and JA biosynthetic pathways, including ICS1, ICS2, NPR1, AOC3, CYP94B, and MYC2, were upregulated in root tissues treated with 0.1% chitosan within 24 h (Lopez-Moya et al. 2017). In industrial hemp (C. sativa), roots treated with 0.2–0.5% chitosan showed increases of total chitinase activity and defense hormone levels, including JA and its derivatives, JA-isoleucine (JA-Ile) and 12-oxo-phytodienoic acid (OPDA), by approximately 2–5 times. Chitosan treatment also induced secretions of defense proteins into the root exudate (Suwanchaikasem et al. 2023a). In tomato (S. lycopersicum), 0.1% chitosan treatment was demonstrated to impair root plasma membrane to induce secretions of defense hormones, including SA, JA and ABA, and growth hormone, IAA, into exudate. Secretions of lipid signaling molecules and defense-related metabolites were also increased after 20 days of chitosan treatment. The exudate with increased secretion of defense metabolites showed 1.5 times reduction of eggs hatched by root-knot nematode, Meloidogyne javanica (Suarez-Fernandez et al. 2020). In date palm (Phoenix dactylifera), roots injected with 0.01–0.1% chitosan solution showed increases in peroxidase and PPO enzymatic activities within 10–20 days after treatment. The levels of phenolic compounds including caffeoylshikimic acid, sinapic, ferulic, and p-coumaric derivatives in root tissues were also increased within 20 days (El Hassni et al. 2004). In ginseng (Panax quinquefolius), callus cultured in the media supplied with 1% chitosan showed 7 times increase of PPO activity and 1.5 times increase of total phenolic contents within 12 h after treatment (Rahman and Punja 2005). Based on microscopic observation, treating cherry tomato (Lycopersicon esculentum) roots with 0.1% chitosan in combination with an endophytic bacteria, Bacillus pumilus, induced callose depositions onto the inner cell wall surface, resulting in mitigation of Fusarium oxysporum fungal infection (Benhamou et al. 1998). In hairy root culture of woad (Isatis tinctoria), adding 0.015% chitosan into suspension media significantly increased total flavonoid production and antioxidant activity. The peak increment was at 36 h after the treatment, where total flavonoid content in the hairy root extract was increased by approximately five times. Individual contents of major flavonoids, such as rutin, quercetin, and isorhamnetin, were increased by approximately 7–13 times (Jiao et al. 2018). In St. John’s wort (H. perforatum), root cultures elicited with 0.02% chitosan showed increases in xanthone production and secretion. The increases were highest at 7 days after treatment, where intracellular and extracellular xanthone levels were approximately 3.5 and 2.5 times higher than control, respectively. The increase of xanthone level in root tissues resulted in enhanced antifungal activity against fungal human pathogens, such as Candida spp. and Cryptococcus neoformans (Tocci et al. 2011). In rice (Oryza sativa) cell suspension cultures, supplying growth media with 0.015% bulk chitosan and chitosan nanoparticles significantly increased total phenolic and flavonoid contents and total activities of antioxidant enzymes, PAL, catalase, and peroxidase in the callus extracts at all timepoints of 7, 14, and 28 days after treatment (Arya et al. 2022).
Rather than monitoring defense mechanisms, some studies demonstrated the effects of root chitosan treatment directly on plant resistance against pathogen infections. In tomato (S. lycopersicum) plantlets, daily irrigation with nutrient solution supplemented with 0.01% chitosan promoted root colonization of beneficial fungus, P. chlamydosporia, leading to reduction of disease severity caused by root-knot nematode, M. javanica. After 56 days of infection, plant shoot and root weights of the chitosan-treated plants were significantly higher than the infected plants without chitosan treatment (Escudero et al. 2017). In another study of tomato (S. lycopersicum), irrigating soil with 0.15% nano-chitosan five days before or during the infection reduced gall number of root-knot nematode, Meloidogyne incognita, on root tissues by 56–68%. After 47 days of infection, shoot and root fresh weight of chitosan-treated plants were approximately 23–29% higher than the infected plants without treatment and relatively comparable to the uninfected plants (Khalil et al. 2022). In carrot, mixing soil with 1% chitosan reduced disease incidence caused by pathogenic fungi: Rhizoctonia solani by 39.2% and Athelia rolfsii by 29.9%. Carrot yields of chitosan-treated plants were improved by approximately 70–90% upon R. solani and A. rolfsii infection as compared to the infected plants without chitosan treatment (Rahman et al. 2021).
All the evidence above confirms the capability of chitosan to induce root defense responses, leading to increased disease resistance against pathogens (Table 1). Chitosan also has additional benefits to disease control with its implicit antimicrobial effects against some species of bacteria, fungi, and viruses (Chandrasekaran et al. 2020; Raafat and Sahl 2009; Riseh et al. 2022). In addition, chitosan treatment can promote plant tolerance against abiotic stresses, such as salinity and drought (Arif et al. 2021; Hidangmayum et al. 2019). These additional properties add support for implementing chitosan in practical agriculture to manage plant biotic and abiotic stresses.
Growth-Defense Tradeoff is a Result of Root Chitosan Treatment
By integrating information from "Root Development is Inhibited by Chitosan Treatment" and "Root Defense is Induced by Chitosan Treatment" sections, it could be concluded that chitosan inhibits root growth but activates root defense (Table 1 and Fig. 3). Once plant roots are exposed to chitosan, root activity is likely to shift from habitual root expansion to consolidating defense systems. This process is termed the “growth-defense tradeoff,” which is an important mechanism enabling plants to adapt and survive in uncertain environmental conditions (He et al. 2022). Both biotic and abiotic stresses are external factors able to turn on or off this alteration (Figueroa-Macias et al. 2021). For example, in the situation when a young seedling is searching for light but is under a dense canopy, it may heighten shoot growth quickly and minimize defense, thereby becoming increasingly vulnerable to pathogen attacks. On the other hand, a mature plant when attacked by a fungal pathogen may promptly activate a hypersensitive defense response and sacrifice the infected cells to limit the spread of the infection. In this case, plants may stop extending physical growth (Agrios 2005; He et al. 2022).
Molecular mechanisms underlying this tradeoff are not fully understood but are likely attributed to the interaction between growth and defense signaling pathways (Eichmann and Schafer 2015). Current understanding in the mechanisms of growth versus defense balance has been reviewed, with the key players proposed being phytohormones (Denancé et al. 2013; Dhar et al. 2020; Huot et al. 2014; Li et al. 2022; Lozano-Durán and Zipfel 2015). Salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) are defense hormones, actively functioning once plants encounter dangers, while auxin, cytokinin, gibberellin (GA), and brassinosteroid (BR) are mainly responsible for growth and development. Nonetheless, they crosstalk to each other and work in concert to manage stresses and maintain plant life (Eichmann and Schafer 2015; Huot et al. 2014; Lozano-Durán and Zipfel 2015). Endogenous phytohormones work like messengers, receiving primary signals to cell membrane receptors and circulating the messages intracellularly to activate gene expressions in nucleus and extracellularly to neighboring cells to communicate the coming of dangers (Berens et al. 2017; Denancé et al. 2013). Activation of mitogen-activated protein kinase (MAPK) cascades upon the binding of chitosan to CERK1 receptor is another important mediator, transducing signals from cell surface to trigger gene transcriptions and protein translations in nucleus (Zhang et al. 2018). Several transcription factors are involved in the processes of chitosan-induced plant growth and defense responses: for example, NPR1, a receptor and regulator of SA signaling; DELLA-family proteins, repressors of GA signaling; JAZ-family proteins, suppressors of JA signaling; and BIN2 kinase, a repressor of BR signaling (Eichmann and Schafer 2015; Huot et al. 2014). Additionally, some transcription factors, for example, homolog of brassinosteroid-enhanced expression2 interacting with IBH1 (HBI1), DP-E2F-like 1 (DEL1) and TL1-binding transcription factor 1 (TBF1), are identified as intermediate molecules, situating between growth and defense pathways and functioning to balance plant growth and defense activities (Chandran et al. 2014; Neuser et al. 2019; Pajerowska-Mukhtar et al. 2012; Wang and Wang 2014). Eventually, signaling processes end in the nucleus, where expression of growth- and defense-related genes are regulated accordingly. Circadian clock-associated 1 (CCA1) and YUCCAs genes are examples of growth-related genes, and pathogenesis-related proteins (PRs), ethylene response factor (ERFs), and plant defensins (PDFs) are some defense-related genes, activated upon growth-defense tradeoff (Cerrudo et al. 2017; Groszmann et al. 2015; He et al. 2022). Nonetheless, further study is required to convert this level of molecular understanding in plant growth-defense tradeoff to the natural conditions, where plants could be subjected to both biotic and abiotic stresses; to return to an earlier example, identifying what signaling pathways are affected when a plant is searching for light and meanwhile attacked by a pathogen (He et al. 2022).
As discussed in "Root Development is Inhibited by Chitosan Treatment" and "Root Defense is Induced by Chitosan Treatment" sections (Table 1), exogenous chitosan can drive this shift from growth to defense in plant roots by arresting root growth and activating defense responses by increasing production of defense metabolites and proteins and thickening cell wall barriers (Lopez-Moya et al. 2019; Sofi et al. 2023). However, plant growth and defense pathways affected by chitosan have not been completely identified. Chitosan may trigger typical plant defense signaling cascades. The process starts when danger signals from pathogens, called pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs), bind to plant cell surface receptors, named pattern recognition receptors (PRRs). The binding activates downstream signaling pathways and eventually induces the production of defense-related compounds. This process is termed the PAMP-triggered immunity (PTI) (Ramirez-Prado et al. 2018; van der Burgh and Joosten 2019). Danger signals to activate PTI are, for example, bacterial flagellin, bacterial elongation factor-Tu (EF-Tu) or fungal chitin (Zhang and Zhou 2010). Chitosan has not been well recognized to bind with PRRs and induce the PTI (Yin et al. 2016). CERK1 is the only plant cell membrane protein identified to date to be associated with the response to chitosan exposure (Gubaeva et al. 2018; Sofi et al. 2023). In root tissues, CERK1 was only known to be regulated upon fungal attack and salt stress (Espinoza et al. 2017; Zhang et al. 2015). Further exploration is required to identify whether enhanced defense responses in root tissues according to chitosan treatment is activated via the interaction with root CERK1 receptor.
In Arabidopsis, root chitosan treatment affected the expression of genes involved in biosynthesis of defense hormones, SA and JA, including ICS1, ICS2, NPR1, AOC3, CYP94B, and MYC2, causing increased levels of SA and JA hormones in root tissues. In industrial hemp (C. sativa), the levels of root ABA, JA, JA-Ile, and OPDA, were increased upon chitosan treatment (Suwanchaikasem et al. 2023a, b). Defense hormones, including SA, JA, and ABA were increasingly secreted into root exudate of tomato (S. lycopersicum) treated with 0.1% chitosan (Suarez-Fernandez et al. 2020). These findings signify that chitosan treatment manipulates defense hormone levels in root tissues. On the other side, chitosan can regulate auxin biosynthesis pathways. In Arabidopsis, genes involved in auxin biosynthesis, including YUC2, AMO1, and AMI1, were upregulated, but genes related to auxin efflux, PIN1, and root elongation, including WOX5 and AQC1, were downregulated upon chitosan treatment, causing auxin accumulation at the root tip (Lopez-Moya et al. 2017). This result suggests that the arrest of root growth in response to chitosan could be activated via auxin hormone signaling pathways.
After all, further research is required to elucidate and define in detail the molecular pathways triggered by chitosan and find the connection between growth and defense hormone crosstalk to fill in the gaps left in Fig. 3. A comprehensive understanding of chitosan effects toward plant root systems would provide a solid evidence basis for the application of chitosan in agriculture. It would also benefit the crop improvement sector who could use this knowledge to identify plant traits that produce high yield and still maintain high disease-resistance profiles (Cunha da Silva et al. 2019).
Methods to Apply Chitosan Onto Plant Root Systems
To date, the effects of chitosan on plant roots have been mostly examined in controlled laboratory settings. Two types of setups were used: soil based and nutrient based. In soil-based experiments, chitosan powder was usually directly mixed with the soil by weight in a concentration of 0.01–1% w/w (Ohta et al. 2004; Safikhan et al. 2018; Xu and Mou 2018). In nutrient-based experiments, chitosan was generally dissolved in weak acids, such as acetic, formic, or lactic acids, and then mixed with plant growth media, such as Murashige and Skoog (MS) medium (Asgari-Targhi et al. 2018; Jiao et al. 2018). In some cases, chitosan was prepared in a colloidal form by dissolving in a strong acid, such as hydrochloric acid (HCl) or orthophosphoric acid (H3PO4), to create a colloidal chitosan suspension. The acidic condition is then neutralized using basic compounds and/or vigorously washed with water to remove excess salts and acids. It could then be dehydrated and finally re-suspended in water, nutrient solution, or low-concentration weak acids (Olicón-Hernández et al. 2017; Palma-Guerrero et al. 2008; Suwanchaikasem et al. 2022). In a few cases, chitosan hydrochloride is created to enhance solubility of chitosan in water (De Vega et al. 2021). The methods of chitosan application on plant root systems are summarized in Fig. 4.
Ideally, chitosan would be dissolved in an aqueous-based solution, which would then enable easy addition to soil components or plant growth media to achieve root treatment. However, chitosan is a water-insoluble polymer, causing some barriers to the application. Chitosan is, in turn, soluble in acid due to its free amino group in the d-glucosamine subunit, which can be readily protonated under acidic conditions, with pKa ≈ 6.3 (Aranaz et al. 2021; Jimenez-Gomez and Cecilia 2020). The solubility of chitosan varies on its chemical properties, such as size, molecular weight, and degree of deacetylation. For example, the higher degree of deacetylation or the higher number of free amino acid groups in chitosan polymeric structure, the easier dissipation of chitosan polymer (Dash et al. 2011). Nonetheless, solubilizing chitosan in acid solutions creates a mild acidic condition, pH 4–5, which can affect plant development. For example, supplying 0.5% acetic acid in MS medium reduced chili (C. annuum) height and number of leaves by 46% and 35%, respectively. When plant roots were treated with 20-ppm low-, medium-, and high-molecular weight chitosan, dissolved in 0.5% acetic acid, root growth was significantly interrupted, which could be a result of media acidity (Chookhongkha et al. 2012). In prairie gentian (E. grandiflorum) culture, supplying MS medium with 0.0025% lactic acid decreased root dry weight by 50%. In the root treatment with 0.001–0.02% chitosan, dissolved in 0.0025% lactic acid, root dry weight was significantly higher than the acid media control but not different from the neutral media control (Ohta et al. 2000). Soil acidity is also known to restrict plant growth because of decreased availabilities of essential nutrients, such as phosphorus, calcium, and molybdenum, and increased levels of certain ions, such as aluminum and manganese to toxic levels (Msimbira and Smith 2020). Soil acidity also affects plant–microbe dynamics, which could alter symbiotic activity between plant roots and surrounding organisms. Therefore, the profits that plants would gain from beneficial microbes, such as nitrogen-fixing bacteria, would be mitigated (Msimbira and Smith 2020). Moreover, dissolving natural chitosan, prepared from black soldier fly (Hermetia illucens), in 1% acetic acid changed the polymeric structure of chitosan, reducing beneficial properties of chitosan in the context of thermal stability, crystallinity, and hydrophobicity (Bilican et al. 2020).
To avoid using acids for chitosan dissolution, several techniques have been applied to improve chitosan solubility, which is important for chitosan application onto plant root systems, especially with irrigation and supplementation into hydroponic solution (Fig. 4). Ionic liquids are an alternative solvent for dissolving chitosan (Li et al. 2019; Zhang et al. 2021). The technique uses non-volatile and reusable solvents, e.g., 1-ethyl-3-methylimidazolium acetate and 1-butyl-3-methylimidazolium chloride, which are considered safe and non-toxic to humans and the environment (Inamuddin and Asiri 2020). However, the method requires further optimization to make it cost-effective for mass production and, more importantly, critical evaluation on the side effects toward plant growth and surrounding environments (de Jesus and Maciel Filho 2022; Goncalves et al. 2021).
Chitosan powder can be transformed into chitosan micro/nanoparticles to improve chitosan physical and chemical properties (Colman et al. 2019; Iglesias et al. 2019). The transformation requires additional steps of preparation but obtains an improved version of chitosan with increased consistency, surface area, and solubility (Mohammed et al. 2017). It also improves chitosan efficiency, in which chitosan nanoparticles can be applied at a lower concentration within a range of 0.001–0.01% w/v, while the applied range of normal-sized chitosan is around 0.01–1% w/v (Asgari-Targhi et al. 2018). Chitosan micro/nanoparticles also have an advantage to incorporate other substances into their core structure, mimicking drug delivery systems to deliver active compounds to target sites. The incorporated compounds are protected from pH instability, enzyme degradation and other detrimental environmental conditions by chitosan nanostructure (Imam et al. 2021; Saberi Riseh et al. 2022a). After transformation, chitosan micro/nanoparticles do not lose plant defense-eliciting and antimicrobial properties but, in turn, show better performance in pest and disease control (Kumaraswamy et al. 2018; Sravani et al. 2023).
Modifying chitosan polymeric structure is another way to improve solubility and biochemical properties (Khan and Alamry 2021; Silva et al. 2021; Zhao et al. 2018). Due to free hydroxyl and amino groups in the d-glucosamine subunit, the polymer can undergo various chemical reactions, such as hydroxylation, alkylation, acylation, esterification, and carboxylation. The modified versions of chitosan, for example, carboxymethyl chitosan and quaternary ammonium chitosan, have greater water-soluble properties than intact chitosan, broadening their applications in biomedical and pharmaceutical fields (Fabiano et al. 2020; Shariatinia 2018). Another technique is a polymer grafting to merge chitosan with other polymers, for example coupling chitosan with polyethylene glycol or oligo lactic acid (Khan and Alamry 2021; Zhao et al. 2018). These chitosan copolymers show an improvement in controlling drug release when used as drug carriers, benefiting research into drug delivery, enhancing physical and biological properties of drug vehicles (Bhattarai et al. 2006; Wang et al. 2008). The possibility of using these new chitosan complexes on plant roots, especially when used in combination with other compounds, is discussed in the next section. Nonetheless, the new chitosan formulations need intensive research to assess their advantages and drawbacks toward plant development before being introduced in practical application.
To summarize this section, water insolubility of chitosan raw material is the major issue of root chitosan treatment. Based on current utilizations, chitosan is either diluted in weak acids, formed salt with strong acid, dispersed in hydroponic solution or directly mixed with soil or organic substrates. To improve chitosan solubility and biological functions, chemical modifications, such as transforming chitosan into nanoparticles, modifying chitosan core structure, or integrating chitosan with other polymers have been studied. However, further research is required to thoroughly examine the effects of the new chitosan materials on plant growth and defense responses. In addition, they might not be currently available and affordable for local users. Therefore, developing simplified procedures for chitosan production and preparation would be essential to underpin the practical usage of chitosan in crop productions.
Combination of Chitosan Treatment with Other Methods for Fungal Disease Control
The combination of multiple treatments onto plant root systems has been demonstrated to enhance the efficiency of disease control (Ons et al. 2020). Several techniques can synergize biological effects. For instance, natural products can be combined with synthetic chemicals to promote plant defense in parallel with targeting the invading pathogens. For example, applying a beneficial fungus, Trichoderma atroviride, in addition to drenching potting substrates with 0.01% fluazinam fungicide enhanced the chance of disease control by approximately 10–30% as compared to the use of fungicide alone to manage white root rot in avocado (Persea americana) (Ruano-Rosa et al. 2018). Drenching soil with beneficial bacteria, Burkholderia cepacia or Bacillus megaterium, along with 0.01% carbendazim fungicide reduced disease occurrence on tomato (S. lycopersicum) by approximately 50–60% as compared to fungicide treatment alone (Omar et al. 2006). Combining different microbes to build microbial consortia has also been revealed to enhance disease control performances against soil-borne fungal pathogens (Xu et al. 2011). The fungal and bacterial species commonly used to form microbial consortia are, for instance, Trichoderma spp., Bacillus spp., Pseudomonas spp., and Azospirillum spp. Combination of these microbes are effective to prevent infections from fungal pathogens, for example, Fusarium oxysporum, Pythium aphanidermatum, Rhizoctonia solani, and Athelia rolfsii (Palmieri et al. 2016; Sarma et al. 2015).
Chitosan has also been applied in combination with other biocontrol agents such as beneficial bacteria, fungi, seaweed extract, essential oils, and phytocompounds to manage plant diseases. For example, drenching soil with 0.01% w/v chitosan and 0.5% or 1.5% v/v brown algae (Ascophyllum nodosum) extract significantly reduced disease incidence and area of infection of Fusarium head blight, caused by Fusarium graminearum, in wheat (Triticum aestivum) leaves by approximately 30–80% as compared to individual chitosan or brown algae extract treatment (Gunupuru et al. 2019). Amending soil with 0.1% chitosan alone reduced disease severities of Fusarium seedling blight, caused by F. culmorum, by approximately 30% in wheat (T. aestivum) and 70% in barley (H. vulgare). By adding beneficial bacteria, Pseudomonas sp. MKB 158 or P. fluorescens MKB 249, into soil, disease incidence was approximately 20–50% lower than the chitosan treatment alone (Khan et al. 2006). Pre-treating tomato seedlings (S. lycopersicum) with 0.05% or 0.1% w/v chitosan and beneficial fungus, Trichoderma harzianum diminished disease incidence caused by F. oxysporum, by 50% as compared to the untreated plants and approximately 16–40% as compared to the single treatment of chitosan or beneficial fungus (El-Mohamedy et al. 2014).
To facilitate the use of chitosan in combination with other biocontrol agents, chitosan can be transformed into nanoparticle formulations to incorporate other biocontrol agents into nanocore structure, creating synergy in disease control. This technique has been investigated in biomedicine using chitosan nanoparticles as a drug carrier to encapsulate and deliver active compounds to target sites (Patra et al. 2018; Sung and Kim 2020). Chitosan nanoparticles can be prepared using various techniques. The preparation procedures along with advantage and drawback of each method have been extensively reviewed in many articles (Divya and Jisha 2017; Kumaraswamy et al. 2018; Singh et al. 2021; Yanat and Schroën 2021). The most favorable method is an ionic gelation, where chitosan powder is dissolved in acetic acid and then cross-linked with tripolyphosphate (TPP). The active compound is gently added before or during the crosslinking step (Hoang et al. 2022). Alternatively, oil-in-water emulsification is a method for combining chitosan nanoparticles with hydrophobic molecules, such as essential oils, using Tween 80 as an emulsifying agent (Das et al. 2019; Hasheminejad et al. 2019; Hosseini et al. 2013). Apart from plant defense-eliciting property, chitosan also has bactericidal and fungicidal properties, giving an extra benefit to the composites (Ke et al. 2021; Xing et al. 2014). Additionally, chitosan can be integrated with metals, such as magnesium, iron, and copper, forming chitosan-metal nanocomposites to promote antimicrobial functions (Ahmed et al. 2021; Bharathi et al. 2019; Rubina et al. 2017; Wang et al. 2005).
Chitosan nanoparticles have been demonstrated to exceed the performances of normal-sized chitosan to promote plant defense (Saberi Riseh et al. 2022a,b). However, most of the studies tested the effects of chitosan nanoparticles on plant shoot tissues or in cell suspension cultures. For example, in rice (O. sativa) suspension culture, 0.015% chitosan nanoparticles significantly increased productions of phenolic and flavonoid compounds by approximately 2.5 times as compared to control and 1.5 times as compared to 0.015% crude chitosan after 14 days of treatment. The activity of antioxidant enzyme, phenylalanine ammonia lyase (PAL), was also increased 2.5 times in chitosan nanoparticle treatment as compared to control and 1.25 times as compared to crude chitosan. The levels of phenolics and flavonoids and PAL enzymatic activity were further increased in the treatment of chitosan nanoparticles loaded with methyl jasmonate (Me-JA), a defense hormone in JA pathway, as compared to unloaded chitosan nanoparticles and crude chitosan loaded with Me-JA (Arya et al. 2022). In soil experiment, soaking cherry tomato (L. esculentum) seeds in the solutions of chitosan nanoparticles merged with vanillin or cinnamaldehyde for 5 h prior to planting diminished disease severity index by 2.5–3.3 times and 1.7–2.8 times against F. oxysporum and Pythium debaryanum pathogens, respectively. After 60 days of infection, shoot fresh and dry weight and number of leaves of the treated plants were higher than those of the infected plants without treatment (Elsherbiny et al. 2022). Mixing chili (C. annuum) seeds with alginate-chitosan nanoparticle beads entrapping bacterium, Bacillus licheniformis decreased disease incidence by approximately 30% as compared to negative control (water) and empty alginate bead after three days of A. rolfsii infection on two-week-old seedlings. The study also showed that the alginate bead containing both chitosan nanoparticles and beneficial bacteria had better performance than the beads containing only chitosan or bacteria alone to promote chili shoot growth in the uninfected condition (Panichikkal et al. 2021). Spraying cherry tomato (L. esculentum) leaves with chitosan nanoparticles loaded with harpin, a natural protein elicitor extracted from Pseudomonas syringae bacteria, reduced disease lesions caused by fungal pathogen R. solani and the DNA copy number of the fungus on plant leaves by more than 70% as compared to the treatments with empty chitosan nanoparticles or harpin elicitor alone. The leaves sprayed with chitosan nanoparticles containing harpin protein also showed increased peroxidase and PAL activities as compared to the treatments with chitosan or harpin alone at 3–4 days after treatment (Nadendla et al. 2018). However, the effects of chitosan nanoparticles have been barely studied on plant root systems, requiring further research to fill this lacking information to verify the benefits of chitosan toward overall plant defenses.
Due to capability to incorporate other molecules into its polymeric structure, chitosan is in a great position for the use in combination with other biocontrol agents to promote plant defense and manage plant diseases. Additional steps of preparation and encapsulation are required to produce a chitosan composite integrated with other molecules. Therefore, further research is needed to streamline and standardize the procedures and to test the efficacy and potential adverse effects of encapsulated chitosan nanoparticles on plant root systems.
Conclusion and Perspective
One ultimate goal of studying chitosan in the context of plant defense activation and disease resistance promotion is to find a substitute for chemical pesticides and fungicides. Application onto plant root systems by mixing with soil, daily irrigation or adding to liquid nutrient, would be convenient methods for chitosan treatment. Considerable research has confirmed the eliciting effects of chitosan on plant root defenses. However, chitosan seems to have a drawback on root growth. Once exposed to chitosan, plant roots are likely to stop physical growth and turn on defensive pathways. This phenomenon has been observed from several studies, conducting plant growth experiments in an in vitro setup using transparent plant growth media as nutrients (see in “Root Development is Inhibited by Chitosan Treatment” section). Nonetheless, the result seems to be inconclusive in soil-based experiments, where several factors, such as growth conditions, timepoint and duration of chitosan application, and plant species could impact the effects of chitosan on both shoot and root growth. This requires further studies to verify and confirm the effects of chitosan on plants, especially agricultural crops, grown in the field under farmland conditions.
Chitosan can be prepared in different formats, such as chitosan suspension, chitosan nanoparticles, and grafted chitosan copolymers. A comparative study is needed to examine their positive and negative impacts on plant tissues side by side. The modified versions of chitosan, including chitosan nanoparticles and graft chitosan copolymers, show improvement in chitosan water-soluble properties, facilitating the application of chitosan on plant root systems. Nonetheless, further research is required to simplify the preparation pipeline to make the modified chitosan a cost-effective material. This would also be useful for large-scale chitosan production for other commercial purposes, such as developing chitosan nanoparticles as film coating or food packaging materials.
Before implementation of chitosan in pest and disease control in agriculture, research is required to confirm the efficacy of chitosan in comparison with currently available commercial fungicides and pesticides. At the earlier stage, chitosan could be used in combination with fungicides and pesticides to reduce the amount of chemicals. It could then be utilized in combination with other techniques, such as beneficial microbes, to promote plant defenses. Good farming practices, such as avoiding fields with disease history, rotating with non-host crops and regularly screening sites and immediately removing infected plants, are still fundamental methods to prevent disease occurrence. By combining these techniques with chitosan treatment, pests and diseases in crop cultivations could be controlled in a more sustainable way to reduce environmental impacts of fungicides and pesticides and improve well-being of farmers and consumers.
References
Acemi A (2020) Chitosan versus plant growth regulators: a comparative analysis of their effects on in vitro development of Serapias vomeracea (Burm.f.) Briq. Plant Cell Tissue Organ Cult 141(2):327–338. https://doi.org/10.1007/s11240-020-01789-3
Agrios GN (2005) How plants defend themselves against pathogens. In: Agrios GN (ed) Plant pathol, 5th edn. Elsevier Academic Press, United States, pp 208–248
Ahmed T, Noman M, Luo J, Muhammad S, Shahid M, Ali MA, Zhang M, Li B (2021) Bioengineered chitosan-magnesium nanocomposite: a novel agricultural antimicrobial agent against Acidovorax oryzae and Rhizoctonia solani for sustainable rice production. Int J Biol Macromol 168:834–845. https://doi.org/10.1016/j.ijbiomac.2020.11.148
Algam SAE, Xie G, Li B, Yu S, Su T, Larsen J (2010) Effects of Paenibacillus strains and chitosan on plant growth promotion and control of ralstonia wilt in tomato. J Plant Pathol 92(3):593–600
Aranaz I, Alcántara AR, Civera MC, Arias C, Elorza B, Heras Caballero A, Acosta N (2021) Chitosan: An overview of its properties and applications. Polymers 13(19):3256. https://doi.org/10.3390/polym13193256
Arif Y, Siddiqui H, Hayat S (2021) Role of chitosan nanoparticles in regulation of plant physiology under abiotic stress. In: Faizan M, Hayat S, Yu F (eds) Sustainable agriculture reviews, vol 53. Springer Nature Publishing, Cham, pp 399–414
Arya SS, Rookes JE, Cahill DM, Lenka SK (2022) Chitosan nanoparticles and their combination with methyl jasmonate for the elicitation of phenolics and flavonoids in plant cell suspension cultures. Int J Biol Macromol 214:632–641. https://doi.org/10.1016/j.ijbiomac.2022.06.145
Asgari-Targhi G, Iranbakhsh A, Ardebili ZO (2018) Potential benefits and phytotoxicity of bulk and nano-chitosan on the growth, morphogenesis, physiology, and micropropagation of Capsicum annuum. Plant Physiol Biochem 127:393–402. https://doi.org/10.1016/j.plaphy.2018.04.013
Asghari-Zakaria R, Maleki-Zanjani B, Sedghi E (2009) Effect of in vitro chitosan application on growth and minituber yield of Solanum tuberosum L. Plant Soil Environ 55(6):252–256. https://doi.org/10.17221/1018-pse
Benhamou N, Kloepper JW, Tuzun S (1998) Induction of resistance against Fusarium wilt of tomato by combination of chitosan with an endophytic bacterial strain: ultrastructure and cytochemistry of the host response. Planta 204:153–168
Berens ML, Berry HM, Mine A, Argueso CT, Tsuda K (2017) Evolution of hormone signaling networks in plant defense. Annu Rev Phytopathol 55:401–425. https://doi.org/10.1146/annurev-phyto-080516-035544
Bharathi D, Ranjithkumar R, Vasantharaj S, Chandarshekar B, Bhuvaneshwari V (2019) Synthesis and characterization of chitosan/iron oxide nanocomposite for biomedical applications. Int J Biol Macromol 132:880–887. https://doi.org/10.1016/j.ijbiomac.2019.03.233
Bhattarai N, Ramay HR, Chou S-H, Zhang M (2006) Chitosan and lactic acid-grafted chitosan nanoparticles as carriers for prolonged drug delivery. Int J Nanomed 1(2):181–187. https://doi.org/10.2147/nano.2006.1.2.181
Bilican I, Pekdemir S, Onses MS, Akyuz L, Altuner EM, Koc-Bilican B, Zang L-S, Mujtaba M, Mulerčikas P, Kaya M (2020) Chitosan loses innate beneficial properties after being dissolved in acetic acid: supported by detailed molecular modeling. ACS Sustain Chem Eng 8(49):18083–18093. https://doi.org/10.1021/acssuschemeng.0c06373
Boamah PO, Onumah J, Aduguba WO, Santo KG (2023) Application of depolymerized chitosan in crop production: A review. Int J Biol Macromol 235:123858. https://doi.org/10.1016/j.ijbiomac.2023.123858
Brasselet C, Pierre G, Dubessay P, Dols-Lafargue M, Coulon J, Maupeu J, Vallet-Courbin A, de Baynast H, Doco T, Michaud P, Delattre C (2019) Modification of chitosan for the generation of functional derivatives. Appl Sci 9(7):1321. https://doi.org/10.3390/app9071321
Cerrudo I, Caliri-Ortiz ME, Keller MM, Degano ME, Demkura PV, Ballaré CL (2017) Exploring growth-defence trade-offs in Arabidopsis: phytochrome B inactivation requires JAZ10 to suppress plant immunity but not to trigger shade-avoidance responses. Plant Cell Environ 40(5):635–644. https://doi.org/10.1111/pce.12877
Chakraborty M, Hasanuzzaman M, Rahman M, Khan MAR, Bhowmik P, Mahmud NU, Tanveer M, Islam T (2020) Mechanism of plant growth promotion and disease suppression by chitosan biopolymer. Agriculture 10(12):624. https://doi.org/10.3390/agriculture10120624
Chandra S, Chakraborty N, Panda K, Acharya K (2017) Chitosan-induced immunity in Camellia sinensis (L.) O. Kuntze against blister blight disease is mediated by nitric-oxide. Plant Physiol Biochem 115:298–307. https://doi.org/10.1016/j.plaphy.2017.04.008
Chandran D, Rickert J, Huang Y, Steinwand MA, Marr SK, Wildermuth MC (2014) Atypical E2F transcriptional repressor DEL1 acts at the intersection of plant growth and immunity by controlling the hormone salicylic acid. Cell Host Microbe 15(4):506–513. https://doi.org/10.1016/j.chom.2014.03.007
Chandrasekaran M, Kim KD, Chun SC (2020) Antibacterial activity of chitosan nanoparticles: a review. Processes 8(9):1173. https://doi.org/10.3390/pr8091173
Chookhongkha N, Miyagawa S, Jirakiattikul Y, Photchanachai S (2012) Chilli growth and seed productivity as affected by chitosan. In: Proceeding of the international conference on agriculture technology and food sciences, Manila, Philippines. 17–18 November 2012, pp 146–149
Colman SL, Salcedo MF, Mansilla AY, Iglesias MJ, Fiol DF, Martín-Saldaña S, Alvarez VA, Chevalier AA, Casalongué CA (2019) Chitosan microparticles improve tomato seedling biomass and modulate hormonal, redox and defense pathways. Plant Physiol Biochem 143:203–211. https://doi.org/10.1016/j.plaphy.2019.09.002
Coqueiro DS, de Souza AA, Takita MA, Rodrigues CM, Kishi LT, Machado MA (2015) Transcriptional profile of sweet orange in response to chitosan and salicylic acid. BMC Genom 16:288. https://doi.org/10.1186/s12864-015-1440-5
Cord-Landwehr S, Moerschbacher BM (2021) Deciphering the ChitoCode: fungal chitins and chitosans as functional biopolymers. Fungal Biol Biotechnol 8(1):19. https://doi.org/10.1186/s40694-021-00127-2
Cunha da Silva A, Lima MdF, Eloy NB, Thiebaut F, Montessoro P, Hemerly AS, Ferreira PCG (2019) The Yin and Yang in plant breeding: the trade-off between plant growth yield and tolerance to stresses. Biotechnol Res Innov 3:73–79. https://doi.org/10.1016/j.biori.2020.02.001
Das S, Singh VK, Dwivedy AK, Chaudhari AK, Upadhyay N, Singh P, Sharma S, Dubey NK (2019) Encapsulation in chitosan-based nanomatrix as an efficient green technology to boost the antimicrobial, antioxidant and in situ efficacy of Coriandrum sativum essential oil. Int J Biol Macromol 133:294–305. https://doi.org/10.1016/j.ijbiomac.2019.04.070
Das SN, Madhuprakash J, Sarma PV, Purushotham P, Suma K, Manjeet K, Rambabu S, Gueddari NE, Moerschbacher BM, Podile AR (2015) Biotechnological approaches for field applications of chitooligosaccharides (COS) to induce innate immunity in plants. Crit Rev Biotechnol 35(1):29–43. https://doi.org/10.3109/07388551.2013.798255
Dash M, Chiellini F, Ottenbrite RM, Chiellini E (2011) Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog Polym Sci 36(8):981–1014. https://doi.org/10.1016/j.progpolymsci.2011.02.001
De Bona GS, Vincenzi S, De Marchi F, Angelini E, Bertazzon N (2021) Chitosan induces delayed grapevine defense mechanisms and protects grapevine against Botrytis cinerea. J Plant Dis Prot 128(3):715–724. https://doi.org/10.1007/s41348-021-00432-3
de Jesus SS, Maciel Filho R (2022) Are ionic liquids eco-friendly? Renew Sust Energ Rev 157:112039. https://doi.org/10.1016/j.rser.2021.112039
de la Fuente CC, Simonin M, King E, Moulin L, Bennett MJ, Castrillo G, Laplaze L (2020) An extended root phenotype: the rhizosphere, its formation and impacts on plant fitness. Plant J 103(3):951–964. https://doi.org/10.1111/tpj.14781
De Vega D, Holden N, Hedley PE, Morris J, Luna E, Newton A (2021) Chitosan primes plant defence mechanisms against Botrytis cinerea, including expression of Avr9/Cf-9 rapidly elicited genes. Plant Cell Environ 44(1):290–303. https://doi.org/10.1111/pce.13921
Denancé N, Sánchez-Vallet A, Goffner D, Molina A (2013) Disease resistance or growth: the role of plant hormones in balancing immune responses and fitness costs. Front Plant Sci 4:155. https://doi.org/10.3389/fpls.2013.00155
Dhar N, Chen JY, Subbarao KV, Klosterman SJ (2020) Hormone signaling and its interplay with development and defense responses in Verticillium-plant interactions. Front Plant Sci 11:584997. https://doi.org/10.3389/fpls.2020.584997
Divya K, Jisha MS (2017) Chitosan nanoparticles preparation and applications. Environ Chem Lett 16(1):101–112. https://doi.org/10.1007/s10311-017-0670-y
Eichmann R, Schafer P (2015) Growth versus immunity—a redirection of the cell cycle? Curr Opin Plant Biol 26:106–112. https://doi.org/10.1016/j.pbi.2015.06.006
El-Mohamedy RSR, Abdel-Kareem F, Jabnoun-Khiareddine H, Daami-Remadi M (2014) Chitosan and Trichoderma harzianum as fungicide alternatives for controlling Fusarium crown and root rot of tomato. Tunis J Plant Prot 9:31–43
El Ghaouth A, Arul J, Grenier J, Benhamou N, Asselin A, Bélanger R (1994) Effect of chitosan on cucumber plants: Suppression of Pythium aphanidermatum and induction of disease reactions. Phytopathology 84(3):313–320
El Hassni M, El Hadrami A, Daayf F, Ait Barka E, El Hadrami I (2004) Chitosan, antifungal product against Fusarium oxysporum f. sp. albedinis and elicitor of defense reactions in date palm roots. Phytopathol Mediterr 43:195–204
Elieh-Ali-Komi D, Hamblin MR (2016) Chitin and chitosan: production and application of versatile biomedical nanomaterials. Int J Adv Res 4(3):411–427
Elsherbiny AS, Galal A, Ghoneem KM, Salahuddin NA (2022) Novel chitosan-based nanocomposites as ecofriendly pesticide carriers: synthesis, root rot inhibition and growth management of tomato plants. Carbohydr Polym 282:119111. https://doi.org/10.1016/j.carbpol.2022.119111
Escudero N, Lopez-Moya F, Ghahremani Z, Zavala-Gonzalez EA, Alaguero-Cordovilla A, Ros-Ibanez C, Lacasa A, Sorribas FJ, Lopez-Llorca LV (2017) Chitosan increases tomato root colonization by Pochonia chlamydosporia and their combination reduces root-knot nematode damage. Front Plant Sci 8:1415. https://doi.org/10.3389/fpls.2017.01415
Espinoza C, Liang Y, Stacey G (2017) Chitin receptor CERK1 links salt stress and chitin-triggered innate immunity in Arabidopsis. Plant J 89(5):984–995. https://doi.org/10.1111/tpj.13437
Fabiano A, Beconcini D, Migone C, Piras AM, Zambito Y (2020) Quaternary ammonium chitosans: The importance of the positive fixed charge of the drug delivery systems. Int J Mol Sci 21(18):6617. https://doi.org/10.3390/ijms21186617
Fan Z, Wang L, Qin Y, Li P (2023) Activity of chitin/chitosan/chitosan oligosaccharide against plant pathogenic nematodes and potential modes of application in agriculture: A review. Carbohydr Polym 306:120592. https://doi.org/10.1016/j.carbpol.2023.120592
Faqir Y, Ma J, Chai Y (2021) Chitosan in modern agriculture production. Plant Soil Environ 67:679–699. https://doi.org/10.17221/332/2021-pse
Figueroa-Macias JP, Garcia YC, Nunez M, Diaz K, Olea AF, Espinoza L (2021) Plant growth-defense trade-offs: molecular processes leading to physiological changes. Int J Mol Sci 22(2):693. https://doi.org/10.3390/ijms22020693
García YH, Zamora OR, Troncoso-Rojas R, Tiznado-Hernández ME, Báez-Flores ME, Carvajal-Millan E, Rascón-Chu A (2021) Toward understanding the molecular recognition of fungal chitin and activation of the plant defense mechanism in horticultural crops. Molecules 26(21):6513. https://doi.org/10.3390/molecules26216513
Ghormade V, Pathan EK, Deshpande MV (2017) Can fungi compete with marine sources for chitosan production? Int J Biol Macromol 104:1415–1421. https://doi.org/10.1016/j.ijbiomac.2017.01.112
Goncalves ARP, Paredes X, Cristino AF, Santos FJV, Queiros CSGP (2021) Ionic liquids-a review of their toxicity to living organisms. Int J Mol Sci 22(11):5612. https://doi.org/10.3390/ijms22115612
Gong BQ, Wang FZ, Li JF (2020) Hide-and-seek: Chitin-triggered plant immunity and fungal counterstrategies. Trends Plant Sci 25(8):805–816. https://doi.org/10.1016/j.tplants.2020.03.006
Górnik K, Grzesik M, Romanowska-Duda B (2008) The effect of chitosan on rooting of grapevine cuttings and on subsuquent plant growth under drought and temperature stress. J Fruit Ornam Plant Res 16:333–343
Groszmann M, Gonzalez-Bayon R, Lyons RL, Greaves IK, Kazan K, Peacock WJ, Dennis ES (2015) Hormone-regulated defense and stress response networks contribute to heterosis in Arabidopsis F1 hybrids. Proc Natl Acad Sci USA 112(46):E6397-6406. https://doi.org/10.1073/pnas.1519926112
Gubaeva E, Gubaev A, Melcher RLJ, Cord-Landwehr S, Singh R, El Gueddari NE, Moerschbacher BM (2018) “Slipped sandwich” model for chitin and chitosan perception in Arabidopsis. Mol Plant Microbe Interact 31(11):1145–1153. https://doi.org/10.1094/MPMI-04-18-0098-R
Gunupuru LR, Patel JS, Sumarah MW, Renaud JB, Mantin EG, Prithiviraj B (2019) A plant biostimulant made from the marine brown algae Ascophyllum nodosum and chitosan reduce Fusarium head blight and mycotoxin contamination in wheat. PLoS ONE 14(9):e0220562. https://doi.org/10.1371/journal.pone.0220562
Hakim S, Naqqash T, Nawaz MS, Laraib I, Siddique MJ, Zia R, Mirza MS, Imran A (2021) Rhizosphere engineering with plant growth-promoting microorganisms for agriculture and ecological sustainability. Front Sustain Food Syst 5:617157. https://doi.org/10.3389/fsufs.2021.617157
Hasheminejad N, Khodaiyan F, Safari M (2019) Improving the antifungal activity of clove essential oil encapsulated by chitosan nanoparticles. Food Chem 275:113–122. https://doi.org/10.1016/j.foodchem.2018.09.085
He Z, Webster S, He SY (2022) Growth-defense trade-offs in plants. Curr Biol 32(12):R634–R639. https://doi.org/10.1016/j.cub.2022.04.070
Hidangmayum A, Dwivedi P, Katiyar D, Hemantaranjan A (2019) Application of chitosan on plant responses with special reference to abiotic stress. Physiol Mol Biol Plants 25(2):313–326. https://doi.org/10.1007/s12298-018-0633-1
Hoang NH, Le Thanh T, Sangpueak R, Treekoon J, Saengchan C, Thepbandit W, Papathoti NK, Kamkaew A, Buensanteai N (2022) Chitosan nanoparticles-based ionic gelation method: a promising candidate for plant disease management. Polymers 14(4):662. https://doi.org/10.3390/polym14040662
Hosseini SF, Zandi M, Rezaei M, Farahmandghavi F (2013) Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: preparation, characterization and in vitro release study. Carbohydr Polym 95(1):50–56. https://doi.org/10.1016/j.carbpol.2013.02.031
Huot B, Yao J, Montgomery BL, He SY (2014) Growth-defense tradeoffs in plants: a balancing act to optimize fitness. Mol Plant 7(8):1267–1287. https://doi.org/10.1093/mp/ssu049
Iglesias MJ, Colman SL, Terrile MC, París R, Martín-Saldaña S, Chevalier AA, Álvarez VA, Casalongué CA (2019) Enhanced properties of chitosan microparticles over bulk chitosan on the modulation of the auxin signaling pathway with beneficial impacts on root architecture in plants. J Agr Food Chem 67(25):6911–6920. https://doi.org/10.1021/acs.jafc.9b00907
Imam SS, Alshehri S, Ghoneim MM, Zafar A, Alsaidan OA, Alruwaili NK, Gilani SJ, Rizwanullah M (2021) Recent advancement in chitosan-based nanoparticles for improved oral bioavailability and bioactivity of phytochemicals: challenges and perspectives. Polymers 13(22):4036. https://doi.org/10.3390/polym13224036
Inamuddin AAM (2020) Nanotechnology-based industrial applications of ionic liquids. Springer Nature Publishing, Cham
Jiao J, Gai QY, Wang X, Qin QP, Wang ZY, Liu J, Fu YJ (2018) Chitosan elicitation of Isatis tinctoria L. hairy root cultures for enhancing flavonoid productivity and gene expression and related antioxidant activity. Ind Crops Prod 124:28–35. https://doi.org/10.1016/j.indcrop.2018.07.056
Jimenez-Gomez CP, Cecilia JA (2020) Chitosan: a natural biopolymer with a wide and varied range of applications. Molecules 25(17):3981. https://doi.org/10.3390/molecules25173981
Katiyar D, Hemantaranjan A, Singh B (2015) Chitosan as a promising natural compound to enhance potential physiological responses in plant: a review. Indian J Plant Physiol 20(1):1–9. https://doi.org/10.1007/s40502-015-0139-6
Ke CL, Deng FS, Chuang CY, Lin CH (2021) Antimicrobial actions and applications of chitosan. Polymers 13(6):904. https://doi.org/10.3390/polym13060904
Khalil MS, Abd El-Aziz MH, Selim RE-S (2022) Physiological and morphological response of tomato plants to nano-chitosan used against bio-stress induced by root-knot nematode (Meloidogyne incognita) and Tobacco mosaic tobamovirus (TMV). Eur J Plant Pathol 163(4):799–812. https://doi.org/10.1007/s10658-022-02516-8
Khan A, Alamry KA (2021) Recent advances of emerging green chitosan-based biomaterials with potential biomedical applications: a review. Carbohydr Res 506:108368. https://doi.org/10.1016/j.carres.2021.108368
Khan MR, Fischer S, Egan D, Doohan FM (2006) Biological control of fusarium seedling blight diseae of wheat and barley. Phytopathology 96:386–394. https://doi.org/10.1094/PHYTO-96-0386
Kumaraswamy RV, Kumari S, Choudhary RC, Pal A, Raliya R, Biswas P, Saharan V (2018) Engineered chitosan based nanomaterials: bioactivities, mechanisms and perspectives in plant protection and growth. Int J Biol Macromol 113:494–506. https://doi.org/10.1016/j.ijbiomac.2018.02.130
Li B, Wang J, Moustafa ME, Yang H (2019) Ecofriendly method to dissolve chitosan in plain water. ACS Biomater Sci Eng 5(12):6355–6360. https://doi.org/10.1021/acsbiomaterials.9b00695
Li C, Xu M, Cai X, Han Z, Si J, Chen D (2022) Jasmonate signaling pathway modulates plant defense, growth, and their trade-offs. Int J Mol Sci 23(7):3945. https://doi.org/10.3390/ijms23073945
Lin W, Hu X, Zhang W, Rogers WJ, Cai W (2005) Hydrogen peroxide mediates defence responses induced by chitosans of different molecular weights in rice. J Plant Physiol 162(8):937–944. https://doi.org/10.1016/j.jplph.2004.10.003
Lopez-Moya F, Escudero N, Zavala-Gonzalez EA, Esteve-Bruna D, Blázquez MA, Alabadí D, Lopez-Llorca LV (2017) Induction of auxin biosynthesis and WOX5 repression mediate changes in root development in Arabidopsis exposed to chitosan. Sci Rep 7(1):16813. https://doi.org/10.1038/s41598-017-16874-5
Lopez-Moya F, Suarez-Fernandez M, Lopez-Llorca LV (2019) Molecular mechanisms of chitosan interactions with fungi and plants. Int J Mol Sci 20(2):332. https://doi.org/10.3390/ijms20020332
Lozano-Durán R, Zipfel C (2015) Trade-off between growth and immunity: role of brassinosteroids. Trends Plant Sci 20(1):12–19. https://doi.org/10.1016/j.tplants.2014.09.003
Malerba M, Cerana R (2016) Chitosan effects on plant systems. Int J Mol Sci 17(7):996. https://doi.org/10.3390/ijms17070996
Malerba M, Cerana R (2018) Recent advances of chitosan applications in plants. Polymers 10(2):118. https://doi.org/10.3390/polym10020118
Mohammed MA, Syeda JTM, Wasan KM, Wasan EK (2017) An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 9(4):53. https://doi.org/10.3390/pharmaceutics9040053
Moon YH, Lee JH, Ahn BK, Choi IY, Cheong SS (2012) Effects of chitosan on red pepper (Capsicum annuum L.) cultivation for eco-friendly agriculture. Korean J Soil Sci Fert 45(4):635–641. https://doi.org/10.7745/kjssf.2012.45.4.635
Morin-Crini N, Lichtfouse E, Torri G, Crini G (2019) Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ Chem Lett 17(4):1667–1692. https://doi.org/10.1007/s10311-019-00904-x
Msimbira LA, Smith DL (2020) The roles of plant growth promoting microbes in enhancing plant tolerance to acidity and alkalinity stresses. Front Sustain Food Syst 4:106. https://doi.org/10.3389/fsufs.2020.00106
Mukarram M, Ali J, Dadkhah-Aghdash H, Kurjak D, Kačík F, Ďurkovič J (2023) Chitosan-induced biotic stress tolerance and crosstalk with phytohormones, antioxidants, and other signalling molecules. Front Plant Sci 14:1217822. https://doi.org/10.3389/fpls.2023.1217822
Muxika A, Etxabide A, Uranga J, Guerrero P, de la Caba K (2017) Chitosan as a bioactive polymer: processing, properties and applications. Int J Biol Macromol 105:1358–1368. https://doi.org/10.1016/j.ijbiomac.2017.07.087
Nadendla SR, Rani TS, Vaikuntapu PR, Maddu RR, Podile AR (2018) HarpinPss encapsulation in chitosan nanoparticles for improved bioavailability and disease resistance in tomato. Carbohydr Polym 199:11–19. https://doi.org/10.1016/j.carbpol.2018.06.094
Neuser J, Metzen CC, Dreyer BH, Feulner C, van Dongen JT, Schmidt RR, Schippers JHM (2019) HBI1 mediates the trade-off between growth and immunity through its impact on apoplastic ROS homeostasis. Cell Rep 28(7):1670–1678. https://doi.org/10.1016/j.celrep.2019.07.029
Noor Rohmah K, Taratima W (2021) Effect of chitosan, coconut water and potato extract on protocorm growth and plantlet regeneration of Cymbidium aloifolium (L.) Sw. Curr Appl Sci 22(2):1–10. https://doi.org/10.55003/cast.2022.02.22.014
Ohta K, Asao T, Hosoki T (2001) Effects of chitosan treatments on seedling growth, chitinase activity and flower quality in Eustoma grandiflorum (Raf.) Shinn, “Kairyou Wakamurasaki.” J Hortic Sci Biotechnol 76(5):612–614. https://doi.org/10.1080/14620316.2001.11511419
Ohta K, Atarashi H, Shimatani Y, Matsumoto S, Asao T, Hosoki T (2000) Effects of chitosan with or without nitrogen treatments on seedlings growth in Eustoma grandiflorum (Raf.) Shinn. Cv. Kairyou Wakamurasaki. J Jpn Soc Hortic Sci 69(1):63–65
Ohta K, Morishita S, Suda K, Kobayashi N, Hosoki T (2004) Effects of chitosan soil mixture treatment in the seedling stage on the growth and flowering of several ornamental plants. J Jpn Soc Hortic Sci 73(1):66–68. https://doi.org/10.2503/jjshs.73.66
Ohta K, Taniguchi A, Konishi N, Hosoki T (1999) Chitosan treatment affects plant growth and flower quality in Eustoma grandiflorum. HortScience 34(2):233–234. https://doi.org/10.21273/HORTSCI.34.2.233
Olicón-Hernández DR, Vázquez-Landaverde PA, Cruz-Camarillo R, Rojas-Avelizapa LI (2017) Comparison of chito-oligosaccharide production from three different colloidal chitosans using the endochitonsanolytic system of Bacillus thuringiensis. Prep Biochem Biotechnol 47(2):116–122. https://doi.org/10.1080/10826068.2016.1181086
Omar I, O’Neill TM, Rossall S (2006) Biological control of fusarium crown and root rot of tomato with antagonistic bacteria and integrated control when combined with the fungicide carbendazim. Plant Pathol 55(1):92–99. https://doi.org/10.1111/j.1365-3059.2005.01315.x
Ons L, Bylemans D, Thevissen K, Cammue BPA (2020) Combining biocontrol agents with chemical fungicides for integrated plant fungal disease control. Microorganisms 8(12):1930. https://doi.org/10.3390/microorganisms8121930
Pajerowska-Mukhtar KM, Wang W, Tada Y, Oka N, Tucker CL, Fonseca JP, Dong X (2012) The HSF-like transcription factor TBF1 is a major molecular switch for plant growth-to-defense transition. Curr Biol 22(2):103–112. https://doi.org/10.1016/j.cub.2011.12.015
Palma-Guerrero J, Jansson HB, Salinas J, Lopez-Llorca LV (2008) Effect of chitosan on hyphal growth and spore germination of plant pathogenic and biocontrol fungi. J Appl Microbiol 104(2):541–553. https://doi.org/10.1111/j.1365-2672.2007.03567.x
Palmieri D, Vitullo D, De Curtis F, Lima G (2016) A microbial consortium in the rhizosphere as a new biocontrol approach against fusarium decline of chickpea. Plant Soil 412:425–439. https://doi.org/10.1007/s11104-016-3080-1
Panichikkal J, Puthiyattil N, Raveendran A, Nair RA, Krishnankutty RE (2021) Application of encapsulated Bacillus licheniformis supplemented with chitosan nanoparticles and rice starch for the control of Sclerotium rolfsii in Capsicum annuum (L.) seedlings. Curr Microbiol 78(3):911–919. https://doi.org/10.1007/s00284-021-02361-8
Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, Habtemariam S, Shin HS (2018) Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol 16(1):71. https://doi.org/10.1186/s12951-018-0392-8
Pichyangkura R, Chadchawan S (2015) Biostimulant activity of chitosan in horticulture. Sci Hortic 196:49–65. https://doi.org/10.1016/j.scienta.2015.09.031
Pongprayoon W, Siringam T, Panya A, Roytrakul S (2022) Application of chitosan in plant defense responses to biotic and abiotic stresses. Appl Sci Eng Prog 15(1):3865. https://doi.org/10.14416/j.asep.2020.12.007
Povero G, Loreti E, Pucciariello C, Santaniello A, Di Tommaso D, Di Tommaso G, Kapetis D, Zolezzi F, Piaggesi A, Perata P (2011) Transcript profiling of chitosan-treated Arabidopsis seedlings. J Plant Res 124(5):619–629. https://doi.org/10.1007/s10265-010-0399-1
Raafat D, Sahl HG (2009) Chitosan and its antimicrobial potential—a critical literature survey. Microb Biotechnol 2(2):186–201. https://doi.org/10.1111/j.1751-7915.2008.00080.x
Rahman M, Mukta JA, Sabir AA, Gupta DR, Mohi-Ud-Din M, Hasanuzzaman M, Miah MG, Rahman M, Islam MT (2018) Chitosan biopolymer promotes yield and stimulates accumulation of antioxidants in strawberry fruit. PLoS ONE 13(9):e0203769. https://doi.org/10.1371/journal.pone.0203769
Rahman M, Punja ZK (2005) Biochemistry of ginseng root tissues affected by rusty root symptoms. Plant Physiol Biochem 43(12):1103–1114. https://doi.org/10.1016/j.plaphy.2005.09.004
Rahman MA, Jannat R, Akanda AM, Khan MAR, Rubayet MT (2021) Role of chitosan in disease suppression, growth and yield of carrot. Eur J Agric Food Sci 3(3):34–40. https://doi.org/10.24018/ejfood.2021.3.3.266
Ramirez-Prado JS, Abulfaraj AA, Rayapuram N, Benhamed M, Hirt H (2018) Plant immunity: From signaling to epigenetic control of defense. Trends Plant Sci 23(9):833–844. https://doi.org/10.1016/j.tplants.2018.06.004
Riseh RS, Hassanisaadi M, Vatankhah M, Babaki SA, Barka EA (2022) Chitosan as a potential natural compound to manage plant diseases. Int J Biol Macromol 220:998–1009. https://doi.org/10.1016/j.ijbiomac.2022.08.109
Román-Doval R, Torres-Arellanes SP, Tenorio-Barajas AY, Gómez-Sánchez A, Valencia-Lazcano AA (2023) Chitosan: properties and its application in agriculture in context of molecular weight. Polymers 15(13):2867. https://doi.org/10.3390/polym15132867
Ruano-Rosa D, Arjona-Girona I, López-Herrera CJ (2018) Integrated control of avocado white root rot combining low concentrations of fluazinam and Trichoderma spp. Crop Prot 112:363–370. https://doi.org/10.1016/j.cropro.2017.06.024
Rubina MS, Vasil’kov AY, Naumkin AV, Shtykova EV, Abramchuk SS, Alghuthaymi MA, Abd-Elsalam KA (2017) Synthesis and characterization of chitosan–copper nanocomposites and their fungicidal activity against two sclerotia-forming plant pathogenic fungi. J Nanostruct Chem 7(3):249–258. https://doi.org/10.1007/s40097-017-0235-4
Saberi Riseh R, Hassanisaadi M, Vatankhah M, Soroush F, Varma RS (2022a) Nano/microencapsulation of plant biocontrol agents by chitosan, alginate, and other important biopolymers as a novel strategy for alleviating plant biotic stresses. Int J Biol Macromol 222:1589–1604. https://doi.org/10.1016/j.ijbiomac.2022.09.278
Saberi Riseh R, Tamanadar E, Hajabdollahi N, Vatankhah M, Thakur VK, Skorik YA (2022b) Chitosan microencapsulation of rhizobacteria for biological control of plant pests and diseases: Recent advances and applications. Rhizosphere 23:100565. https://doi.org/10.1016/j.rhisph.2022.100565
Safikhan S, Khoshbakht K, Chaichi MR, Amini A, Motesharezadeh B (2018) Role of chitosan on the growth, physiological parameters and enzymatic activity of milk thistle (Silybum marianum (L.) Gaertn.) in a pot experiment. J Appl Res Med Aroma 10:49–58. https://doi.org/10.1016/j.jarmap.2018.06.002
Sarma BK, Yadav SK, Singh S, Singh HB (2015) Microbial consortium-mediated plant defense against phytopathogens: Readdressing for enhancing efficacy. Soil Biol Biochem 87:25–33. https://doi.org/10.1016/j.soilbio.2015.04.001
Sathiyabama M, Akila G, Charles RE (2014) Chitosan-induced defence responses in tomato plants against early blight disease caused by Alternaria solani (Ellis and Martin) Sorauer. Arch Phytopathol 47(16):1963–1973. https://doi.org/10.1080/03235408.2013.863497
Shahbaz U, Basharat S, Javed U, Bibi A, Yu XB (2022) Chitosan: a multipurpose polymer in food industry. Polym Bull 80(4):3547–3569. https://doi.org/10.1007/s00289-022-04269-0
Shariatinia Z (2018) Carboxymethyl chitosan: Properties and biomedical applications. Int J Biol Macromol 120:1406–1419. https://doi.org/10.1016/j.ijbiomac.2018.09.131
Sharif R, Mujtaba M, Ur Rahman M, Shalmani A, Ahmad H, Anwar T, Tianchan D, Wang X (2018) The multifunctional role of chitosan in horticultural crops: a review. Molecules 23(4):872. https://doi.org/10.3390/molecules23040872
Sharma A, Kumar V, Shahzad B, Tanveer M, Sidhu GPS, Handa N, Kohli SK, Yadav P, Bali AS, Parihar RD, Dar OI, Singh K, Jasrotia S, Bakshi P, Ramakrishnan M, Kumar S, Bhardwaj R, Thukral AK (2019) Worldwide pesticide usage and its impacts on ecosystem. SN Appl Sci 1(11):1446. https://doi.org/10.1007/s42452-019-1485-1
Silva AO, Cunha RS, Hotza D, Machado RAF (2021) Chitosan as a matrix of nanocomposites: a review on nanostructures, processes, properties, and applications. Carbohydr Polym 272:118472. https://doi.org/10.1016/j.carbpol.2021.118472
Singh A, Mittal A, Benjakul S (2021) Chitosan nanoparticles: preparation, food applications and health benefits. ScienceAsia 47(1):1–10. https://doi.org/10.2306/scienceasia1513-1874.2021.020
Sofi KG, Metzger C, Riemann M, Nick P (2023) Chitosan triggers actin remodelling and activation of defence genes that is repressed by calcium influx in grapevine cells. Plant Sci 326:111527. https://doi.org/10.1016/j.plantsci.2022.111527
Sravani B, Dalvi S, Narute TK (2023) Role of chitosan nanoparticles in combating Fusarium wilt (Fusarium oxysporum f. sp. ciceri) of chickpea under changing climatic conditions. J Phytopathol 171:67–81. https://doi.org/10.1111/jph.13159
Sripinyowanich S, Petchsri S, Tongyoo P, Lee TK, Lee S, Cho WK (2023) Comparative transcriptomic analysis of genes in the 20-hydroxyecdysone biosynthesis in the fern Microsorum scolopendria towards challenges with foliar application of chitosan. Int J Mol Sci 24(3):2397. https://doi.org/10.3390/ijms24032397
Stasińska-Jakubas M, Hawrylak-Nowak B (2022) Protective, biostimulating, and eliciting effects of chitosan and its derivatives on crop plants. Molecules 27(9):2801. https://doi.org/10.3390/molecules27092801
Suarez-Fernandez M, Marhuenda-Egea FC, Lopez-Moya F, Arnao MB, Cabrera-Escribano F, Nueda MJ, Gunsé B, Lopez-Llorca LV (2020) Chitosan induces plant hormones and defenses in tomato root exudates. Front Plant Sci 11:572087. https://doi.org/10.3389/fpls.2020.572087
Sung YK, Kim SW (2020) Recent advances in polymeric drug delivery systems. Biomater Res 24:12. https://doi.org/10.1186/s40824-020-00190-7
Suwanchaikasem P, Idnurm A, Selby-Pham J, Walker R, Boughton BA (2022) Root-TRAPR: a modular plant growth device to visualize root development and monitor growth parameters, as applied to an elicitor response of Cannabis sativa. Plant Methods 18(1):46. https://doi.org/10.1186/s13007-022-00875-1
Suwanchaikasem P, Nie S, Idnurm A, Selby-Pham J, Walker R, Boughton BA (2023a) Effects of chitin and chitosan on root growth, biochemical defense response and exudate proteome of Cannabis sativa. Plant-Environ Interact 4(3):115–133. https://doi.org/10.1002/pei3.10106
Suwanchaikasem P, Nie S, Selby-Pham J, Walker R, Boughton BA, Idnurm A (2023b) Hormonal and proteomic analyses of southern blight disease caused by Athelia rolfsii and root chitosan priming on Cannabis sativa in an in vitro hydroponic system. Plant Direct 7(9):e528. https://doi.org/10.1002/pld3.528
Tocci N, Simonetti G, D'Auria FD, Panella S, Palamara AT, Valletta A, Pasqua G (2011) Root cultures of Hypericum perforatum subsp. angustifolium elicited with chitosan and production of xanthone-rich extracts with antifungal activity. Appl Microbiol Biotechnol 91(4):977–987. https://doi.org/10.1007/s00253-011-3303-6
Tsalidis GA (2022) Human health and ecosystem quality benefits with life cycle assessment due to fungicides elimination in agriculture. Sustainability 14(2):846. https://doi.org/10.3390/su14020846
van der Burgh AM, Joosten MHAJ (2019) Plant immunity: Thinking outside and inside the box. Trends Plant Sci 24(7):587–601. https://doi.org/10.1016/j.tplants.2019.04.009
Wang Q, Zhang N, Hu X, Yang J, Du Y (2008) Chitosan/polyethylene glycol blend fibers and their properties for drug controlled release. J Biomed Mater Res A 85(4):881–887. https://doi.org/10.1002/jbm.a.31544
Wang R, He F, Ning Y, Wang GL (2020) Fine-tuning of RBOH-mediated ROS signaling in plant immunity. Trends Plant Sci 25(11):1060–1062. https://doi.org/10.1016/j.tplants.2020.08.001
Wang W, Wang ZY (2014) At the intersection of plant growth and immunity. Cell Host Microbe 15(4):400–402. https://doi.org/10.1016/j.chom.2014.03.014
Wang X, Du Y, Fan L, Liu H, Hu Y (2005) Chitosan- metal complexes as antimicrobial agent: Synthesis, characterization and structure-activity study. Polym Bull 55:105–113. https://doi.org/10.1007/s00289-005-0414-1
Watt M, Kirkegaard JA, Passioura JB (2006) Rhizosphere biology and crop productivity—a review. Aust J Soil Res 44(4):299–317. https://doi.org/10.1071/sr05142
Xing K, Zhu X, Peng X, Qin S (2014) Chitosan antimicrobial and eliciting properties for pest control in agriculture: a review. Agron Sustain Dev 35(2):569–588. https://doi.org/10.1007/s13593-014-0252-3
Xu C, Mou B (2018) Chitosan as soil amendment affects lettuce growth, photochemical efficiency, and gas exchange. Hort Technol 28(4):476–480. https://doi.org/10.21273/horttech04032-18
Xu XM, Jeffries P, Pautasso M, Jeger MJ (2011) Combined use of bicontrol agents to manage plant diseases in theory and practice. Phytopathology 101:1024–1031. https://doi.org/10.1094/PHYTO-08-10-0216
Yanat M, Schroën K (2021) Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React Funct Polym 161:104849. https://doi.org/10.1016/j.reactfunctpolym.2021.104849
Yin H, Du Y, Dong Z (2016) Chitin oligosaccharide and chitosan oligosaccharide: Two similar but different plant elicitors. Front Plant Sci 7:522. https://doi.org/10.3389/fpls.2016.00522
York LM, Carminati A, Mooney SJ, Ritz K, Bennett MJ (2016) The holistic rhizosphere: integrating zones, processes, and semantics in the soil influenced by roots. J Exp Bot 67(12):3629–3643. https://doi.org/10.1093/jxb/erw108
Younes I, Rinaudo M (2015) Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs 13(3):1133–1174. https://doi.org/10.3390/md13031133
Yu J, Wang D, Geetha N, Khawar KM, Jogaiah S, Mujtaba M (2021) Current trends and challenges in the synthesis and applications of chitosan-based nanocomposites for plants: A review. Carbohydr Polym 261:117904. https://doi.org/10.1016/j.carbpol.2021.117904
Zhang H, Kong M, Jiang Q, Hu K, Ouyang M, Zhong F, Qin M, Zhuang L, Wang G (2021) Chitosan membranes from acetic acid and imidazolium ionic liquids: Effect of imidazolium structure on membrane properties. J Mol Liq 340:117209. https://doi.org/10.1016/j.molliq.2021.117209
Zhang J, Zhou JM (2010) Plant immunity triggered by microbial molecular signatures. Mol Plant 3(5):783–793. https://doi.org/10.1093/mp/ssq035
Zhang M, Su J, Zhang Y, Xu J, Zhang S (2018) Conveying endogenous and exogenous signals: MAPK cascades in plant growth and defense. Curr Opin Plant Biol 45:1–10. https://doi.org/10.1016/j.pbi.2018.04.012
Zhang X, Dong W, Sun J, Feng F, Deng Y, He Z, Oldroyd GE, Wang E (2015) The receptor kinase CERK1 has dual functions in symbiosis and immunity signalling. Plant J 81(2):258–267. https://doi.org/10.1111/tpj.12723
Zhao D, Yu S, Sun B, Gao S, Guo S, Zhao K (2018) Biomedical applications of chitosan and its derivative nanoparticles. Polymers 10(4):462. https://doi.org/10.3390/polym10040462
Acknowledgements
PS received a Melbourne Research Scholarship and Gretna Weste Plant Pathology and Mycology Scholarship (University of Melbourne Botany Foundation).
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
PS, AI, JSW, RW and BAB framed the idea of the review. PS performed literature search and drafted the manuscript. AI, JSW, RW, BAB provided comments and feedback. All authors edited and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, I declare that there is no conflict of interest. No fundings, grants or other support was received for conducting this review.
Additional information
Handling Editor: Golam Jalal Ahammed.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suwanchaikasem, P., Idnurm, A., Selby-Pham, J. et al. The Impacts of Chitosan on Plant Root Systems and Its Potential to be Used for Controlling Fungal Diseases in Agriculture. J Plant Growth Regul (2024). https://doi.org/10.1007/s00344-024-11356-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00344-024-11356-1