Abstract
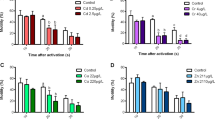
We evaluated the effect of pH on larval development in larval Pacific oyster (Crassostrea gigas) and blood cockle (Arca inflata Reeve). The larvae were reared at pH 8.2 (control), 7.9, 7.6, or 7.3 beginning 30 min or 24 h post fertilization. Exposure to lower pH during early embryonic development inhibited larval shell formation in both species. Compared with the control, larvae took longer to reach the D-veliger stage when reared under pH 7.6 and 7.3. Exposure to lower pH immediately after fertilization resulted in significantly delayed shell formation in the Pacific oyster larvae at pH 7.3 and blood cockle larvae at pH 7.6 and 7.3. However, when exposure was delayed until 24 h post fertilization, shell formation was only inhibited in blood cockle larvae reared at pH 7.3. Thus, the early embryonic stages were more sensitive to acidified conditions. Our results suggest that ocean acidification will have an adverse effect on embryonic development in bivalves. Although the effects appear subtle, they may accumulate and lead to subsequent issues during later larval development.
Similar content being viewed by others
References
Anthony K R N, Kline D I, Diaz-Pulido G, Dove S, Hoegh-Guldberg O. 2008. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proceedings of the National Academy of Sciences of the United States of America, 105(45): 17 442–17 446.
Baumann H, Talmage S C, Gobler C J. 2012. Reduced early life growth and survival in a fish in direct response to increased carbon dioxide. Nat. Clim. Chang., 2(1): 38–41.
Beaufort L, Probert I, de Garidel-Thoron T, Bendif E M, Ruiz-Pino D, Metzl N, Goyet C, Buchet N, Coupel P, Grelaud M, Rost B, Rickaby R E M, de Vargas C. 2011. Sensitivity of coccolithophores to carbonate chemistry and ocean acidification. Nature, 476(7358): 80–83.
Beniash E, Ivanina A, Lieb N S, Kurochkin I, Sokolova I M. 2010. Elevated level of carbon dioxide affects metabolism and shell formation in oysters Crassostrea virginica. Marine Ecology Progress Series, 419: 95–108.
Branch T A, DeJoseph B M, Ray L J, Wagner C A. 2013. Impacts of ocean acidification on marine seafood. Trends in Ecology & Evolution, 28(3): 178–186.
Comeau S, Gorsky G, Jeffree R, Teyssie J L, Gattuso J P. 2009. Impact of ocean acidification on a key Arctic pelagic mollusc (Limacina helicina). Biogeosciences, 6(9): 1 877–1 882.
De’ath G, Lough J M, Fabricius K E. 2009. Declining coral calcification on the Great Barrier Reef. Science, 323(5910): 116–119.
Dineshram R, Thiyagarajan V, Lane A, Yu Z, Shu X, Leung P T Y. 2013. Elevated CO2 alters larval proteome and its phosphorylation status in the commercial oyster, Crassostrea hongkongensis. Marine Biology, 160(8): 2 189–2 205.
Dineshram R, Wong K K W, Xiao S, Yu Z, Qian P Y, Thiyagarajan V. 2012. Analysis of Pacific oyster larval proteome and its response to high-CO2. Marine Pollution Bulletin, 64(10): 2 160–2 167.
Doney S C, Fabry V J, Feely R A, Kleypas J A. 2009. Ocean Acidification: The Other CO2 Problem. In: Annual Review of Marine Science. Annual Reviews, Palo Alto. p.169–192.
Doney S C, Ruckelshaus M, Duffy J E, Barry J P, Chan F, English C A, Galindo H M, Grebmeier J M, Hollowed A B, Knowlton N, Polovina J, Rabalais N N, Sydeman W J, Talley L D. 2012. Climate Change Impacts on Marine Ecosystems. In: Carlson C A, Giovannoni S J eds. Annual Review of Marine Science, Vol.411–437.
Fabry V J, Seibel B A, Feely R A, Orr J C. 2008. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES Journal of Marine Science, 65(3): 414–432.
Gazeau F, Gattuso J P, Dawber C, Pronker A E, Peene F, Peene J, Heip C H R, Middelburg J J. 2010. Effect of ocean acidification on the early life stages of the blue mussel Mytilus edulis. Biogeosciences, 7(7): 2 051–2 060.
Gazeau F, Quiblier C, Jansen J M, Gattuso J P, Middelburg J J, Heip C H R. 2007. Impact of elevated CO2 on shellfish calcification. Geophys. Res. Lett., 34(7).
Gobler C J, Talmage S C. 2013. Short- and long-term consequences of larval stage exposure to constantly and ephemerally elevated carbon dioxide for marine bivalve populations. Biogeosciences, 10(4): 2 241–2 253.
Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt K B, Tignor M, Miller H L. 2007. Climate Change 2007: The Physical Science Basis: Working Group I Contribution to the Fourth Assessment Report of the IPCC. Cambridge University Press, Cambridge, UK.
Kroeker K J, Kordas R L, Crim R, Hendriks I E, Ramajo L, Singh G S, Duarte C M, Gattuso J P. 2013. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Global Change Biology, 19(6): 1 884–1 896.
Kurihara H, Asai T, Kato S, Ishimatsu A. 2009. Effects of elevated p CO2 on early development in the mussel Mytilus galloprovincialis. Aquatic Biology, 4(3): 225–233.
Li J, Jiang Z, Zhang J, Qiu J-W, Du M, Bian D, Fang J. 2013. Detrimental effects of reduced seawater pH on the early development of the Pacific abalone. Marine Pollution Bulletin, 74(1): 320–324.
Miller A W, Reynolds A C, Sobrino C, Riedel G F. 2009. Shellfish face uncertain future in high CO2 world: influence of acidification on oyster larvae calcification and growth in estuaries. PloS One, 4(5).
Moy A D, Howard W R, Bray S G, Trull T W. 2009. Reduced calcification in modern Southern Ocean planktonic foraminifera. Nat. Geosci., 2(4): 276–280.
Narita D, Rehdanz K, Tol R S J. 2012. Economic costs of ocean acidification: a look into the impacts on global shellfish production. Climatic Change, 113(3–4): 1 049–1 063.
Norris R D, Turner S K, Hull P M, Ridgwell A. 2013. Marine ecosystem responses to cenozoic global change. Science, 341(6145): 492–498.
Orr J C, Fabry V J, Aumont O, Bopp L, Doney S C, Feely R A, Gnanadesikan A, Gruber N, Ishida A, Joos F, Key R M, Lindsay K, Maier-Reimer E, Matear R, Monfray P, Mouchet A, Najjar R G, Plattner G K, Rodgers K B, Sabine C L, Sarmiento J L, Schlitzer R, Slater R D, Totterdell I J, Weirig M F, Yamanaka Y, Yool A. 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature, 437(7059): 681–686.
Parker L M, Ross P M, O’Connor W A. 2010. Comparing the effect of elevated p CO2 and temperature on the fertilization and early development of two species of oysters. Marine Biology, 157(11): 2 435–2 452.
Parker L M, Ross P M, O’Connor W A. 2011. Populations of the Sydney rock oyster, Saccostrea glomerata, vary in response to ocean acidification. Marine Biology, 158(3): 689–697.
Pierrot D, Lewis E, Wallace D. 2006. MS Excel program developed for CO2 system calculations. ORNL/CDIAC-105. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge, Tennessee.
Polidoro B, Carpenter K. 2013. Dynamics of coral reef recovery. Science, 340(6128): 34–35.
Raven J, Caldeira K, Elderfield H, Hoegh-Guldberg O, Liss P, Riebesell U, Shepherd J, Turley C, Watson A. 2005. Ocean acidification due to increasing atmospheric carbon dioxide.
Sokal R R, Rohlf F J. 1995. Biometry: The Principles and Practice of Statistics in Biological Research. 3rd edn. WH Freeman and Company, New York.
Stumpp M, Wren J, Melzner F, Thorndyke M C, Dupont S T. 2011. CO2 induced seawater acidification impacts sea urchin larval development I: elevated metabolic rates decrease scope for growth and induce developmental delay. Comparative Biochemistry and Physiology A — Molecular & Integrative Physiology, 160(3): 331–340.
Talmage S C, Gobler C J. 2009. The effects of elevated carbon dioxide concentrations on the metamorphosis, size, and survival of larval hard clams (Mercenaria mercenaria), bay scallops (Argopecten irradians), and Eastern oysters (Crassostrea virginica). Limnology and Oceanography, 54(6): 2 072–2 080.
Talmage S C, Gobler C J. 2010. Effects of past, present, and future ocean carbon dioxide concentrations on the growth and survival of larval shellfish. Proceedings of the National Academy of Sciences of the United States of America, 107(40): 17 246–17 251.
Talmage S C, Gobler C J. 2011. Effects of elevated temperature and carbon dioxide on the growth and survival of larvae and juveniles of three species of northwest Atlantic bivalves. PloS One, 6(10).
Todgham A E, Hofmann G E. 2009. Transcriptomic response of sea urchin larvae Strongylocentrotus purpuratus to CO2-driven seawater acidification. Journal of Experimental Biology, 212(16): 2 579–2 594.
Van Colen C, Debusschere E, Braeckman U, Van Gansbeke D, Vincx M. 2012. The early life history of the clam Macoma balthica in a High CO2 World. PloS One, 7(9).
Wood H L, Spicer J I, Widdicombe S. 2008. Ocean acidification may increase calcification rates, but at a cost. Proceedings of the Royal Society B-Biological Sciences, 275(1644): 1 767–1 773.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Special Scientific Research Funds for Central Non-Profit Institutes, CAFS (No. 2014A01YY01), the National Basic Research Program of China (973 Program) (No. 2011CB409805), the Earmarked Fund for Modern Agro-Industry Technology Research System (No. CARS-48), and the National Key Technology R&D Program of China (No. 2011BAD45B01)
Rights and permissions
About this article
Cite this article
Li, J., Jiang, Z., Zhang, J. et al. The potential of ocean acidification on suppressing larval development in the Pacific oyster Crassostrea gigas and blood cockle Arca inflata Reeve. Chin. J. Ocean. Limnol. 32, 1307–1313 (2014). https://doi.org/10.1007/s00343-014-3317-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-014-3317-x