Abstract
Exposure to more frequent ocean warming events is driving the loss of coral reef cover as the window of recovery between episodes of bleaching reduces. Coral propagation via in situ nurseries and subsequent outplanting have increased worldwide to support replenishing coral cover on degraded reefs. However, challenges in identifying fast-growing and bleaching-resistant target corals have limited how informative we can be regarding the resilience of outplanted corals. Here, we employed short-term thermal stress assays using the Coral Bleaching Automated Stress System (CBASS) to assess the thermal threshold of a fast-growing coral pre- and post-propagation on in situ nursery frames. We show that year-long nursery-propagated corals exhibit a statistically significant reduction in thermal thresholds (i.e., ED50s) compared to their corresponding reef-based donor colonies based on dose–response modelling of dark acclimated photosynthetic efficiency. RNA-Seq was then used to assess the underlying drivers of this thermotolerance reduction, identifying that processes involved in metabolic and oxidative stress management were disrupted in nursery versus donor heat-treated corals. Whether trade-offs during potential growth-focused phases (post-fragmentation), nursery conditions, and/or a consecutively high summer heat-load drove the lower thermal capacity remains to be determined. However, nursery corals expressed genes associated with telomere maintenance, which are typically expressed in stress-sensitive fast-growing corals under seasonal environmental stress, suggesting consecutively high summer heat-loading contributed to the observed patterns. Our results highlight that thermal tolerance is (i) variable and (ii) subject to acclimation to varying degrees across colonies. Thus, a path forward for reef practitioners to improve propagation efforts may entail the initial screening of a larger reef population from which thermally superior colonies can be selected for propagation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent decades, ocean warming events and disease outbreaks have resulted in a rapid decline of coral reef populations worldwide at a rate at which natural recovery alone is likely ineffective in maintaining healthy-functioning reefs (Hughes et al. 2018; Kleypas et al. 2021; Ortiz et al. 2018). Even under optimistic scenarios of ~ 1.5 °C global warming, only 10–30% of coral reefs are predicted to survive by the end of the century (Cheung et al. 2021; Intergovernmental Panel on Climate Change (IPCC) 2022; Smale et al. 2019). Such a dire outlook for coral reefs has motivated intensive efforts to implement active restoration interventions in a bid to enhance existing reef management towards rehabilitating damaged or degraded reef ecosystems (Anthony et al. 2017; Boström-Einarsson et al. 2020; Shaver et al. 2022; van Oppen et al. 2017).
Various active restorative approaches have been developed, spanning selective breeding, larval reseeding, artificial structure deployment, and transplantation of coral colonies (Bayraktarov et al. 2019; Boström-Einarsson et al. 2020; van Oppen et al. 2017; Voolstra et al. 2021a, b). Among these methods, propagation-based techniques have gained widespread popularity as a means to generate coral biomass faster than natural recruitment allows (Boström-Einarsson et al. 2020), including leveraging accelerated growth stimulated by micro-fragmentation (Forsman et al. 2015; Rinkevich 2000) and by high-flow nurseries (e.g. (Nuñez Lendo et al. 2023). Typically during propagation, wild donor coral colonies are fragmented and grown either on an in situ nursery structure or in a land-based facility. Once fragments reach an optimal threshold size, they are outplanted onto sites that are degraded or areas with reduced coral cover (e.g. Hein et al. 2020; Howlett et al. 2022). Propagation-based restoration has now been initiated in every reef bioregion to support diverse restoration goals (Boström-Einarsson et al. 2020), but only relatively recently on the Great Barrier Reef (GBR) where in situ nursery propagation of corals has been adopted by tourism operators across multiple ‘high value’ sites to support local stewardship of high-value reef sites (Howlett et al. 2022; Suggett et al. 2023) to accelerate reef recovery or retain site health (Roper et al. 2022).
Evidence to date suggests in situ coral nurseries can yield relatively high survivorship on an annual basis, but variability in growth rates exist between coral species (Boström-Einarsson et al. 2020; Howlett et al. 2021; Suggett et al. 2019). Most nursery propagation programmes have predominantly focused on genera of fast-growing branching corals (including those with branchlet-forming plates) which also readily fragment, for example the genus Acropora (Boström-Einarsson et al. 2020; Madin et al. 2023). In some cases, such logistically desirable traits have been associated with a greater susceptibility to thermal stress (Lirman et al. 2011; Loya et al. 2001; McClanahan 2004) where an enhanced growth rate can result in trade-offs, e.g. limited energy for processes related to stress mitigation (Carturan et al. 2022; Cornwell et al. 2021; Cunning et al. 2015; Hazraty-Kari et al. 2023; Morikawa & Palumbi 2019). Prior stress-hardening, for example via thermal preconditioning (DeMerlis et al. 2022; Majerova et al. 2021; Martell 2022; Putnam and Gates 2015) or microbiome manipulation (Damjanovic et al. 2019; Peixoto et al. 2019, 2021), of such fast-growing corals is now under development. However, some naturally occurring individuals and populations have already been observed to exhibit greater thermal bleaching resistance irrespective of their relatively fast growth rates and represent an ideal genetic resource for immediate selective propagation or breeding (i.e. assisted gene flow methods; Cunning et al. 2021; Evensen et al. 2022; Lachs et al. 2023; Morikawa & Palumbi 2019; Palacio-Castro et al. 2023; Riegl et al. 2011; Voolstra et al. 2021a, b). These corals are thought to grow and mature faster, and facilitate a more rapid adjustment of individuals to a changing environment. Tracking natural bleaching events can provide an opportunity to identify such bleaching-resistant individuals within a coral population (Barott et al. 2021); however, a more rapid means of screening for such individuals is required to drive more time-effective active restoration programmes and avoid cases when environmental conditions provide thermal refuge rather than enhanced tolerance (Gardner et al. 2019).
The Coral Bleaching Automated Stress System (CBASS) was recently developed to rapidly resolve fine-scale differences in the coral thermotolerance via short-term acute heat stress assays (Evensen et al. 2021; Voolstra et al. 2020; Voolstra et al. 2021a, b). While CBASS can be used to screen coral colonies with superior thermal tolerance as targets for restoration, it is unclear whether corals actually retain their thermal tolerance after an in situ nursery propagation phase (Cunning et al. 2021). In partnership with the Coral Nurture Program (CNP), a tourism-research restoration initiative on the GBR (Australia) we investigated whether any changes to the initial assessment of the coral thermal threshold occurred after propagation of a fast-growing key reef-building coral species, Acropora hyacinthus, on in situ nursery frames. Here, we applied the CBASS to coral fragments from donor colonies originating at Opal Reef (Northern GBR), a CNP site that has been the focus of intensive coral propagation and restoration (Howlett et al. 2022, 2023; Suggett et al. 2023). A subset of fragments was then attached to a nursery frame adjacent to the collection site, i.e. an in situ coral nursery. After one year of propagation, the CBASS was performed on the nursery-propagated corals and the thermal threshold was compared to their donor colonies assessed the previous year. Notably, during the propagation phase, there was a consecutively high summer heat-load (> 4 degrees heating weeks; DHW) and a cyclone. Measurements of maximum photosystem II quantum yield were used to show how the coral thermal threshold (by means of effective dose 50 (ED50) scores, i.e. standardised thermal thresholds; (Evensen et al. 2021; Voolstra et al. 2020) significantly reduced after the nursery propagation phase despite survivorship and apparent growth. To elucidate these alternate thermal thresholds, RNA-Seq analysis was performed to compare the differences in heat stress responses pre- and post-propagation.
Materials and methods
Coral samples and site location
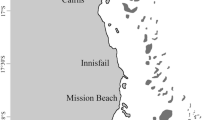
Opal reef on the great barrier reef (GBR) has been a ‘high-value’ site of focus for coral restoration as part of the Coral Nurture Program (CNP) since 2018 (Howlett et al. 2021). The CNP has planted over 100,000 corals (https://www.coralnurtureprogram.org/activity-to-date), but to-date, preferential selection of corals based on differences in thermal resilience has not been done. To assess how such an approach could be integrated into restoration practitioner activities such as that of the CNP, we undertook a year-long study focusing on a fast-growing tabular coral species used within the CNP restoration activities, Acropora hyacinthus (Nuñez Lendo et al. 2023). In February 2021, five parent (i.e. donor) colonies of A. hyacinthus were sampled for partial colonies (< 30 cm in diameter) from a back reef site with a depth of 4–6 m named Mojo (16° 12.390' S 145° 53.892' E; Fig. 1) on Opal Reef. Sample collection consisted of colonies at least 5 m apart to minimise the potential of sampling clonal genotypes (Baums et al. 2006). Partial colonies were further fragmented (5–8 cm in diameter), and a subset of these fragments (n = 5 genets per treatment tank, a total of 20) was used in thermal tolerance assessment using a rapid, acute heat stress assay (Evensen et al. 2023; Voolstra et al. 2020), while the remaining 20 fragments were attached to a CNP nursery frame (ca. 5 m depth) as described in Howlett et al. (2021) and tracked using tags and photographs (Olympus Tough TG-6). All fragmentation, assembly and deployment of coral nurseries were conducted under permit G18/40023.1 issued by the Great Barrier Reef Marine Park Authority. In February 2022, nursery fragments (i.e. new nursery partial colonies) were re-sampled and fragmented into a new subset of fragments (< 5 cm in diameter) for a repeat heat stress assay to compare the thermal tolerance threshold of the nursery-propagated (2022) and their corresponding donor (2021) colonies. Unfortunately, the tags identifying the donor colonies were lost as a result of a cyclone and re-sampling was not possible in 2022. As such, the comparison here is simply nursery material at time zero (donor 2021) versus a year-long propagation phase (nursery 2022) without an on-reef (donor) control, which is discussed later.
Map of study site on Mojo, Opal Reef, Great Barrier Reef, Australia. Red box indicates the study site (Opal Reef), and the yellow box shows photographs of an example Acropora hyacinthus donor colony and the corresponding partial colonies propagated on the in situ nursery frame located at the back reef site on Opal Reef. Note, the nursery frame was positioned within 30 m of the donor collection site at a similar depth. Blue points on map represent reference reefs from the Global Distribution of Coral Reefs dataset (UNEP-WCMC, WorldFish Centre, WRI, TNC 2021) and were plotted using reefMapMaker (Hume and Voolstra 2021)
Coral Bleaching Automated Stress System (CBASS) experimental design and setup
We used the previously established Coral Bleaching Automated Stress System (CBASS) for the heat stress assays (Voolstra et al. 2020; Voolstra et al. 2021a, b). Fragments were transferred directly into the CBASS experimental tanks (n = 5 genets per tank) without any acclimatisation time and randomly assigned to 1 of 4 temperature treatments: baseline (ambient temperature; 30 °C) which was close to the local historical maximum monthly mean temperature (MMM; 29 °C, determined by averaging climatology data between 1985–2012 from the NOAA Coral Reef Watch 5 km database for the Opal Reef site; (Liu et al. 2014)) and three heat treatments at + 3 °C, + 7 °C and + 9 °C above baseline. Note, baseline temperature was slightly higher than the historical MMM typically used for CBASS to take into consideration the much greater ‘Degrees Heating Weeks’ (DHW) experienced in the most recent decade and push the surviving corals to their thermal limit (Fig. S6a). In 2021, due to an overload of the power supply, only three temperature treatments were achieved: 30 °C, 33 °C and 37 °C. In 2022, the following four temperatures were selected to ensure comparability with 2021: 30 °C, 33 °C, 37 °C and 39 °C. The additional temperature treatment (39 °C) in 2022 was important to use for more accurate modelling of the dose–response curves as described in following sections. A further modified ‘basic’ CBASS setup (i.e. lacking automatic temperature controllers and chillers) used for this project (across both years) was a portable and low-cost design created to be feasible for use by reef practitioners globally (Evensen et al. 2023). Each treatment tank consisted of a 21-L flow-through tank connected to an independent sump, supplied with native seawater (Eheim CompactON 5000 L/hr) and fitted with a 600 L/hr powerhead (Aqua One Moray 360) to circulate water (Fig. S1). A standardised temperature profile coordinated to the local diel cycle was applied as per Voolstra et al. (2020). The 18-h profile consisted of a 3-h ramp up to the desired treatment temperature, 3-h hold, 1-h ramp down to baseline temperature and then maintained at baseline temperature for an overnight recovery phase (11 h; Fig. S2). The temperature of each sump was controlled with a custom-made titanium bar heater connected to a thermostat and temperature probe. During ramping and cool-down periods, temperature was also monitored manually with a handheld temperature probe (Multimeter 340 WTW, Weilheim, Germany), and manual water adjustments of the sump were performed to help maintain the targeted temperature. Seawater was supplied to the treatment tanks using a pump (Eheim CompactON 2500 L/hr) at a rate of approximately 0.6 L/min (turnover ~ 1.7 h). Seawater was circulated within each tank using a submersible powerhead (Aqua One 320 lph), and temperature and light were continuously measured using inter-calibrated HOBO™ Pendant Data Loggers (5-min interval, Microdaq, USA). Across all tanks irradiance was set as 350 μmol quanta m−2 s−1 (white Hydra 52 HD LED, Aqua Illumination, Ames, IA, USA) as measured with a 4π LI-190SA Quantum Sensor (LI-COR, Lincoln, NE, USA).
Pulse amplitude modulated (PAM) fluorometry (Diving-PAM underwater fluorometer; Heinz Walz GmbH, Effeltrich Germany) was used to determine the dark acclimated maximum photosynthetic efficiency of PSII (Fv/Fm) of the coral symbiotic algae, typically used as a proxy of coral bleaching (Evensen et al. 2021; Ralph et al. 2015; Voolstra et al. 2021a, b; Warner et al. 1996). All fragments were dark acclimated for at least 1 h prior to measurements post-ramp down, i.e. 7 h into the experiment. All measurements were standardised using a fibre optic adaptor at a constant distance from the coral (3 mm) and consisted of three replicates per fragment from the exposed tip to the base to capture the variation in light acclimation (Suggett et al. 2022). Optimised PAM settings were used as follows: measuring light intensity = 8, saturation pulse intensity = 11, saturating width = 0.8, damp = 2, and gain = 4. Fragments from baseline, + 3 °C and + 7 °C treatment were then sampled at 18 h (end of experiment) for gene expression (RNA-Seq) analyses by flash-freezing in liquid nitrogen. Due to the limited sample number for this project, destructive sampling for RNA-Seq was taken at the end of the experiment rather than directly after the heat stress exposure (7 h into profile). Therefore, the transcriptional response may miss some key transient heat stress gene regulation.
RNA extraction and sequencing
Total RNA was extracted using the Ambion PureLink RNA Mini kit (Thermo Fisher Scientific, Australia). Coral tissue was air-brushed from frozen fragments held in sterile zip-lock bags on dry ice using 200 µl of ice-cold 0.2 µm filtered seawater. The tissue slurry was then added to 1 mL of TRIzol and homogenised using a bead beater at 50 Hz for 1 min. The homogenate was then processed through a QIAshredder tube (Qiagen, Germany), centrifuged at 12,000 g for 5 min and added to 160 µl bromochloropropane. This mixture was then processed through the Ambion PureLink RNA Mini Kit with PureLink DNase (Ambion, Texas) following manufacturer’s protocol. RNA quality and quantity was evaluated using gel electrophoresis (18S and 28S bands) and spectrophotometry (NanoDrop), respectively. Total RNA for each sample was shipped to generate 2 × 150 bp paired end mRNA libraries. Sequencing was performed on the NovaSeq 6000 platform. RNA-Seq data are available under NCBI BioProject PRJNA1011835 (available at https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1011835).
RNA-Seq analysis
Demultiplexed reads were quality-checked using FASTQC (Andrews 2010) before and after read trimming with Trimmomatic v0.38 (Bolger et al. 2014) to remove Illumina adapters, low-quality reads and reads shorter than 50 bp. Each read was scanned using a 4-base window and cut if the quality of Phred score dropped below 15 (SLIDINGWINDOW: 4:15). Leading and trailing bases were removed if quality dropped below a score of 3 (LEADING:3 TRAILING:3). Each sample retained > 89% of paired end read counts (Table S1). Trimmed reads were then mapped using STAR 2.7.10b (Dobin et al. 2013) to the reference genomic gene set (n = 23,148 genes) of A. hyacinthus (Shinzato et al. 2021; available at https://marinegenomics.oist.jp/). Read counts were quantified using the STAR—quantMode GeneCounts. Samples with < 5 million mapped reads, a typical read depth threshold for reliable statistical power in differential expression (Conesa et al. 2016), were not considered for downstream analysis, resulting in the loss of one sample from + 3 °C and most of + 7 °C (therefore, all + 7 °C samples were not considered) as given in Table S1. Aligned reads were assembled into transcripts, and the abundance estimates of transcripts associated with genes were calculated using StringTie (Pertea et al. 2015). Count data were considered the coral host as the reference genome was sequenced from samples of aposymbiotic coral sperm (Shinzato et al. 2021); therefore, no further taxonomic filtering was applied. To visualise general patterns of gene expression, variance-stabilising transformed counts were used for principal component analysis (PCA) and plotted using the R package ggplot2 (Wickham 2016). Differential gene expression analysis based on the raw count data was performed using the R package DESeq2 (Love et al. 2014). Normalisation for sequencing depth was applied through the DESeq2 dispersion function. Wald testing for significance difference of coefficients with a negative binomial general linear model (GLM) was applied in DESeq2. P values were corrected using Benjamini–Hochberg (BH) at a default false discovery rate (FDR) cut-off of 0.05 (Table S2). Gene ontology (GO) enrichment analyses for all differentially expressed gene (DEG) lists were performed using the R package TopGO (Alexa & Rahnenführer 2019) with a recommended weighted Fisher p value cut-off of < 0.001. Venn diagrams of the common and unique DEGs between comparisons were created using the R package ggVennDiagram (Gao et al. 2021). The EggNOG v5.0 ortholog database by EMBL Data Library was used to annotate the reference genomic gene set (Huerta-Cepas et al. 2019) on the coding sequence level and infer functional annotation of DEGs. Heatmap table of GO-enriched terms across comparison groups was created using the R package ggplot2 (Wickham 2016), displaying manually curated categories. Note that the more general GO-enriched terms were not included, see Data S1 for the complete list. All scripts can be accessed on GitHub at https://github.com/RachelCAlderdice/CBASS_Nursery.
DNA extractions and Symbiodiniaceae ITS2 amplification
Frozen coral fragments were processed as per (Grima et al. 2022), by air-picking with 10 mL of phosphate-buffered saline (PBS; using 3 tablets/100 mL) to remove the tissue from the skeleton. Resulting slurry was poured into 15-mL tubes and centrifuged for 5 min at 4 °C with 3500 RPM to separate the symbiont and host fractions. Algal symbiont pellets were stored at -80 °C until DNA extraction was performed. Total DNA was extracted from a fragment of each donor colony (n = 5 genets, independent fragments from RNA samples) using the DNeasy PowerPlant Kit (Qiagen, Germany) following manufacturer’s instructions with modifications as per (Camp et al. 2019). Polymerase chain reaction (PCR) amplification of the internal transcribed spacer 2 (ITS2) region was performed using the primers ITSintfor2 and ITS2-reverse and following the PCR conditions of (Arif et al. 2014). For individual reactions, 1 μL of DNA was added to 12.5 μL Qiagen Mix, 0.4 μM each forward and reverse primers, and volume adjusted to 25 μL with DNase-free water. To visualise successful amplification, 3 μL of each PCR product was run on a 1% agarose gel for 40 min at 80 V. Samples were sequenced by the Australian Genome Research Facility using 250 bp paired end sequencing on a MiSeq instrument (Illumina).
ITS2-based Symbiodiniaceae profiling
ITS2 sequences were submitted to SymPortal for quality control and ITS2-type profile analysis (https://symportal.org) as described in Hume et al. (2019). In brief, Symbiodiniaceae genera were identified through BLAST querying a database containing representatives of each Symbiodiniaceae genus; subgeneric ITS2-type profiles were designated by SymPortal based on the presence and abundance of the ITS2 sequences across samples and within the SymPortal database. These profiles were characterised by unique combinations of defining intragenomic variants (DIVs). Refer to Data S2 for SymPortal ITS2-type profiles output file; all other output files are available at https://zenodo.org/record/8431668.
Thermal threshold assessment
Temperature tolerance thresholds were determined, for both nursery-propagated fragments (2022) and their corresponding donor colonies (2021), as the mean temperature (across all genets) at which Fv/Fm dropped to 50% of the value measured at baseline temperature, here defined as the Effective Dose 50 or ED50 (Evensen et al. 2021) using the DRC package in R (Ritz et al. 2015). Note that the average holding temperatures were rounded for simplicity in reporting, while the more accurate values were used for modelling: 30.3 °C, 33.2 °C, 36.7 °C, and 39.1 °C. Given failure to achieve the highest temperature treatment of the heat stress assay in 2021, the corresponding colony data from the highest temperature in 2022 was used to model the 2021 data. In the case of hypothetically higher Fv/Fm values for this temperature in 2021, we also modelled the Fv/Fm data using 0.15 (an arbitrary value) rather than values closer to zero to assess how conservative the approach was (Fig. S3). Using Fv/Fm values closer to zero was a more conservative modelling approach regarding the extent in which the mean ED50 differed between donor versus nursery-propagated corals. In both cases, mean ED50s were significantly different between donor and nursery corals. Statistical differences of ED50s were assessed via Welch’s unequal variances (two-tailed) t test with genotype-based ED50s as the response variable and treatment (i.e. one year in nursery) as the respective factor (Table S3). The script and data are available at https://github.com/RachelCAlderdice/CBASS_Nursery.
Environmental data
Seawater temperature was monitored at both the nursery (~ 80 m away from nursery corals, ca. 5 m depth) and the reef collection site using HOBO™ Pendant Data Loggers (30-min interval, Microdaq, USA) during only the last three months of propagation given failure of HOBO™ loggers (Fig. S4). Thus, remotely sensed sea surface temperature (SST) from the GIOVANNI online system for satellite-derived data maintained by NASA (http://disc.sci.gsfc.nasa.gov/giovanni) was extracted to provide a time-series of monthly area-averaging SST bound to 145.92E, 16.36S, 145.96E, and 16.32S (and hence capturing the entire study area around Opal Reef of ca. 30 km2) between October 2020 and April 2022 (Fig. S5) to include months prior to stress testing in both years. Degrees Heating Weeks (DHW) determined from the NOAA Coral Reef Watch daily global 5 km satellite database as a product of accumulating coral bleaching hotspots greater than 1 °C over a 12-week window was also extracted to support the consecutively high summer heat-load in 2020–2021 and 2021–2022 (Liu et al. 2014).
Results
Reduced coral bleaching thermal thresholds after one year in situ propagation with a consecutively high summer heat-load
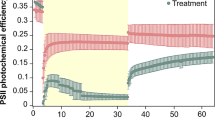
To assess a putative effect of the nursery propagation phase on their thermal threshold, we ran short-term heat stress assays using the CBASS on Acropora hyacinthus colonies collected from a Northern GBR reef site in 2021 (donors) and compared their values to corresponding nursery-propagated fragments in 2022 (i.e. the same colonies which had been propagated on an in situ coral nursery frame for one year). We subsequently determined the effective dose 50 (ED50) thermal threshold to obtain a standardised measure of coral thermal tolerance based on photosynthetic efficiency (Fv/Fm) of the coral symbiotic algae. Despite growth and no visible paling of the nursery coral tissue (Fig. S7), ED50Fv/Fm thresholds were notably lower after one year on the in situ nursery frames (donor = 37.26 °C ± 0.09 versus nursery = 36.44 °C ± 0.24 [mean ± SE]; Fig. 2c). This difference was significant despite the small sample size (n = 5 genets; t = 3.24, df = 5.08, p value = 0.02). The variance in Fv/Fm values along the slope (specifically at 37 °C) of the dose–response curve was greater for the nursery versus donor corals (Fig. 2b). The mean colony-based ED50 values did not maintain a similar ranking after one year of propagation, i.e. the donor colony with the highest ED50 was not the colony that had the highest ED50 after propagation (Fig. S8). Notably, average monthly sea surface temperatures for both years (2021 and 2022) exceeded the historical MMM for this site during the summer months. This was also reflected by the ‘Degrees Heating Weeks’ (DHW, i.e. accumulation of greater than 1 °C over a 12-week window) reported for the Northern GBR exceeding 4 DHW, a threshold which typically induces heat stress and coral bleaching, in January and February for both years (Fig. S5 and S6). ITS2-based Symbiodiniaceae profiling using the SymPortal analytical framework (Hume et al. 2019) identified uniform symbiont algal assemblage across donor corals, with samples associated with Cladocopium (C3k/C50a-C3-C3ba-C50q-C50f-C3dq). However, whether the nursery corals maintained the same profile post-propagation remains undetermined.
Loss of coral thermotolerance following year-long in situ nursery propagation. a Short-term thermal stress assay profiles (CBASS) with respective 3 h heat-hold temperatures at 30 °C (baseline/ambient), 33 °C, 37 °C, and 39 °C. Measurements of maximum photosynthetic efficiency of PSII (Fv/Fm) of the fragments were taken following 1-h dark acclimation as indicated by the grey shading, and samples for RNA-Seq analysis were taken at the end of the night-time recovery phase. b Log-logistic regression of maximum photosynthetic efficiency of PSII (Fv/Fm) in relation to temperature for donor and nursery corals with n = 5 genets for each and determined ED50 thermal tolerance thresholds as a proxy for coral bleaching susceptibility (sensu (Evensen et al. 2022; Voolstra et al. 2020). Solid lines reflect the log-logistic model with 95% confidence intervals represented by the shaded areas. c Boxplots of colony-level ED50s with asterisks representing significance of p value < 0.05. d Principal component analysis (PCA) of 23,148 genes comparing all donor (D) and nursery (N) samples across baseline temperature and + 3 °C (n = 5 per group, except for 33_D with one less). The x- and y-axes indicate the per cent of the variance explained by the first and second principal component, respectively
To further explore differences between the heat stress responses of in situ pre- and post-nursery-propagated coral, we evaluated the expression profiles of transcripts mapped to A. hyacinthus genomic gene set (n = 23,148) for baseline and + 3 °C samples (+ 7 °C samples were not analysed given the low number of mapped reads) using RNA-Seq data. Principal component analysis (PCA) revealed that samples were separated by genotype, with PC1 explaining 32% of the variation, and also whether the sample was a donor or nursery coral, with PC2 explaining 15% of the variation (Fig. 2d). In general, the heat treatment of + 3 °C did not have a strong effect on the gene expression (as typically found in CBASS studies; Savary et al. 2021). However, the heat stress response for the nursery corals was more pronounced than for the donors as baseline and + 3 °C treated samples from the same colony consistently clustered closer together for the donors compared to the nursery corals.
Corals exhibit a greater stress response to heating after year-long nursery propagation with a consecutively high summer heat-load
To elucidate how the heat stress response of donor corals differs following one-year propagation on an in situ nursery, we assessed differential gene expression between baseline temperature and + 3 °C samples for donor colonies from year 2021 and corresponding nursery fragments propagated for a year in 2022 in which a consecutively high summer heat-load was experienced (Fig. 3; Fig. S6). In agreement with the PCA clustering (Fig. 1d), a greater heat stress response was exhibited in the nursery corals compared to the donors with 918 and only 42 DEGs (FDR < 0.05), respectively (Fig. 3a, Table S2). When comparing the same temperature treatments between donor and nursery-propagated corals, the number of DEGs is more than double that, with the highest number of DEGs found expressed between baseline temperatures (2391, 47% of total; Fig. 3b), highlighting how the greatest difference is between donor and nursery corals on a whole as suggested by the PCA analysis. Within the baseline temperature comparison, nursery corals upregulated functionally annotated genes involved in processes associated with histone modifications and chromatin remodelling (Table S4).
Corals exhibit a different response to heat stress after the nursery propagation phase. a Number of differentially expressed genes that were up- (white) and down- (grey) regulated for each group comparison (30/33 = temperature treatment, D/N = donors or nursery-propagated colonies, respectively). b Number (and percentage) of differentially expressed (DE) genes at FDR < 0.05 that were common to or unique between donor and nursery corals when comparing heating temperatures (30 °C and 33 °C). c Heat map of gene ontology (GO)-enriched terms of differentially expressed genes (p value < 0.001). Comparisons (top panel) represent 30/33 = temperature treatment, D/N = donors or nursery-propagated colonies. Categories (left) manually curated for the GO terms (right). The colour gradient indicates P values. The smaller the P value is, the less likely the observed annotation of the particular GO term to a group of genes occurred by chance. White space indicates no GO enrichment.
Examination of DEGs via gene ontology (GO) enrichment analysis revealed categorical expression patterns for groups comparing the heat treatments between and within donor versus nursery corals to assess the baseline differences in the response to an in situ nursery propagation phase as well as their heat stress response (Fig. 3c). Samples at baseline temperature (30 °C) exhibited a difference in mitochondrial activity, an immune response, iron homeostasis, and the response to starvation with most of these DEGs upregulated in donor colonies. Examples of gene regulation associated with starvation involved glucose sensing via Glucokinase (GCK FClog2 = 1.09, FDR < 0.05; Table S5), gluconeogenesis via phosphoenolpyruvate carboxykinase (PCK1 FClog2 = 1.13, FDR < 0.05) and nitrogen level signalling via Gamma-aminobutyric acid A receptors (GABARAP FClog2 = 1.36, FDR < 0.05), whereas samples exposed to + 3 °C were characterised by differences in responses to reactive oxygen species (ROS; predominantly hydrogen peroxide) and DNA repair. Such genes were mostly upregulated in donor colonies and included a suite of different antioxidants (e.g. superoxide dismutase, glutathione peroxidase, and peroxiredoxin; donor vs nursery SOD1, GSHPX, PRDX5 FClog2 = 1.30, 1.66, 1.93, respectively, FDR < 0.05) and Bcl-2 adenovirus E1B (BNIP3 FClog2 = 1.96, FDR < 0.05), which is involved in mitophagy (i.e. programmed death of ROS-producing mitochondria). No GO enrichment was found in the heat stress comparison within donor corals, while for the nursery corals, DEGs associated with glycolysis, immunity, and different stress responses including protein chaperoning and telomere regulation were enriched. Examples of gene regulation involved upregulation of Alpha-crystallin B chain protein belonging to the small heat shock protein 20 family (CRYAB FClog2 = 1.71, FDR < 0.05; Table S5) and telomere maintaining dyskerin protein (DKC1 FClog2 = 1.13, FDR < 0.05) in samples exposed to + 3 °C.
Discussion
Continued degradation of coral reefs worldwide and a narrowing window of natural recovery under intensifying climate warming calls for the use of active restoration approaches, such as coral nursery propagation (Boström-Einarsson et al. 2020; Kleypas et al. 2021; Rinkevich 2008; Smale et al. 2019). However, nursery propagation programmes focusing on fast-growing corals have been subject to critique, with many concluding that efforts should also consider sexual propagation and how stress tolerant the nursery-propagated corals are in order to achieve long-lasting reefs rather than only ephemeral recovery phases (Côté & Darling 2010; Rinkevich 2015; Shaver et al. 2022; Voolstra et al. 2021a, b). The challenges in identifying corals which are fast-growing and bleaching-resistant in an efficient manner at scale has limited our ability to predict the resilience of outplants used in restoration programmes. Recently, the Coral Bleaching Automated Stress System (CBASS; Voolstra et al. 2020), a standardised rapid heat stress assay, was applied in coral nurseries to explicitly test thermal thresholds of propagated colonies in search for those which are more thermally tolerant within a population and therefore, more likely to sustain a reef (Cunning et al. 2021). The efficiency of coral propagation programmes could be improved by preselecting coral colonies with known higher thermal tolerance to grow in the nursery (Caruso et al. 2021; Lachs et al. 2023; Klepac et al. 2024); however, it is unclear whether and to what extent thermotolerance varies post-propagation phase. Therefore, here we used CBASS to test whether the initial thermal threshold assessment of donor corals of the fast-growing Acropora hyacinthus is retained after a year-long nursery propagation phase on in situ reef frames. We found the thermal threshold reduced by 0.8 °C after one year of growing through consecutive years of high summer heat-load (> 4 DHW), where the propagated corals expressed genes suggestive of a greater sensitivity to even ‘mild’ heat stress (i.e. + 3 °C above baseline temperature).
Loss of coral thermotolerance after year-long propagation on in situ nursery frame
Although mostly limited to one or two years of monitoring, evidence so far suggests that corals propagated on in situ coral nurseries have a relatively high chance of survival (> 70%; (Boström-Einarsson et al. 2020), with recorded losses often occurring due to detachment, as opposed to disease or predation (Bongiorni et al. 2011; Shafir et al. 2006). In agreement, we observed all A. hyacinthus nursery corals to survive and exhibit a ‘healthy’ state after one year based on their brown coloration and absence of paling tissue (Siebeck et al. 2006), and also increase in size (diameter; Fig. S5). In terms of heat tolerance, only a few recent studies have incorporated heat stress testing into their nursery assessments and implied that the heat stress response of propagated (Morikawa & Palumbi 2019) or transplanted (Barott et al. 2021) corals could be a relatively fixed trait. For example, donor colonies were subjected to a 3-h heat stress performance test and those designated as more heat-tolerant corals were reported to propagate seemingly more bleaching-resistant nursery corals than less heat-tolerant donors during natural bleaching events (Morikawa & Palumbi 2019). However, as similar performance tests were not conducted on the nursery corals it remained unclear whether their capacity to resist thermal bleaching was altered. Here, we performed repeated CBASS threshold testing pre- and post-propagation and found the thermal threshold of A. hyacinthus nursery-propagated corals to be significantly reduced by 0.8 °C according to ED50Fv/Fm over the propagation process of one year in which a consecutively high summer of heat-loading was endured (Fig. 2, Fig. S5 and Fig. S6).
In general, corals do not cope well with changes in their environment (Chen 2021); however, changes in conditions created by adjacent in situ coral nursery frames have been reported to be minimal or even beneficial to the propagated corals (Nuñez Lendo et al. 2023). For example, a multi-trait analysis of A. hyacinthus propagated in Opal coral nursery frame environments (Nuñez Lendo et al. 2023) identified a high growth rate in association with conditions that likely promote heterotrophic feeding which is conducive to enhancing coral energy reserves (Levy et al. 2006; Wooldridge 2014). Whether such energy would then be made available for a coral’s heat stress response remained undetermined. However, DEGs associated with a starvation response were only upregulated in the donor corals (Fig. 3c) suggesting that energy reserves may have been higher in the nursery corals or that glucose sensing was dysregulated. Many different environmental factors not typically recorded in studies, including our study, can impact growth and stress tolerance (Suggett et al. 2019). Temperature is a well-established determining factor, and even though the nursery in question was adjacent to the reef collection site (within 30 m) arguably exhibiting similar thermal conditions (Fig. S4), the overall accumulative warming exceeded 4 DHW in both years (Fig. S5 and S6). Notably, such seasonal warming did not result in observations of coral bleaching (by the CNP tourist operator, John Edmondson, on a weekly basis) on the reef or nursery within the year of propagation but non-fatal bleaching was observed on the reef after stress testing in 2022. Such consecutive years exceeding 4 DHW were only ever reported for this reef in 2016–2017 (Fig. S6). While we cannot currently verify the specific cause of the lowered thermal threshold, it is conceivable that such accumulative stress is able to reduce a coral’s capacity to manage the acute heat stress during the CBASS stress testing (Spady et al. 2022; Travesso et al. 2023). There is evidence of lowered thresholds in the form of legacy effects, e.g. following episodes of heat stress (Dörr et al. 2023; Evensen et al. 2022), highlighting the importance of considering more recent as well as historical (1985–2012) local climatic data when modelling the heat stress tolerance of nursery-propagated corals. To better understand whether the change in thermal threshold is a result of growth-focused coral functioning (post-fragmentation), nursery frame conditions, and/or recurrently high annual heat-loading—parallel stress testing of the nursery and donor colonies would be an important reference to establish in future studies.
Not only was the mean thermal threshold lower after propagation, but the individual colony thermal threshold ED50s also did not maintain their ranking across tested years indicating how the heat stress response can vary after a propagation phase and/or high heat-loading when energy resource strategies are likely altered (Fig. S8). This is in contrast with previous work using a larger sample size (n = 27) which reported a strong correlation between repeated ED50Fv/Fm values for Acropora cervicornis across different seasons within the same year (Cunning et al. 2021). Therefore, it would be worthwhile to expand the sample size of our study to assess whether it is possible to consistently rank groups of corals by their ED50Fv/Fm on an annual basis.
Corals exhibit a greater molecular stress response to heating after one year in nursery
In agreement with the overall gene expression patterns (Fig. 2d), the largest proportion of differentially expressed genes (DEGs) was observed when comparing corals exposed to baseline temperature or heat stress (+ 3 °C) between donors and the corresponding year-long nursery-propagated corals (Fig. 3a). For baseline temperature corals, differences were associated with genes involved in mitochondrial activity, iron homeostasis and the response to starvation (e.g. glucose sensing and gluconeogenesis; Fig. 3c). These genes were upregulated in the donor colonies suggesting that fundamental processes for survival under metabolic pressures were disrupted following the propagation phase. In the heat-stressed corals, genes associated with oxidative stress, such as antioxidants (e.g. superoxide dismutase, glutathione peroxidase, and peroxiredoxin), were significantly upregulated in the donor colonies which are typically reported in corals under thermal stress (Dias et al. 2019; Majerová & Drury 2022). This is not surprising given that corals typically express antioxidants under heat stress (Cziesielski et al. 2019) and more heat-tolerant corals tend to express a higher level of antioxidants (Howells et al. 2016; Jin et al. 2016; Majerová & Drury 2022). Notably, at baseline temperature nursery-propagated corals, compared to donor, upregulated DEGs involved in epigenetic processes such as histone modifications and chromatin remodelling which typically occur in corals in an attempt to respond and adapt quickly to environmental stress (Liew et al. 2018; Rinkevich 2021). These findings provide evidence for relatively heat stress susceptible nursery-propagated corals in terms of metabolic and oxidative stress management. However, given that these corals were observed to survive and grow in the nursery, resist bleaching (absence of tissue paling) under consecutive years of high summer heating (> 4 DHW) and express genes involved in epigenetic mechanisms at baseline temperature indicate a level of heat stress resilience in the propagated corals. Therefore, it is important to also consider possible non-fatal phenotypic plasticity of coral thermal tolerance, i.e. thermal acclimation, when tracking the thermal threshold of corals on an annual basis in which there is exposure to repeatedly high but not too high DHW of 4–8. Such DHW conventional thresholds also require further investigation given the changes in heat stress frequency and intensity in recent decades. Retesting of the donor colonies after the propagation phase would be critical to exclude the loss in thermal tolerance to be associated with the restoration procedures or setting.
The large difference in the transcriptional response between donor and nursery corals was accompanied by a greater number of DEGs between baseline temperature and heat stress (+ 3 °C) treated nursery corals compared to their donors. Such DEGs were functionally annotated for genes associated with commonly reported coral stress responses including glycolysis, immune signalling and protein chaperoning (Alderdice et al. 2022; Barshis et al. 2013; Cziesielski et al. 2019), highlighting how even under relatively minor heating (+ 3 °C) in the CBASS, the nursery corals already elicited a stress response. Of particular interest, we also found genes associated with the maintenance of telomeres, repeat regions of DNA that function to protect chromosomes from damage, which can be detrimentally shortened under environmental stress (Blackburn et al. 2015). Recent work has reported telomere length regulation in more stress-sensitive corals in response to seasonal stress (Rouan et al. 2023). Therefore, such telomere regulation in the nursery corals (between baseline and during their heat stress response) could support the reduction in the ED50Fv/Fm-derived thermal threshold between the propagated corals and their donors to be greatly influenced by a consecutively high summer heat-loading stress. Further investigations should more explicitly examine the relationship between the extent of telomere length regulation, ED50Fv/Fm-derived thermal thresholds, and longer-term survival of nursery corals.
Notably, coral genotype appears to be an overall consistent driver of the gene expression profiles (Fig. 2d) where baseline and heat stress corals cluster relatively close together in the PCA analysis. This agrees with many previous studies reporting genotype-specific responses to different stressors (Barott et al. 2021; Klepac et al. 2023; Maneval et al. 2021) and emphasises the need to screen a larger number of individuals to identify the individuals within coral populations, which will be fast-growing and more bleaching-tolerant to thermal stress, serving as the most ideal candidates for nursery propagation programmes.
Conclusions
In partnership with the Coral Nurture Program (CNP), we used the Coral Bleaching Automated Stress System (CBASS) to show that after a year-long in situ nursery propagation during a consecutively high summer heat-load, the fast-growing Acropora hyacinthus can exhibit a lower thermal threshold. Subsequent RNA-Seq analysis suggests that processes involved in metabolic and oxidative stress management were disrupted in the nursery versus donor corals, likely attributing to the increased thermal sensitivity. Whether trade-offs during potential growth-focused phases, nursery condition, and/or the consecutive years of high summer heat-loading drove the lower thermal capacity remains to be determined. However, the nursery corals expressed genes associated with telomere maintenance, which are typically expressed in stress-sensitive fast-growing corals under seasonal environmental stress, suggesting the support of seasonal heat-loading to be a key contributing factor in this case. Future investigations should consider how telomere length and coral thermal thresholds differ across different stages of nursery propagation and outplanting. In addition, more thorough characterisation of the nursery conditions, parallel tracking of donor colonies as well as long-term monitoring of nursery-propagated coral transplanted back to the field in future studies will help to better understand the impact of the in situ nursery propagation process on the extent of thermal tolerance. This study ultimately emphasises the need for the pre-screening of a larger coral population for the most ideal propagation candidates which are both fast-growing and more thermally tolerant to improve the success rate of rebuilding long-lasting reefs.
Data availability
Sequence data determined in this study are available under NCBI BioProject PRJNA1011835 (at https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1011835). SymPortal ITS2 analysis output files and various input files used for RNA-Seq are available at https://zenodo.org/record/8431668. Scripts and all other input files used in RNA‐Seq analysis and for DRC models are available at GitHub https://github.com/RachelCAlderdice/CBASS_Nursery.
References
Alderdice R, Perna G, Cárdenas A, Hume BCC, Wolf M, Kühl M, Pernice M, Suggett DJ, Voolstra CR (2022) Deoxygenation lowers the thermal threshold of coral bleaching. Sci Rep 12(1):18273. https://doi.org/10.1038/s41598-022-22604-3
Alexa A, Rahnenführer J, Lengauer T (2006) Improved scoring of functional groups from gene expression data by decorrelating GO graph structure, Bioinformatics 22(13): 1600–1607. https://doi.org/10.1093/bioinformatics/btl140
Andrews S (2010) Babraham Bioinformatics - FastQC a quality control tool for high throughput sequence data. https://doi.org/10.1016/0038-0717(73)90093-X
Anthony K, Bay LK, Costanza R, Firn J, Gunn J, Harrison P, Heyward A, Lundgren P, Mead D, Moore T, Mumby PJ, Van Oppen MJH, Robertson J, Runge MC, Suggett DJ, Schaffelke B, Wachenfeld D, Walshe T (2017) New interventions are needed to save coral reefs. Nat Ecol Evol 1(10):1420–1422. https://doi.org/10.1038/s41559-017-0313-5
Arif C, Daniels C, Bayer T, Banguera-Hinestroza E, Barbrook A, Howe CJ, LaJeunesse TC, Voolstra CR (2014) Assessing Symbiodinium diversity in scleractinian corals via next-generation sequencing-based genotyping of the ITS2 rDNA region. Mol Ecol 23(17):4418–4433. https://doi.org/10.1111/mec.12869
Barott KL, Huffmyer AS, Davidson JM, Lenz EA, Matsuda SB, Hancock JR, Innis T, Drury C, Putnam HM, Gates RD (2021) Coral bleaching response is unaltered following acclimatization to reefs with distinct environmental conditions. Proc Natl Acad Sci USA 118(22):e2025435118. https://doi.org/10.1073/pnas.2025435118
Barshis DJ, Ladner JT, Oliver TA, Seneca FO, Traylor-Knowles N, Palumbi SR (2013) Genomic basis for coral resilience to climate change. Proc Natl Acad Sci USA 110(4):1387–1392. https://doi.org/10.1073/pnas.1210224110
Baums IB, Miller MW, Hellberg ME (2006) Geographic variation in clonal structure in a reef-building caribbean coral, Acropora Palmata. Ecol Monogr 76(4):503–519. https://doi.org/10.1890/0012-9615(2006)076[0503:gvicsi]2.0.co;2
Bayraktarov E, Stewart-Sinclair PJ, Brisbane S, Boström-Einarsson L, Saunders MI, Lovelock CE, Possingham HP, Mumby PJ, Wilson KA (2019) Motivations, success, and cost of coral reef restoration. Restor Ecol 27(5):981–991. https://doi.org/10.1111/rec.12977
Blackburn EH, Epel ES, Lin J (2015) Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science 350(6265):1193–1198. https://doi.org/10.1126/science.aab3389
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30(15):2114–2120. https://doi.org/10.1093/bioinformatics/btu170
Bongiorni L, Giovanelli D, Rinkevich B, Pusceddu A, Chou LM, Danovaro R (2011) First step in the restoration of a highly degraded coral reef (Singapore) by in situ coral intensive farming. Aquaculture 322–323:191–200. https://doi.org/10.1016/j.aquaculture.2011.09.024
Boström-Einarsson L, Babcock RC, Bayraktarov E, Ceccarelli D, Cook N, Ferse SCA, Hancock B, Harrison P, Hein M, Shaver E, Smith A, Suggett D, Stewart-Sinclair PJ, Vardi T, McLeod IM (2020) Coral restoration - a systematic review of current methods, successes, failures and future directions. PLoS ONE 15(1):e0226631. https://doi.org/10.1371/journal.pone.0226631
Camp EF, Edmondson J, Doheny A, Rumney J, Grima AJ, Huete A, Suggett DJ (2019) Mangrove lagoons of the Great Barrier Reef support coral populations persisting under extreme environmental conditions. Mar Ecol Prog Ser 625:1–14. https://doi.org/10.3354/meps13073
Carturan BS, Parrott L, Pither J (2022) Functional richness and resilience in coral reef communities. Front Ecol Evol. 10. https://doi.org/10.3389/fevo.2022.780406
Caruso C, Hughes K, Drury C (2021) Selecting heat-tolerant corals for proactive reef restoration. Front Mar Sci 8. https://doi.org/10.3389/fmars.2021.632027
Chen D (2021) Impact of climate change on sensitive marine and extreme terrestrial ecosystems: recent progresses and future challenges: this article belongs to Ambio’s 50th Anniversary Collection Theme: climate change impact. Ambio 50(6):1141–1144. https://doi.org/10.1007/s13280-020-01446-1
Cheung MWM, Hock K, Skirving W, Mumby PJ (2021) Cumulative bleaching undermines systemic resilience of the Great Barrier Reef. Curr Biol 31(23):5385–5392.e4. https://doi.org/10.1016/j.cub.2021.09.078
Conesa A, Madrigal P, Tarazona S, Gomez-Cabrero D, Cervera A, McPherson A, Szcześniak MW, Gaffney DJ, Elo LL, Zhang X, Mortazavi A (2016) A survey of best practices for RNA-seq data analysis. Genome Biol 17:13. https://doi.org/10.1186/s13059-016-0881-8
Cornwell B, Armstrong K, Walker NS, Lippert M, Nestor V, Golbuu Y, Palumbi SR (2021) Widespread variation in heat tolerance and symbiont load are associated with growth tradeoffs in the coral Acropora hyacinthus in Palau. eLife 10. https://doi.org/10.7554/eLife.64790
Côté IM, Darling ES (2010) Rethinking ecosystem resilience in the face of climate change. PLoS Biol 8(7):e1000438. https://doi.org/10.1371/journal.pbio.1000438
Cunning R, Gillette P, Capo T, Galvez K, Baker AC (2015) Growth tradeoffs associated with thermotolerant symbionts in the coral Pocillopora damicornis are lost in warmer oceans. Coral Reefs 34(1):155–160. https://doi.org/10.1007/s00338-014-1216-4
Cunning R, Parker KE, Johnson-Sapp K, Karp RF, Wen AD, Williamson OM, Bartels E, D’Alessandro M, Gilliam DS, Hanson G, Levy J, Lirman D, Maxwell K, Million WC, Moulding AL, Moura A, Muller EM, Nedimyer K, Reckenbeil B, Baker AC (2021) Census of heat tolerance among Florida’s threatened staghorn corals finds resilient individuals throughout existing nursery populations. Proc R Soc B Biol Sci 288(1961). https://doi.org/10.1098/rspb.2021.1613
Cziesielski MJ, Schmidt-Roach S, Aranda M (2019) The past, present, and future of coral heat stress studies. Ecol Evol 9(17):10055–10066. https://doi.org/10.1002/ece3.5576
Damjanovic K, van Oppen MJH, Menéndez P, Blackall LL (2019) Experimental inoculation of coral recruits with marine bacteria indicates scope for microbiome manipulation in Acropora tenuis and Platygyra daedalea. Front Microbiol 10:1702. https://doi.org/10.3389/fmicb.2019.01702
DeMerlis A, Kirkland A, Kaufman ML, Mayfield AB, Formel N, Kolodziej G, Manzello DP, Lirman D, Traylor-Knowles N, Enochs IC (2022) Pre-exposure to a variable temperature treatment improves the response of Acropora cervicornis to acute thermal stress. Coral Reefs 41(2):435–445. https://doi.org/10.1007/s00338-022-02232-z
Dias M, Ferreira A, Gouveia R, Madeira C, Jogee N, Cabral H, Diniz M, Vinagre C (2019) Long-term exposure to increasing temperatures on scleractinian coral fragments reveals oxidative stress. Mar Environ Res 150:104758. https://doi.org/10.1016/j.marenvres.2019.104758
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29(1):15–21. https://doi.org/10.1093/bioinformatics/bts635
Dörr M, Denger J, Maier CS, Kirsch JV, Manns H, Voolstra CR (2023) Short-term heat stress assays resolve effects of host strain, repeat stress, and bacterial inoculation on Aiptasia thermal tolerance phenotypes. Coral Reefs 45:1271–1281. https://doi.org/10.1007/s00338-023-02427-y
Evensen NR, Fine M, Perna G, Voolstra CR, Barshis DJ (2021) Remarkably high and consistent tolerance of a Red Sea coral to acute and chronic thermal stress exposures. Limnol Oceanogr 66(5):1718–1729. https://doi.org/10.1002/lno.11715
Evensen NR, Voolstra CR, Fine M, Perna G, Buitrago-López C, Cárdenas A, Banc-Prandi G, Rowe K, Barshis DJ (2022) Empirically derived thermal thresholds of four coral species along the Red Sea using a portable and standardized experimental approach. Coral Reefs 41(2):239–252. https://doi.org/10.1007/s00338-022-02233-y
Evensen NR, Parker KE, Oliver TA, Palumbi SR, Logan CA, Ryan JS, Klepac CN, Perna G, Warner ME, Voolstra CR, Barshis DJ (2023) The coral bleaching automated stress system (CBASS): a low-cost, portable system for standardized empirical assessments of coral thermal limits. Limnol Oceanogr Methods/ASLO 21(7):421–434. https://doi.org/10.1002/lom3.10555
Forsman ZH, Page CA, Toonen RJ, Vaughan D (2015) Growing coral larger and faster: micro-colony-fusion as a strategy for accelerating coral cover. PeerJ 3:e1313. https://doi.org/10.7717/peerj.1313
Gao CH, Yu G, Cai P (2021) ggVennDiagram: an intuitive, easy-to-use, and highly customizable R package to generate venn diagram. Front Genet 12:1–7. https://doi.org/10.3389/fgene.2021.706907
Gardner SG, Camp EF, Smith DJ, Kahlke T, Osman EO, Gendron G, Hume BCC, Pogoreutz C, Voolstra CR, Suggett DJ (2019) Coral microbiome diversity reflects mass coral bleaching susceptibility during the 2016 El Niño heat wave. Ecol Evol 9(3):938–956. https://doi.org/10.1002/ECE3.4662
Grima AJ, Clases D, Gonzalez de Vega R, Nitschke MR, Goyen S, Suggett DJ, Camp EF (2022) Species-specific elementomes for scleractinian coral hosts and their associated Symbiodiniaceae. Coral Reefs 41(4):1115–1130. https://doi.org/10.1007/s00338-022-02259-2
Hazraty-Kari S, Morita M, Tavakoli-Kolour P, Nakamura T, Harii S (2023) Reactions of juvenile coral to three years of consecutive thermal stress. Sci Total Environ 863:161227. https://doi.org/10.1016/j.scitotenv.2022.161227
Hein MY, Beeden R, Birtles A, Gardiner NM, Le Berre T, Levy J, Marshall N, Scott CM, Terry L, Willis BL (2020) Coral restoration effectiveness: multiregional snapshots of the long-term responses of coral assemblages to restoration. Diversity 12(4):153. https://doi.org/10.3390/d12040153
Howells EJ, Abrego D, Meyer E, Kirk NL, Burt JA (2016) Host adaptation and unexpected symbiont partners enable reef-building corals to tolerate extreme temperatures. Glob Change Biol 22(8):2702–2714. https://doi.org/10.1111/gcb.13250
Howlett L, Camp EF, Edmondson J, Henderson N, Suggett DJ (2021) Coral growth, survivorship and return-on-effort within nurseries at high-value sites on the Great Barrier Reef. PLoS ONE 16(1):e0244961. https://doi.org/10.1371/journal.pone.0244961
Howlett L, Camp EF, Edmondson J, Edmondson J, Agius T, Hosp R, Coulthard P, Edmondson S, Suggett DJ (2022) Adoption of coral propagation and out-planting via the tourism industry to advance site stewardship on the northern Great Barrier Reef. Ocean Coast Manag 225:106199. https://doi.org/10.1016/j.ocecoaman.2022.106199
Howlett L, Camp EF, Edmondson J, Hosp R, Taylor B, Coulthard P, Suggett DJ (2023) Active coral propagation outcomes on coral communities at high-value Great Barrier Reef tourism sites. Biol Cons 279:109930. https://doi.org/10.1016/j.biocon.2023.109930
Huerta-Cepas J, Szklarczyk D, Heller D, Hernández-Plaza A, Forslund SK, Cook H, Mende DR, Letunic I, Rattei T, Jensen LJ, Von Mering C, Bork P (2019) EggNOG 5.0: A hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res 47(D1):D309–D314. https://doi.org/10.1093/NAR/GKY1085
Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, Baird AH, Baum JK, Berumen ML, Bridge TC, Claar DC, Eakin CM, Gilmour JP, Graham NAJ, Harrison H, Hobbs JPA, Hoey AS, Hoogenboom M, Lowe RJ, Wilson SK (2018) Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359(6371):80–83. https://doi.org/10.1126/science.aan8048
Hume BCC, Smith EG, Ziegler M, Warrington HJM, Burt JA, LaJeunesse TC, Wiedenmann J, Voolstra CR (2019) SymPortal: a novel analytical framework and platform for coral algal symbiont next-generation sequencing ITS2 profiling. Mol Ecol Resour 19(4):1063–1080. https://doi.org/10.1111/1755-0998.13004
Hume BCC, Voolstra CR (2021) reefMapMaker - convenient creation of user-defined regional maps with coral reef locations. https://doi.org/10.5281/zenodo.4434696
Intergovernmental Panel on Climate Change (IPCC) (2022) Summary for policymakers. In: Global warming of 1.5°C: IPCC special report on impacts of global warming of 1.5°C above pre-industrial levels in context of strengthening response to climate change, sustainable development, and efforts to eradicate poverty. Cambridge University Press 1–24. https://doi.org/10.1017/9781009157940.001
Jin YK, Lundgren P, Lutz A, Raina J-B, Howells EJ, Paley AS, Willis BL, van Oppen MJH (2016) Genetic markers for antioxidant capacity in a reef-building coral. Sci Adv 2(5):e1500842. https://doi.org/10.1126/sciadv.1500842
Klepac CN, Eaton KR, Petrik CG, Arick LN, Hall ER, Muller EM (2023) Symbiont composition and coral genotype determines massive coral species performance under end-of-century climate scenarios. Front Mar Sci 10. https://doi.org/10.3389/fmars.2023.1026426
Klepac CN, Petrik CG, Karabelas E, Owens J, Hall ER, Muller EM (2024) Assessing acute thermal assays as a rapid screening tool for coral restoration. Sci Rep 14(1):1898. https://doi.org/10.1038/s41598-024-51944-5
Kleypas J, Allemand D, Anthony K, Baker AC, Beck MW, Hale LZ, Hilmi N, Hoegh-Guldberg O, Hughes T, Kaufman L, Kayanne H, Magnan AK, Mcleod E, Mumby P, Palumbi S, Richmond RH, Rinkevich B, Steneck RS, Voolstra CR, Gattuso J-P (2021) Designing a blueprint for coral reef survival. Biol Conserv 257:109107. https://doi.org/10.1016/j.biocon.2021.109107
Lachs L, Humanes A, Pygas DR, Bythell JC, Mumby PJ, Ferrari R, Figueira WF, Beauchamp E, East HK, Edwards AJ, Golbuu Y, Martinez HM, Sommer B, van der Steeg E, Guest JR (2023) No apparent trade-offs associated with heat tolerance in a reef-building coral. Commun Biol 6(1):400. https://doi.org/10.1038/s42003-023-04758-6
Levy O, Dubinsky Z, Achituv Y, Erez J (2006) Diurnal polyp expansion behavior in stony corals may enhance carbon availability for symbionts photosynthesis. J Exp Mar Biol Ecol 333(1):1–11. https://doi.org/10.1016/j.jembe.2005.11.016
Liew YJ, Zoccola D, Li Y, Tambutté E, Venn A, Michell C, Cui G, Deutekom E, Kaandorp J, Voolstra C, Forêt S, Allemand D, Tambutté S, Aranda M (2018) Epigenome-associated phenotypic acclimatization to ocean acidification in a reef-building coral. Sci Adv 4(6):eaar8028. https://doi.org/10.1101/188227
Lirman D, Schopmeyer S, Manzello D, Gramer LJ, Precht WF, Muller-Karger F, Banks K, Barnes B, Bartels E, Bourque A, Byrne J, Donahue S, Duquesnel J, Fisher L, Gilliam D, Hendee J, Johnson M, Maxwell K, McDevitt E, Thanner S (2011) Severe 2010 cold-water event caused unprecedented mortality to corals of the Florida reef tract and reversed previous survivorship patterns. PloS One 6(8):e23047. https://doi.org/10.1371/journal.pone.0023047
Liu G, Heron SF, Mark Eakin C, Muller-Karger FE, Vega-Rodriguez M, Guild LS, de la Cour JL, Geiger EF, Skirving WJ, Burgess TFR, Strong AE, Harris A, Maturi E, Ignatov A, Sapper J, Li J, Lynds S (2014) Reef-scale thermal stress monitoring of coral ecosystems: New 5-km global products from NOAA coral reef watch. Remote Sens 6(11):11579–11606. https://doi.org/10.3390/rs61111579
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15(12):550. https://doi.org/10.1186/s13059-014-0550-8
Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, Van Woesik R (2001) Coral bleaching: the winners and the losers. Ecol Lett 4(2):122–131. https://doi.org/10.1046/J.1461-0248.2001.00203.X
Madin JS, McWilliam M, Quigley K, Bay LK, Bellwood D, Doropoulos C, Fernandes L, Harrison P, Hoey AS, Mumby PJ, Ortiz JC, Richards ZT, Riginos C, Schiettekatte NMD, Suggett DJ, van Oppen MJH (2023) Selecting coral species for reef restoration. J Appl Ecol 60(8)1537–1544. https://doi.org/10.1111/1365-2664.14447
Majerova E, Carey FC, Drury C, Gates RD (2021) Preconditioning improves bleaching tolerance in the reef-building coral Pocillopora acuta through modulations in the programmed cell death pathways. Mol Ecol 30(14):3560–3574. https://doi.org/10.1111/MEC.15988
Majerová E, Drury C (2022) Thermal preconditioning in a reef-building coral alleviates oxidative damage through a BI-1-mediated antioxidant response. Front Mar Sci 9. https://doi.org/10.3389/fmars.2022.971332
Maneval P, Jacoby CA, Harris HE, Frazer TK (2021) Genotype, nursery design, and depth influence the growth of Acropora cervicornis fragments. Front Mar Sci 8. https://doi.org/10.3389/fmars.2021.670474
Martell HA (2022) Thermal priming and bleaching hormesis in the staghorn coral, Acropora cervicornis (Lamarck 1816). J Exp Mar Biol Ecol 151820. https://doi.org/10.1016/j.jembe.2022.151820
McClanahan TR (2004) The relationship between bleaching and mortality of common corals. Mar Biol 144(6):1239–1245. https://doi.org/10.1007/s00227-003-1271-9
Morikawa MK, Palumbi SR (2019) Using naturally occurring climate resilient corals to construct bleaching-resistant nurseries. Proc Natl Acad Sci USA 116(21):10586–10591. https://doi.org/10.1073/PNAS.1721415116/-/DCSUPPLEMENTAL
Nuñez Lendo CI, Camp EF, Edmondson J, Hughes DJ, Kuzhiumparambil U, Clases D, De Vega RG, Suggett DJ (2023) Multiple trait approach to inform ecosystem service value of corals propagated for restoration on the great barrier reef. Res Square https://doi.org/10.21203/rs.3.rs-2030847/v1
Ortiz J-C, Wolff NH, Anthony KRN, Devlin M, Lewis S, Mumby PJ (2018) Impaired recovery of the Great Barrier Reef under cumulative stress. Sci Adv 4(7):eaar6127. https://doi.org/10.1126/sciadv.aar6127
Palacio-Castro AM, Smith TB, Brandtneris V, Snyder GA, van Hooidonk R, Maté JL, Manzello D, Glynn PW, Fong P, Baker AC (2023) Increased dominance of heat-tolerant symbionts creates resilient coral reefs in near-term ocean warming. Proc Natl Acad Sci USA 120(8):e2202388120. https://doi.org/10.1073/pnas.2202388120
Peixoto RS, Sweet M, Bourne DG (2019) Customized medicine for corals. Front Mar Sci 6. https://doi.org/10.3389/fmars.2019.00686
Peixoto RS, Sweet M, Villela HDM, Cardoso P, Thomas T, Voolstra CR, Høj L, Bourne DG (2021) Coral probiotics: premise, promise. Prospect Annu Rev Anim Biosci 9(1):265–288. https://doi.org/10.1146/annurev-animal-090120-115444
Pertea M, Pertea GM, Antonescu CM, Chang T-C, Mendell JT, Salzberg SL (2015) StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol 33(3):290–295. https://doi.org/10.1038/nbt.3122
Putnam HM, Gates RD (2015) Preconditioning in the reef-building coral Pocillopora damicornis and the potential for trans-generational acclimatization in coral larvae under future climate change conditions. J Exp Biol 218(Pt 15):2365–2372. https://doi.org/10.1242/jeb.123018
Ralph PJ, Hill R, Doblin MA, Davy SK (2015) Theory and application of pulse amplitude modulated chlorophyll fluorometry in coral health assessment. In: Diseases of Coral, Wiley, 506–523. https://doi.org/10.1002/9781118828502.ch38
Riegl BM, Purkis SJ, Al-Cibahy AS, Abdel-Moati MA, Hoegh-Guldberg O (2011) Present limits to heat-adaptability in corals and population-level responses to climate extremes. PLoS ONE 6(9):e24802. https://doi.org/10.1371/journal.pone.0024802
Rinkevich B (2000) Steps towards the evaluation of coral reef restoration by using small branch fragments. Mar Biol 136(5):807–812. https://doi.org/10.1007/s002270000293
Rinkevich B (2008) Management of coral reefs: we have gone wrong when neglecting active reef restoration. Mar Pollut Bull 56(11):1821–1824. https://doi.org/10.1016/j.marpolbul.2008.08.014
Rinkevich B (2015) Climate change and active reef restoration—ways of constructing the “Reefs of Tomorrow.” J Mar Sci Eng 3(1):111–127. https://doi.org/10.3390/jmse3010111
Rinkevich B (2021) Augmenting coral adaptation to climate change via coral gardening (the nursery phase). J Environ Manag 291:112727. https://doi.org/10.1016/j.jenvman.2021.112727
Ritz C, Baty F, Streibig JC, Gerhard D (2015) Dose-response analysis using R. Plos One 10(12):e0146021. https://doi.org/10.1371/JOURNAL.PONE.0146021
Roper CD, Camp EF, Edmondson J, Suggett DJ (2022) Combined impacts of natural recruitment and active propagation for coral population recovery on the Great Barrier Reef. Mar Ecol Prog Ser 700:95–109. https://doi.org/10.3354/meps14184
Rouan A, Pousse M, Djerbi N, Porro B, Bourdin G, Carradec Q, Hume BC, Poulain J, Lê-Hoang J, Armstrong E, Agostini S, Salazar G, Ruscheweyh H-J, Aury J-M, Paz-García DA, McMinds R, Giraud-Panis M-J, Deshuraud R, Ottaviani A, Gilson E (2023) Telomere DNA length regulation is influenced by seasonal temperature differences in short-lived but not in long-lived reef-building corals. Nat Commun 14(1):3038. https://doi.org/10.1038/s41467-023-38499-1
Savary R, Barshis DJ, Voolstra CR, Cárdenas A, Evensen NR, Banc-Prandi G, Fine M, Meibom A (2021) Fast and pervasive transcriptomic resilience and acclimation of extremely heat-tolerant coral holobionts from the northern Red Sea. Proc Natl Acad Sci USA 118(19):e2023298118. https://doi.org/10.1073/pnas.2023298118
Shafir S, Van Rijn J, Rinkevich B (2006) Steps in the construction of underwater coral nursery, an essential component in reef restoration acts. Mar Biol 149(3):679–687. https://doi.org/10.1007/s00227-005-0236-6
Shaver EC, McLeod E, Hein MY, Palumbi SR, Quigley K, Vardi T, Mumby PJ, Smith D, Montoya-Maya P, Muller EM, Banaszak AT, McLeod IM, Wachenfeld D (2022) A roadmap to integrating resilience into the practice of coral reef restoration. Glob Change Biol 28(16):4751–4764. https://doi.org/10.1111/gcb.16212
Shinzato C, Khalturin K, Inoue J, Zayasu Y, Kanda M, Kawamitsu M, Yoshioka Y, Yamashita H, Suzuki G, Satoh N (2021) Eighteen coral genomes reveal the evolutionary origin of Acropora strategies to accommodate environmental changes. Mol Biol Evol 38(1):16–30. https://doi.org/10.1093/molbev/msaa216
Siebeck UE, Marshall NJ, Klüter A, Hoegh-Guldberg O (2006) Monitoring coral bleaching using a colour reference card. Coral Reefs 25(3):453–460. https://doi.org/10.1007/s00338-006-0123-8
Smale DA, Wernberg T, Oliver ECJ, Thomsen M, Harvey BP, Straub SC, Burrows MT, Alexander LV, Benthuysen JA, Donat MG, Feng M, Hobday AJ, Holbrook NJ, Perkins-Kirkpatrick SE, Scannell HA, Sen Gupta A, Payne BL, Moore PJ (2019) Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat Clim Chang 9(4):306–312. https://doi.org/10.1038/s41558-019-0412-1
Spady BL, Skirving WJ, Liu G, De La Cour JL, McDonald CJ, Manzello DP (2022) Unprecedented early-summer heat stress and forecast of coral bleaching on the Great Barrier Reef. F1000Res 11:127. https://doi.org/10.12688/f1000research.108724.4
Suggett DJ, Camp EF, Edmondson J, Boström-Einarsson L, Ramler V, Lohr K, Patterson JT (2019) Optimizing return-on-effort for coral nursery and outplanting practices to aid restoration of the Great Barrier Reef. Restor Ecol 27(3):683–693. https://doi.org/10.1111/rec.12916
Suggett DJ, Nitschke MR, Hughes DJ, Bartels N, Camp EF, Dilernia N, Edmondson J, Fitzgerald S, Grima A, Sage A, Warner ME (2022) Toward bio-optical phenotyping of reef-forming corals using Light-Induced Fluorescence Transient-Fast Repetition Rate fluorometry. Limnol Oceanogr Methods/ASLO 20(3):172–191. https://doi.org/10.1002/LOM3.10479
Suggett DJ, Edwards M, Cotton D, Hein M, Camp EF (2023) An integrative framework for sustainable coral reef restoration. One Earth 6(6):666–681. https://doi.org/10.1016/j.oneear.2023.05.007
Travesso M, Missionário M, Cruz S, Calado R, Madeira D (2023) Combined effect of marine heatwaves and light intensity on the cellular stress response and photophysiology of the leather coral Sarcophyton cf. glaucum. Sci Total Environ 861:160460. https://doi.org/10.1016/j.scitotenv.2022.160460
UNEP-WCMC, WorldFish Centre, WRI, TNC (2021) In: Global distribution of warm-water coral reefs, compiled from multiple sources including the Millennium Coral Reef Mapping Project. Version 4.1. Includes contributions from IMaRS-USF and IRD (2005), IMaRS-USF (2005) and Spalding et al. (2001). Cambridge (UK): UN Environment World Conservation Monitoring Centre. https://doi.org/10.34892/t2wk-5t34
van Oppen MJH, Gates RD, Blackall LL, Cantin N, Chakravarti LJ, Chan WY, Cormick C, Crean A, Damjanovic K, Epstein H, Harrison PL, Jones TA, Miller M, Pears RJ, Peplow LM, Raftos DA, Schaffelke B, Stewart K, Torda G, Putnam HM (2017) Shifting paradigms in restoration of the world’s coral reefs. Glob Change Biol 23(9):3437–3448. https://doi.org/10.1111/gcb.13647
Voolstra CR, Buitrago-López C, Perna G, Cárdenas A, Hume BCC, Rädecker N, Barshis DJ (2020) Standardized short-term acute heat stress assays resolve historical differences in coral thermotolerance across microhabitat reef sites. Glob Change Biol 26(8):4328–4343. https://doi.org/10.1111/gcb.15148
Voolstra CR, Suggett DJ, Peixoto RS, Parkinson JE, Quigley KM, Silveira CB, Sweet M, Muller EM, Barshis DJ, Bourne DG, Aranda M (2021a) Extending the natural adaptive capacity of coral holobionts. Nat Rev Earth Environ 2(11):747–762. https://doi.org/10.1038/s43017-021-00214-3
Voolstra CR, Valenzuela JJ, Turkarslan S, Cárdenas A, Hume BCC, Perna G, Buitrago-López C, Rowe K, Orellana MV, Baliga NS, Paranjape S, Banc-Prandi G, Bellworthy J, Fine M, Frias-Torres S, Barshis DJ (2021b) Contrasting heat stress response patterns of coral holobionts across the Red Sea suggest distinct mechanisms of thermal tolerance. Mol Ecol 30(18):4466–4480. https://doi.org/10.1111/mec.16064
Warner ME, Fitt WK, Schmidt GW (1996) The effects of elevated temperature on the photosynthetic efficiency of zooxanthellae in hospite from four different species of reef coral: A novel approach. Plant, Cell Environ 19(3):291–299. https://doi.org/10.1111/j.1365-3040.1996.tb00251.x
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York. https://doi.org/10.1007/978-3-319-24277-4
Wooldridge SA (2014) Formalising a mechanistic linkage between heterotrophic feeding and thermal bleaching resistance. Coral Reefs 33(4):1131–1136. https://doi.org/10.1007/s00338-014-1193-7
Acknowledgements
The authors wish to express immense thanks to the Great Barrier Reef Marine Park Authority, whose support established the permit for the coral nurseries at Opal Reef (G18/40023.1), as well as staff from Wavelength Reef Cruises, who have continuously supported the operations and data collection. We express our gratitude to the past, present and emerging Gadigal people of the Eora Nation, Yidinji, Gunggandji, Yirrganydji, and Kuku Yalanji Traditional Owners of the land and sea country where our research was conducted. Work was funded by a L’Oreal-UNESCO Women in Science Awarded to E.F.C. R.A. and C.R.V. were supported by Paul G. Allen Family Foundation (‘Global Search’).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
E.F.C and C.R.V designed the experiment; S.G and C.I.N.L (2021), and S.G, T.H and E.F.C (2022) conducted the CBASS experiments with support from J.E. In addition, J.E monitored the nursery frames over the one-year study period; S.G and C.B processed RNA extractions; R.A and C.R.V analysed physiological and RNA-Seq data. R.A generated all figures. R.A, C.R.V and E.F.C wrote the manuscript with input from all authors. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alderdice, R., Voolstra, C.R., Lendo, C.I.N. et al. Loss of coral thermotolerance following year-long in situ nursery propagation with a consecutively high summer heat-load. Coral Reefs (2024). https://doi.org/10.1007/s00338-024-02505-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00338-024-02505-9