Abstract
The direct nanoimprinting of silver nanoparticles (Ag NPs) generated a plasmonic metallic nanostructure. The Ag NPs used in this study have been synthesized by a phase transfer method. The procedure comprised the utilisation of sodium borohydride to reduce silver nitrate in the presence of a stabilizing agent, which facilitated the transfer of the NPs from the aqueous phase to an organic phase, resulting in uniform and stable particles. The size and shape of the Ag NPs were characterized using electron microscopy, revealing spherical particles with an average diameter of 9 nm. The XRD examination revealed that the nanoparticles exhibit a high degree of crystallinity and possess a cubic geometric phase. The UV-visible absorption spectra demonstrate a significant level of homogeneity in the size. The silicon surface imprinted with Ag NPs exhibits hydrophobic behaviour towards water molecules. This approach allows for precise control of Ag NPs on the substrate, resulting in a robust and effective platform for future applications in sensor technology. Furthermore, the hydrophobic nature of the imprinted surface suggests potential applications in water-repellent coatings or self-cleaning materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanoimprinting lithography (NIL) is a potential nanopatterning method used to fabricate large-area nanoscale patterns [1]. It involves the creation of a master template with the desired nanopattern, followed by the replication of this pattern onto a substrate through the process of molding or embossing. This technology offers high resolution patterning capabilities, allowing for the creation of intricate nanostructures with precise control over dimensions and spacing, making it a popular choice for various applications in electronics and photonics [2]. In the development of nanophotonic devices, this technique has been used to fabricate optical components with subwavelength features, leading to advancements in ultrafast communication and sensing technologies [3]. Bio-imaging agents and nanosensors have also benefited from nanoimprinting lithography, as it enables the precise patterning of materials with specific functionalities for detecting and imaging biological molecules [4]. In the realm of nanosensors, this technology has been instrumental in the creation of highly sensitive and selective sensors for detecting various analytes in environmental, medical, and industrial applications [5]. Overall, the versatility and precision of nanoimprinting lithography make it a valuable tool for advancing research and innovation in various fields. The fabrication of nanomaterials into specific patterns or configurations, their properties and behaviour can be further optimized for desired applications [6].
Nanomaterials possess distinct characteristics resulting from their higher surface-to-volume ratio, heightened surface energy, and spatial confinement, which are absent in bulk materials. Nanoparticles that are well-dispersed offer an opportunity of tune the physical properties of the material through minimal variations to the nanoparticle’s composition, size, and loading. There was a considerable difference in the physical, chemical, and biological characteristics of nanoparticles of noble metals like silver compared to their bulk counterparts [7]. These unique properties have led to a wide range of applications in various fields including medicine, electronics, and environmental science [8]. In electronics, they are used for their excellent conductivity and stability, enhancing the performance of electronic devices. The versatility and effectiveness of noble metal nanoparticles make them a valuable tool in advancing technology and improving quality of life [9].
Silver nanoparticles play an important part in several industries due to their exceptional properties such as quantum confinement, high reactivity and high electrical conductivity [10]. These unique characteristics make silver nanoparticles ideal for use in various applications, including electronics [11], healthcare [12], and environmental remediation [13]. In the electronics industry, silver nanoparticles are commonly used in conductive inks and pastes for printing circuits on flexible substrates [14]. In healthcare, silver nanoparticles are utilized for their antimicrobial properties in wound dressings, medical devices, and even in antibacterial coatings for surfaces. Additionally, silver nanoparticles are being explored for their potential in water treatment and air purification due to their ability to effectively remove pollutants and pathogens.
In this study, we examined a direct nanoimprinting procedure using nanoparticles in order to form metal nanopatterns on rigid silicon substrate. To accomplish this, Ag NPs were synthesized using both aqueous and organic phase transfer techniques [15]. A PDMS mold has been used to replicate a silicon master plate, since it facilitates the evaporation of organic solvents and significantly reduces the residual layer by ensuring excellent contact between the PDMS mold and the substrate throughout the nanoimprinting process. The imprinted Ag NPs on silicon substrate show excellent hydrobobity. This high hydrophobicity makes the surface ideal for use in various industries such as electronics, medical devices, and sensors.
2 Experimental procedure
Silver nitrate (AgNO3) was purchased from Aldrich (99+% purity), Trisodium citrate from S.D Fine Chemicals Limited, Octadecylamine from Central Drug House (P) Ltd. and Toluene from HiMedia Laboratories Pvt. Ltd. Sylgard 184 base polymer purchased from sigma and curing agent from Dow Corning. All other reagents and solvents were obtained from commercial suppliers and used as received. All aqueous solutions were prepared using Milli-Q water. All Si substrates were cleaned in a 1:1 piranha solution (H2O2:H2SO4) and rinsed with Milli-Q water and finally blown dry using N2.
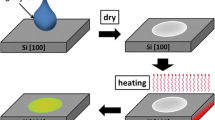
To a 1 L solution of 10− 3 M Ag(NO)3 in Milli-Q water, few drops of 10− 1 M salt of NaBH4 was added followed by vigorous stirring. This reaction is accompanied by the immediate appearance of yellowish brown colour characteristic of synthesis of silver (Ag) nanoparticles. This solution was allowed to stand for a period of 24 h before further use. Further, as-synthesized Ag nanoparticle was transformed into organic phase by adding 10− 3 M octadecylamine (ODA) into 10 ml of 10− 3 M toluene. The Ag nanoparticle was concentrated by rotatory evaporator for characterization and further use in nanoimprinting experiments. UV-Vis spectroscopy measurements of Ag nanoparticles were carried out on an Agilent dual beam spectrophotometer operated at a resolution of 1 nm in the wavelength range between 200 and 800 nm. The size and shape analysis of Ag nanoparticles were done by TEM analysis using JEOL, JEM-2010 operated at an acceleration voltage of 200 kV with a lattice resolution of 0.14 nm. For this purpose, the sample by drop coating the particles suspended in aqueous medium on carbon coated copper grids was prepared. The crystallinity of Ag NPs synthesize using phase transfer method and imprinted Ag NPs nanostructures was analyzed using X-ray powder diffraction (XRD). XRD patterns were recorded using a Philips PW 3710 powder diffractometer (Philips Analytical, Alm eao, Netherlands) with a Cu-Kα radiation source (λ = 1.5405 Å). The imprint images of Ag nanoparticles were examine using field-emission scanning electron microscopy (Hitachi S-4300). The complete imprinting process of the Ag nanoparticles on silicon wafer shown in Fig. 1. Polydimethylsiloxane (PDMS) was selected as the mold material for Ag nanoparticles nanoimprint lithography, since it has relatively low surface energy, high replication fidelity, adequate mechanical hardness, high chemical stability, and flexibility. For this, commercially available Sylagrd 184 and its curing agent were mixed in a volume ratio of 10:1 and poured onto a sapphire master stamp with conical structural patterns. After pouring the mixture, PDMS was degassed in a vacuum and cured at 80oC for 2 h. The PDMS stamp was then peeled from the sapphire master stamp. A polydimethylsiloxane (PDMS) mold with nanopatterns having conical structural (with array a diameter of 300 nm, pitch of 500 nm, and height of 200 nm) was replicated from a silicon master mold. The Ag NPs solution was then spin-coated onto the silicon substrate at a rate of 1,000 rpm for 15 s. The PDMS replica mold was immediately contacted with the spin-coated Ag NP resin and then held at a pressure of 5 bar for 30 min at 700 C to ensure vaporization of the Ag NPs solvent and complete filling of the PDMS replica mold. After detaching the PDMS mold, the nanoscale pattern was printed on a silicone substrate.
3 Results and discussion
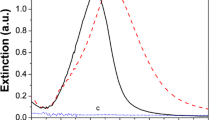
In Fig. 2 shows the UV-visible absorption spectra of the silver nanoparticles in the organic phase. The strong absorption at ca. 413 nm arises due to the excitation of surface plasmons in the Ag nanoparticles [16]. The absorption curve is quite narrow, indicates a high degree of uniformity in the size and shape of the nanoparticles in the organic phase. Further, in inset in Fig. 2 showing vial containing silver nanoparticles having yellowish brown colour which indicating the formation of Ag nanoparticles. The yellowish-brown color is a result of the surface plasmon resonance of the nanoparticles, which is influenced by their size and shape. Additionally, the formation of Ag nanoparticles can be confirmed through techniques such as transmission electron microscopy.
To determine, shape and size of Ag nanoparticles transformed into organic phase, transmission electron micrograph was done and represented in Fig. 3, which reveal that the nanoparticles have overall spherical morphology. The particle size histogram shows that size of nanoparticles within the range of 5–14 nm maximum nanoparticles are of 9 nm. It’s clearly showing from TEM image, nanoparticles are well separated from each other and have almost uniform morphology.
In order to confirm the crystalline structure of Ag nanoparticles synthesized through the phase transfer process illustrated in Fig. 4(a), we have analyses the XRD data acquired from drop-cast films of Ag nanoparticles on a glass substrate. The graph reveals strong peaks that correspond to the {111}, {200}, {220}, and {311} crystallographic planes [17]. The peaks pattern confirmed that the Ag nanoparticles produced are highly crystalline and have a face-centred cubic structure with cell parameters (a = b = c = 4.086 and α = β = γ = 900) [18].
In Fig. 5 (a-d), shown in the top view of the SEM micrograph, nanoimprint Ag nanoparticles on Si substrate with a negative h/s PDMS stamp. Furthermore, as shown, conical array patterns (with an array diameter of 300 nm, a pitch of 500 nm, and a height of 200 nm) were obtained. The SEM images reveal a high level of precision in the placement of the Ag nanoparticles on the Si substrate. The resulting structures exhibit uniformity and high aspect ratios, making them suitable for various applications in nanotechnology and photonics. In a mechanistic approach, Ag NPs show thermomechanical nanomolding (TMNM), where pressure takes the role of the driving force for the molding process and temperature is the enabler to reduce viscosity. The nanoimprinting of Ag NPs at low temperatures occurs because of the nanosize effect, which increases the surface-to-volume ratio of nanoparticles and further leads to the high surface energy that shows good sintered nanostructures at such low temperatures. Such size effects in properties can be significant for the high surface diffusion activity of Ag NPs and the high surface tension; hence, they must be considered in nanomolding [19, 20].
Figure 6 (a-b) shows SEM images of the cross-sectional view of the Ag NPs nanopattern, which consists of conical structures, on the silicon substrate. The Ag NPs are densely packed within each conical structure, creating a highly ordered nanopattern. Overall, the nanopattern appears to exhibit a high degree of structural uniformity and organization, which may have important implications for its potential applications in nanotechnology.
To explore the adhesion and wetting properties, the contact angle of liquid was measured on imprinted Ag NPs and a pristine silicon substrate. The contact angle refers to the angle formed between the tangent line of the drop’s contour at the point of contact and the flat surface of the solid [21]. From the contact angle, the physical properties of the interaction between the solid and liquid, like wettability, affinity, adhesiveness, and repellency, can be studied [22]. When we take liquid water and a solid silicon substrate, we find that the contact angle at the silicon surface is 48.60, which shows the silicon surface is hydrophilic and wettable (Fig 7a). Whereas, when we take liquid water and an Ag NPs-imprinted silicon surface (Fig 7b), the contact angle is 86.80. This shows that the Ag NPs imprinted show hydrophobicity towards the water molecules.
4 Conclusions
The study demonstrates a plasmonic metallic nanostructure by imprinting silver nanoparticles (Ag NPs) using a phase transfer technique. The nanoparticles showed uniformity in size with spherical in shape and had a well-defined crystalline structure. The silicon surface imprinted with Ag NPs with uniform distribution and high purity and exhibited a hydrophobic nature when interacting with water molecules. This method allows for precise manipulation of Ag NPs, providing a solid foundation for future sensor technology applications. Additionally, the hydrophobic nature of the imprinted surface could make it ideal for applications in water-repellent coatings.
Data availability
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
L.J. Guo, Nanoimprint lithography: methods and material requirements. Adv. Mater. 19(4), 495–513 (2007)
A. Capitaine, M. Bochet-Modaresialam, P. Poungsripong, C. Badie, V. Heresanu, O. Margeat, L. Santinacci, D. Grosso, E. Garnett, B. Sciacca, Nanoparticle imprint Lithography: from Nanoscale Metrology to Printable Metallic Grids. ACS nano. 17(10), 9361–9373 (2023)
S. Jiang, M. Saito, M. Murahashi, E. Tamiya, Pressure free nanoimprinting lithography using ladder-type HSQ material for LSPR Biosensor chip. Sens. Actuators B 242, 47–55 (2017)
R. Zandi Shafagh, J.X. Shen, S. Youhanna, W. Guo, V.M. Lauschke, van der W. Wijngaart, T. Haraldsson, Facile nanoimprinting of robust high-aspect-ratio nanostructures for human cell biomechanics. ACS Appl. Bio Mater. 3(12), 8757–8767 (2020)
N. Karimian, D. Campagnol, M. Tormen, A.M. Stortini, P. Canton, P. Ugo, Nanoimprinted arrays of glassy carbon nanoelectrodes for improved electrochemistry of enzymatic redox-mediators. J. Electroanal. Chem. 932, 117240 (2023)
S. Fruncillo, X. Su, H. Liu, L.S. Wong, Lithographic processes for the scalable fabrication of micro-and nanostructures for biochips and biosensors. ACS Sens. 6(6), 2002–2024 (2021)
S.H. Ko, I. Park, H. Pan, C.P. Grigoropoulos, A.P. Pisano, C.K. Luscombe, J.M. Fréchet, Direct nanoimprinting of metal nanoparticles for nanoscale electronics fabrication. Nano Lett. 7(7), 1869–1877 (2007)
D.V. Talapin, E.V. Shevchenko, Introduction: nanoparticle chemistry. Chem. Rev. 116(18), 10343–10345 (2016)
Z. Liu, One-step fabrication of crystalline metal nanostructures by direct nanoimprinting below melting temperatures. Nat. Commun. 8(1), 14910 (2017)
M.A. Gracia-Pinilla, E. Pérez-Tijerina, J. Antúnez-García, C. Fernández-Navarro, A. Tlahuice-Flores, S. Mejía-Rosales, J.M. Montejano-Carrizales, M. José-Yacamán, On the structure and properties of silver nanoparticles. J. Phys. Chem. C 112(35), 13492–13498 (2008)
A.H. Alshehri, M. Jakubowska, A. Młożniak, M. Horaczek, D. Rudka, C. Free, J.D. Carey, Enhanced electrical conductivity of silver nanoparticles for high frequency electronic applications. ACS Appl. Mater. Interfaces. 4(12), 7007–7010 (2012)
A. Nene, M. Galluzzi, L. Hongrong, P. Somani, S. Ramakrishna, X.F. Yu, Synthetic preparations and atomic scale engineering of silver nanoparticles for biomedical applications. Nanoscale. 13(33), 13923–13942 (2021)
V. Rocha, P. Ferreira-Santos, Z. Genisheva, E. Coelho, I.C. Neves, T. Tavares, Environmental remediation promoted by silver nanoparticles biosynthesized by eucalyptus leaves extract. J. Water Process. Eng. 56, 104431 (2023)
H. Jiang, C. Tang, Y. Wang, L. Mao, Q. Sun, L. Zhang, H. Song, F. Huang, C. Zuo, Low content and low-temperature cured silver nanoparticles/silver ion composite ink for flexible electronic applications with robust mechanical performance. Appl. Surf. Sci. 564, 150447 (2021)
A. Kumar, H. Joshi, R. Pasricha, A.B. Mandale, M. Sastry, Phase transfer of silver nanoparticles from aqueous to organic solutions using fatty amine molecules. J. Colloid Interface Sci. 264(2), 396–401 (2003)
I. Uddin, M. Rashid, T. Ali, A. Naqvi, (2018). Bio-inspired Synthesis of Nanoherbal Cream and its Antimicrobial Effect. Current Nanomedicine (Formerly: Recent Patents on Nanomedicine), 8(2), 147–155
I. Uddin, Mechanistic approach to study conjugation of nanoparticles for biomedical applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 202, 238–243 (2018)
The XRD patterns, were indexed with reference to the crystal structures from the PCPDFWIN: Ag (#04- 0784)
Z. Liu, G. Han, S. Sohn, N. Liu, J. Schroers, Nanomolding of crystalline metals: the smaller the easier. Phys. Rev. Lett. 122(3), 036101 (2019)
Z. Liu, N. Liu, J. Schroers, Nanofabrication through molding. Prog. Mater. Sci. 125, 100891 (2022)
R. Akbari, C. Antonini, Contact angle measurements: from existing methods to an open-source tool. Adv. Colloid Interface Sci. 294, 102470 (2021)
T. Huhtamäki, X. Tian, J.T. Korhonen, R.H. Ras, Surface-wetting characterization using contact-angle measurements. Nat. Protoc. 13(7), 1521–1538 (2018)
Acknowledgements
Imran Uddin thanks Center for Global Health Research, Saveetha Institute of Medical and Technical Sciences (SIMATS), Chennai, Tamil Nadu, India for the research support.
Funding
Open access funding provided by University of Pannonia.
Author information
Authors and Affiliations
Contributions
Imran Uddin: Experiment, write, edit, analysis and supervise.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no confict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Uddin, I. Metallic nanostructures arrays by direct nanoimprinting of silver nanoparticles. Appl. Phys. A 130, 479 (2024). https://doi.org/10.1007/s00339-024-07624-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-024-07624-2