Abstract
Objectives
Angioarchitectural analysis of brain arteriovenous malformations (BAVMs) is qualitative and subject to interpretation. This study quantified the morphology of and signal changes in the nidal and perinidal areas by using MR radiomics and compared the performance of MR radiomics and angioarchitectural analysis in detecting epileptic BAVMs.
Materials and methods
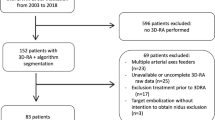
From 2010 to 2020, a total of 111 patients with supratentorial BAVMs were retrospectively included and grouped in accordance with the initial presentation of seizure. Patients’ angiograms and MR imaging results were analyzed to determine the corresponding angioarchitecture. The BAVM nidus was contoured on time-of-flight MR angiography images. The perinidal brain parenchyma was contoured on T2-weighted images, followed by radiomic analysis. Logistic regression analysis was performed to determine the independent risk factors for seizure. ROC curve analysis, decision curve analysis (DCA), and calibration curve were performed to compare the performance of angioarchitecture-based and radiomics-based models in diagnosing epileptic BAVMs.
Results
In multivariate analyses, low sphericity (OR: 2012.07, p = .04) and angiogenesis (OR: 5.30, p = .01) were independently associated with a high risk of seizure after adjustment for age, sex, temporal location, and nidal volume. The AUC for the angioarchitecture-based, MR radiomics-based, and combined models was 0.672, 0.817, and 0.794, respectively. DCA confirmed the clinical utility of the MR radiomics-based and combined models.
Conclusions
Low nidal sphericity and angiogenesis were associated with high seizure risk in patients with BAVMs. MR radiomics-derived tools may be used for noninvasive and objective measurement for evaluating the risk of seizure due to BAVM.
Clinical relevance statement
Low nidal sphericity was associated with high seizure risk in patients with brain arteriovenous malformation and MR radiomics may be used as a noninvasive and objective measurement method for evaluating seizure risk in patients with brain arteriovenous malformation.
Key Points
• Low nidal sphericity was associated with high seizure risk in patients with brain arteriovenous malformation.
• The performance of MR radiomics in detecting epileptic brain arteriovenous malformations was more satisfactory than that of angioarchitectural analysis.
• MR radiomics may be used as a noninvasive and objective measurement method for evaluating seizure risk in patients with brain arteriovenous malformation.
Graphical abstract







Similar content being viewed by others
Abbreviations
- BAVM:
-
Brain arteriovenous malformation
- DCA:
-
Decision curve analysis
- DSA:
-
Digital subtraction angiography
- ICC:
-
Intraclass correlation coefficient
- LASSO:
-
Least absolute shrinkage and selection operator
- RLN:
-
Run length nonuniformity
- SD:
-
Standard deviation
- TOF:
-
Time-of-flight
References
Al-Shahi R, Warlow C (2001) A systematic review of the frequency and prognosis of arteriovenous malformations of the brain in adults. Brain 124:1900–1926. https://doi.org/10.1093/brain/124.10.1900
Josephson CB, Bhattacharya JJ, Counsell CE et al (2012) Seizure risk with AVM treatment or conservative management: prospective, population-based study. Neurology 79:500–507. https://doi.org/10.1212/WNL.0b013e3182635696
Tong X, Wu J, Lin F et al (2016) The effect of age, sex, and lesion location on initial presentation in patients with brain arteriovenous malformations. World Neurosurg 87:598–606. https://doi.org/10.1016/j.wneu.2015.10.060
Abecassis IJ, Xu DS, Batjer HH, Bendok BR (2014) Natural history of brain arteriovenous malformations: a systematic review. Neurosurg Focus 37:E7. https://doi.org/10.3171/2014.6.Focus14250
Garcin B, Houdart E, Porcher R et al (2012) Epileptic seizures at initial presentation in patients with brain arteriovenous malformation. Neurology 78:626–631. https://doi.org/10.1212/WNL.0b013e3182494d40
Stapf C, Khaw AV, Sciacca RR et al (2003) Effect of age on clinical and morphological characteristics in patients with brain arteriovenous malformation. Stroke 34:2664–2669. https://doi.org/10.1161/01.Str.0000094824.03372.9b
Jiang P, Lv X, Wu Z et al (2011) Characteristics of brain arteriovenous malformations presenting with seizures without acute or remote hemorrhage. Neuroradiol J 24:886–888. https://doi.org/10.1177/197140091102400610
Sun Y, Tian RF, Am Li et al (2016) Unruptured epileptogenic brain arteriovenous malformations. Turk Neurosurg 26:341–346. https://doi.org/10.5137/1019-5149.Jtn.9190-13.1
Hoh BL, Chapman PH, Loeffler JS, Carter BS, Ogilvy CS (2002) Results of multimodality treatment for 141 patients with brain arteriovenous malformations and seizures: factors associated with seizure incidence and seizure outcomes. Neurosurgery 51:303–309. https://doi.org/10.1097/00006123-200208000-00004. (discussion 309–311)
Chen CJ, Shabo LM, Ding D et al (2019) Seizure presentation in patients with brain arteriovenous malformations treated with stereotactic radiosurgery: a multicenter study. World Neurosurg 126:e634–e640. https://doi.org/10.1016/j.wneu.2019.02.104
Mast H, Mohr JP, Osipov A et al (1995) ‘Steal’ is an unestablished mechanism for the clinical presentation of cerebral arteriovenous malformations. Stroke 26:1215–1220. https://doi.org/10.1161/01.str.26.7.1215
Zammar SG, Hamade YJ, Aoun RJ et al (2014) Precision medicine in brain arteriovenous malformation management: arteries steal the show but veins may hold the crystal ball. Neurosurgery 75:N13–N14. https://doi.org/10.1227/01.neu.0000457193.02158.c9
Shankar JJS, Menezes RJ, Pohlmann-Eden B, Wallace C, terBrugge K, Krings T (2013) Angioarchitecture of brain AVM determines the presentation with seizures: proposed scoring system. AJNR Am J Neuroradiol 34:1028–1034. https://doi.org/10.3174/ajnr.A3361
Galletti F, Costa C, Cupini LM et al (2014) Brain arteriovenous malformations and seizures: an Italian study. J Neurol Neurosurg Psychiatry 85:284–288. https://doi.org/10.1136/jnnp-2013-305123
Turjman F, Massoud TF, Sayre JW, Viñuela F, Guglielmi G, Duckwiler G (1995) Epilepsy associated with cerebral arteriovenous malformations: a multivariate analysis of angioarchitectural characteristics. AJNR Am J Neuroradiol 16:345–350
Benson JC, Chiu S, Flemming K, Nasr DM, Lanzino G, Brinjikji W (2020) MR characteristics of unruptured intracranial arteriovenous malformations associated with seizure as initial clinical presentation. J Neurointervent Surg 12:186–191. https://doi.org/10.1136/neurintsurg-2019-015021
Lu CF, Hsu FT, Hsieh KLC et al (2018) Machine learning-based radiomics for molecular subtyping of gliomas. Clin Cancer Res 24:4429–4436. https://doi.org/10.1158/1078-0432.Ccr-17-3445
Yang HC, Wu CC, Lee CC et al (2021) Prediction of pseudoprogression and long-term outcome of vestibular schwannoma after Gamma Knife radiosurgery based on preradiosurgical MR radiomics. Radiother Oncol 155:123–130. https://doi.org/10.1016/j.radonc.2020.10.041
Zwanenburg A, Vallières M, Abdalah MA et al (2020) The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology 295:328–338. https://doi.org/10.1148/radiol.2020191145
Lin TM, Yang HC, Lee CC et al (2019) Stasis index from hemodynamic analysis using quantitative DSA correlates with hemorrhage of supratentorial arteriovenous malformation: a cross-sectional study. J Neurosurg 132:1574–1582. https://doi.org/10.3171/2019.1.Jns183386
Atkinson RP, Awad IA, Batjer HH et al (2001) Reporting terminology for brain arteriovenous malformation clinical and radiographic features for use in clinical trials. Stroke 32:1430–1442. https://doi.org/10.1161/01.str.32.6.1430
Taeshineetanakul P, Krings T, Geibprasert S et al (2012) Angioarchitecture determines obliteration rate after radiosurgery in brain arteriovenous malformations. Neurosurgery 71:1071–1078. https://doi.org/10.1227/NEU.0b013e31826f79ec. (discussion 1079)
Soldozy S, Norat P, Yağmurlu K et al (2020) Arteriovenous malformation presenting with epilepsy: a multimodal approach to diagnosis and treatment. J Neurosurg Focus 48:E17. https://doi.org/10.3171/2020.1.Focus19899
Koo TK, Li MY (2016) A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med 15:155–163. https://doi.org/10.1016/j.jcm.2016.02.012
Kramer AA, Zimmerman JE (2007) Assessing the calibration of mortality benchmarks in critical care: The Hosmer-Lemeshow test revisited. Crit Care Med 35:2052–2056. https://doi.org/10.1097/01.CCM.0000275267.64078.B0
Sturiale CL, Rigante L, Puca A et al (2013) Angioarchitectural features of brain arteriovenous malformations associated with seizures: a single center retrospective series. Eur J Neurol 20:849–855. https://doi.org/10.1111/ene.12085
Liu S, Chen HX, Mao Q, You C, Xu JG (2015) Factors associated with seizure occurrence and long-term seizure control in pediatric brain arteriovenous malformation: a retrospective analysis of 89 patients. BMC Neurol 15:155. https://doi.org/10.1186/s12883-015-0402-5
Stapf C, Mohr JP, Sciacca R et al (2000) Incident hemorrhage risk of brain arteriovenous malformations located in the arterial borderzones. Stroke 31:2365–2368. https://doi.org/10.1161/01.str.31.10.2365
Loo JK, Hu YS, Lin TM et al (2022) Shortened cerebral circulation time correlates with seizures in brain arteriovenous malformation: a cross-sectional quantitative digital subtraction angiography study. Eur Radiol 32:5402–5412. https://doi.org/10.1007/s00330-022-08690-x
Hu YS, Lee CC, Wu HM et al (2020) Stagnant venous outflow predicts brain arteriovenous malformation obliteration after gamma knife radiosurgery without prior intervention. Neurosurgery 87:338–347. https://doi.org/10.1093/neuros/nyz507
Limkin EJ, Reuzé S, Carré A et al (2019) The complexity of tumor shape, spiculatedness, correlates with tumor radiomic shape features. Sci Rep 9:4329. https://doi.org/10.1038/s41598-019-40437-5
Friedlander RM (2007) Arteriovenous malformations of the brain. N Engl J Med 356:2704–2712. https://doi.org/10.1056/NEJMcp067192
Chou CJ, Lee CC, Chen CJ, Yang HC, Peng SJ (2021) Displacement of gray matter and incidence of seizures in patients with cerebral cavernous malformations. Biomedicines 9:1872. https://doi.org/10.3390/biomedicines9121872
Van Calster B, Wynants L, Verbeek JFM et al (2018) Reporting and Interpreting Decision Curve Analysis: A Guide for Investigators. Eur Urol 74:796–804. https://doi.org/10.1016/j.eururo.2018.08.038
Zhang Y, Yan P, Liang F et al (2019) Predictors of epilepsy presentation in unruptured brain arteriovenous malformations: a quantitative evaluation of location and radiomics features on T2-weighted imaging. World Neurosurg 125:e1008–e1015. https://doi.org/10.1016/j.wneu.2019.01.229
Zhao S, Zhao Q, Jiao Y et al (2021) Radiomics analysis for predicting epilepsy in patients with unruptured brain arteriovenous malformations. Front Neurol 12:767165. https://doi.org/10.3389/fneur.2021.767165
Jha AK, Mithun S, Jaiswar V et al (2021) Repeatability and reproducibility study of radiomic features on a phantom and human cohort. Sci Rep 11:2055. https://doi.org/10.1038/s41598-021-81526-8
Mouraviev A, Detsky J, Sahgal A et al (2020) Use of radiomics for the prediction of local control of brain metastases after stereotactic radiosurgery. Neuro Oncol 22:797–805. https://doi.org/10.1093/neuonc/noaa007
Acknowledgements
We sincerely thank Hsin-Yi Huang (Biostatistics Task Force, Taipei Veterans General Hospital) for providing assistance in performing statistical analysis.
Funding
This study has received funding by Taipei Veterans General Hospital (grant number: V111C- 073) and Taiwan’s Ministry of Science and Technology (grant number: MOST- 109–2628-B-010–014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Chung-Jung Lin.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Hsin-Yi Huang (Biostatistics Task Force, Taipei Veterans General Hospital) kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
The protocol was reviewed and approved by the Institutional Review Board (3) of Taipei Veterans General Hospital. The protocol was implemented after review and approval by the Human Research Protection Center of TPEVGH (IRB No.: 2020–06-005C).
Study subjects or cohorts overlap
None.
Methodology
• Retrospective
• Cross-sectional study
• Performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, JY., Lu, CF., Hu, YS. et al. Magnetic resonance radiomics-derived sphericity correlates with seizure in brain arteriovenous malformations. Eur Radiol 34, 588–599 (2024). https://doi.org/10.1007/s00330-023-09982-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09982-6