Abstract
Objectives
To systematically assess the early detection rate of biochemical prostate cancer recurrence using choline, fluciclovine, and PSMA.
Methods
Under the guidance of the Preferred Reporting Items for Systematic reviews and Meta-Analysis Diagnostic Test Accuracy guidelines, literature that assessed the detection rates (DRs) of choline, fluciclovine, and PSMA in prostate cancer biochemical recurrence was searched in PubMed and EMBASE databases for our systematic review from 2012 to July 15, 2021. In addition, the PSA-stratified performance of detection positivity was obtained to assess the DRs for various methods, including fluciclovine, PSMA, or choline PET/CT, with respect to biochemical recurrence based on different PSA levels.
Results
In total, 64 studies involving 11,173 patients met the inclusion criteria. Of the studies, 12, 7, and 48 focused on choline, fluciclovine, and PSMA, respectively. The pooled DRs were 24%, 37%, and 44%, respectively, for a PSA level less than 0.5 ng/mL (p < 0.001); 36%, 44%, and 60% for a PSA level of 0.5–0.99 ng/mL (p < 0.001); and 50%, 61%, and 80% for a PSA level of 1.0–1.99 ng/mL (p < 0.001). The DR with 18F-labeled PSMA was higher than that with 68Ga-labeled PSMA, and the DR was 58%, 72%, and 88% for PSA levels < 0.5 ng/mL, 0.5–0.9 ng/mL, and 1.0–1.99 ng/mL, respectively.
Conclusion
The DRs of PSMA-radiotracers were greater than those of choline-radiotracers and fluciclovine-radiotracers at the patient level. 18F-labeled PSMA achieved a higher DR than 68Ga-labeled PSMA.
Key Points
• The DRs of PSMA-radiotracers were greater than those of choline-radiotracers and fluciclovine-radiotracers at the patient level.
• 18 F-labeled PSMA achieved a higher DR than 68 Ga-labeled PSMA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is the most common malignancy of the male genitourinary system worldwide [1]. Radical prostatectomy or radiation therapy remains the most widely used treatment for localized PCa with intermediate and high risks [2]. After definitive therapy, biochemical recurrence (BCR) of PCa still recurs approximately 39–41% of the time [3, 4]. At this stage of the disease, it is still essential to define the location and extent of metastasis and initial recurrence to help urologists make further treatment plans [5]. The detection of subtle or occult recurrence and metastasis after treatment continues to pose a challenge [6]. In this setting, PET/CT is particularly superior to conventional imaging modalities such as CT (computed tomography)/MRI (magnetic resonance imaging) because of its higher detection rate (DR) for low-volume metastatic or locally initial recurrence [7, 8].
To date, of the radiolabels that have been evaluated, 11C-choline and 18F-fluciclovine were approved by the Food and Drug Administration (FDA) in 2012 and 2016 [9]. Prostate-specific membrane antigen (PSMA), a cell surface protein, is highly expressed in the majority of PCa cells and in PCa recurrence [10, 11]. 68Ga- and 18F-labeled PSMA are promising new radiotracers for detecting the BCR of PCa and radio-nuclear therapy. [68Ga]Ga-PSMA-11 was the first PSMA PET tracer that was approved by the FDA [6]. Moreover, 18F-labeled PSMA agents have also been employed in clinical practice.
Previous evidence-based studies have compared the diagnostic performance of choline, fluciclovine, and PSMA PET/CT in PCa patients with BCR, and particularly at a PSA level less than 2 ng/mL6. However, they only concentrated on long-half radionuclides as 18F-labeled PET tracers and compared their diagnostic performance in detecting patients with BCR. To our knowledge, a comprehensive comparative meta-analysis of choline, fluciclovine, and PSMA for detecting PCa patients with BCR and low PSA levels has not been performed. Furthermore, several studies have shown that the higher image resolution of 18F, as a longer half-life nuclide, is slightly better than that of 68Ga [12, 13]. However, evidence-based data based on 18F-labeled and 68Ga-labeled PSMA are still lacking. Therefore, the aims of this meta-analysis were to compare the DRs of radiotracers, including choline, fluciclovine, and PSMA PET/CT, for biochemical recurrence with PSA levels less than 2 ng/mL and to perform subgroup analyses based on 18F-labeled and 68Ga-labeled PSMA.
Materials and methods
Search strategy
This meta-analysis was performed in accordance with the PRISMA-DTA statement [14]. Two reviewers searched the library databases of PubMed and Embase that involved the DR of PET/CT using choline, fluciclovine, and PSMA agents between 2012 and July 2021. The search terms included the following: prostatic neoplasms, prostate cancer, recurrence, biochemical recurrence, 18F-choline, 11C-choline, fluoromethylcholine, [18F]fluciclovine, [18F]FACBC, [18F]PSMA-1007, [18F]DCFPyl, [18F]DCFBC, [68Ga]Ga-PSMA-11, [18F]PSMA-11, [68Ga]Ga-PSMA-I&T, [68Ga]Ga-THP-PSMA, [64Cu]Cu-PSMA-617, [18F]JK-PSMA-7, and [68Ga]Ga-HBED-CC. To expand our search, the lists of references from the retrieved articles were also checked. Two reviewers independently reviewed the references in the included studies.
Study selection
Both retrospective and prospective studies involving males with PCa with BCR who underwent PET/CT using choline, fluciclovine, and PSMA agents between 2012 and July 2021 were included. In addition, single-arm trials, comparative, single-center, multicenter, and clinical trials were also included. Studies were excluded as follows: abstracts, comments, letters, conference records, case reports, reviews, and meta-analyses, non-English articles, studies for staging purposes, and studies assessing specific types of metastatic disease, such as that of bones or lymph nodes. If the studies included patients from the same group, the largest sample was reviewed.
Data extraction and quality assessment
Two reviewers independently extracted and confirmed the data. Information was recorded from each study, including year of publication, radiotracer, imaging protocols, country of origin, study design (prospective or retrospective, multicenter or single center), patient age, sample size, treatment, PSA stratified into tiers (PSA level less than 0.5 ng/mL ng/mL, 0.5–0.99 ng/mL, and 1–1.99 ng/mL), and DR. We used the revised Quality Assessment of Studies of Diagnostic Accuracy, which was included in the QUADAS-2 tool for quality assessment [15]. Each item was judged as “yes,” “no,” or “unclear.” Any disagreements were resolved by consensus.
Statistical analysis
The pooled estimates with 95% CIs were the DRs of PSA-stratified patients with biochemical recurrence after treatment. The pooled estimates for the DRs of different radiotracers were compared using a chi-square test. Forest plots with 95% confidence intervals were used to visually assess the results. The inconsistency index (I2) was used to assess statistical heterogeneity of the included studies. The Cochrane Q with p < 0.05, and I2 > 50% indicated significant heterogeneity. A random-effects model was applied, and marked heterogeneity was observed. Statistical significance was set at a p-value less than 0.05. Egger’s test and funnel plot tests were conducted to assess the publication bias. The open-source statistical software R was used to conduct all statistical analyses (version 3.6.3; www.r-project.org/). The QUADAS quality evaluation was conducted using RevMan (version 5.3).
Results
Literature search
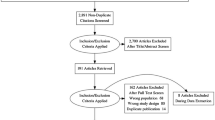
Figure 1 presents an overview of the inclusion process. Initially, 1759 total articles were identified through PubMed and Embase databases using the search terms and keywords (1396 in PubMed, and 363 in Embase). In total, 324 duplicate records were removed. After screening titles and abstracts, 1345 records were excluded; 900 because they were not relevant to the study; 90 as they were abstracts or conference records; 180 as they were letters, reviews, or meta-analyses; and 175 as they were case reports and comments.
Of the remaining studies, 90 full-texts were reviewed, and 64 studies were included in this meta-analysis. Of these, 48 studies focused on the performance of PSMA PET/CT for patients with BCR. Seven and 12 studies were included for fluciclovine and choline PET/CT, respectively. Of these, two studies evaluated both PSMA and choline [16, 17], and one study evaluated both chorine and fluciclovine [18]. The characteristics of the included articles are presented in Tables 1, 2 and 3. Figure 2 shows the proportion of different tracers in the included studies under PSA stratification.
Publication bias and heterogeneity and quality assessment
A symmetrical funnel-shaped distribution of PSA < 0.5 ng/mL (p = 0.96) and PSA levels of 0.5–1.0 ng/mL (p = 0.12) indicated that there was no significant publication bias. However, publication bias was found in the cohorts with PSA levels of 1–1.99 ng/mL (p < 0.001). The Egger test was used to quantify significant asymmetry.
The forest plots revealed strong heterogeneity for the fluciclovine cohort, as the I2 was 64%, 70%, and 72% for a PSA level < 0.5 ng/mL (p < 0.05), 0.5–0.9 ng/mL (p < 0.01), and 1.0–1.99 ng/mL (p < 0.01), respectively. The I2 was 78%, 66%, and 78% for a PSA level < 0.5 ng/mL (p < 0.01), 0.5–0.9 ng/mL (p < 0.01), and 1.0–1.99 ng/mL (p < 0.01), respectively, in the choline cohort. I2 values of the overall pooled DR were 81%, 78%, and 75% for a PSA level < 0.5 ng/mL (p < 0.01), 0.5–0.9 ng/mL (p < 0.01), and 1.0–1.99 ng/mL (p < 0.01), respectively, in the PSMA cohort. The quality assessment of the included studies is shown in Fig. 3. QUADAS-2 revealed that the majority of included studies were at a moderate risk of bias. Because all studies had consistent qualified patient selection criteria, patient selection was not considered the major potential source of bias. For the reference standard, some articles were marked as unclear or high levels because of the lack of consistent reference standards and clinical follow-up times.
Detection rates of choline, [18F]fluciclovine, and PSMA PET/CT
Pooled DRs of choline, 18F-fluciclovine, and PSMA were 24% (95% CI: 11%, 37%), 37% (95% CI: 0%, 49%), and 47% (95% CI: 42%, 52%) for PSA levels < 0.5 ng/mL (p < 0.001), respectively (Fig. 4); 36% (95% CI: 27%, 44%), 44% (95% CI: 32%, 56%), and 60% (95% CI: 54%, 65%) for a level of 0.5–0.9 ng/mL (p < 0.001) (Fig. 5); and 50% (95% CI: 39%, 61%), 61% (95% CI: 46%, 100%), and 80% (95% CI: 76%, 100%) for a level of 1–1.99 ng/mL (p < 0.001), respectively (Fig. 6).
Comparison of 18F-labeled vs 68Ga-labeled PSMA studies
Table 4 shows the results of the point estimates of the pooled DRs for the difference between 18F-labeled and 68Ga-labeled PSMA. The DR with 18F-labeled PSMA was higher than that with 68Ga-labeled PSMA. In addition, the DR showed an increasing magnitude with an increase in the PSA level, which was significant for all PSA levels.
Discussion
Whereas other meta-analyses have investigated the diagnostic roles of tracers applied in PCa with BCR [2, 6, 7, 78, 79], to our knowledge, this is the first comparative meta-analysis that focuses on all three relevant tracers for the early detection of this disease. This meta-analysis showed a higher pooled DR for PCa with BCR using PSMA compared to that with fluciclovine and choline PET/CT for three PSA levels, and we observed a significant difference. These results are in accordance with a previous meta-analysis that included only 18F-labeled choline, fluciclovine, and PSMA, which reported that PSMA was better than choline and fluciclovine [6]. A meta-analysis recently showed that there is a trend but no significant difference when the PSA level is < 0.5 ng/mL and 0.5–0.9 ng/mL. However, our study found an absolute statistical difference when comparing the DRs of PSMA and fluciclovine. A possible reason for this is the limited number of studies. In general, PET/CT imaging is more likely to be negative with low PSA values.
Radiation therapy remains the gold standard for intermediate- and high-risk, localized prostate cancer. While these are effective forms of management, approximately 30–40% of cancers still recur following treatment, manifesting as a rising prostate-specific antigen (PSA). The key issue for patients with BCR is the early and correct identification of recurrent or metastatic disease. Conventional imaging modalities consisting of CT, bone scan, and MRI have been used for patients with PCa, but their diagnostic performance in detecting minimal or occult lesions is limited. At this stage of the disease, it is important to determine the location and extent of metastases to determine the next course of management. PET is an established, non-invasive, molecular imaging modality that uses different radiolabeled tracers, a combination of a radionuclide and a biologically active molecule, targeted to specific receptors to localize disease. PET/CT has a higher detection rate of intra-prostatic tumors that might have clinical implications regarding focal therapy such as radiotherapy and surgical planning. The current study has demonstrated that PSMA-based tracer PET/CT imaging seems to be a promising tool and shows clear superiority in the detection of PCa with BCR and PSA when compared to choline- and fluciclovine-based tracers. In clinical practice, choline PET/CT is the most commonly used radioactive tracer [17]. Significantly, choline PET/CT exhibits a higher DR only at high PSA levels [80]. A previous study has shown that the DR of PCa with BCR and PSA < 1.5 ng/mL was only below 30% when using choline-based tracers PET/CT [81, 82], which is in accordance with our findings.
In our study, 18F-fluciclovine also showed a higher DR than choline (37% vs 24% for 0.2–0.5 ng/mL, 44% vs 36% for 0.5–1.0 ng/mL). Similarly, in a prospective study, the authors showed that the overall performance with [18F]FACBC, a relatively new radiotracer, was higher than that of 11C-choline, and they found that this difference in DR was particularly significant for PCa with BCR and a PSA level < 1 ng/mL18. Furthermore, current EAU guidelines recommend that choline PET/CT only be used for non-early PCa recurrence with serum PSA levels > 1 ng/mL [83]. Afshar-Oromieh et al calculated the detection rate of [68Ga]Ga-PSMA-11 to be 46% (32/69) for PSA < 0.2 ng/mL, 46% (50/108) for 0.2–0.5 ng/mL, and 73% (87/119) for 0.5–1.0 ng/mL [43]. In addition, a higher detection rate of PCa with BCR with low PSA levels was suggested by other research [59]. Considering these aspects, PSMA should be the preferred tracer choice, especially for patients with low PSA (≤ 2.0 ng/mL).
However, PSMA PET/CT is an increasingly used tracer for patients with BCR and achieves a high DR for early PCa recurrence (PSA ≤ 2.0 ng/mL) [84]. Furthermore, the strength of evidence was limited by publication bias, multiple reference standards, and a lack of consistent clinical follow-up times. In particular, our meta-analyses also performed subgroup analyses based on 18F-labeled and 68Ga-labeled PSMA. We observed statistically significant differences when comparing 18F-labeled and 68Ga-labeled PSMA (PSA < 0.5 ng/mL: 58% vs 44%, p < 0.01; PSA level 0.5–1.0 ng/mL: 72% vs 56%, p < 0.01; PSA 1.0–1.99 ng/mL: 88% vs 78%, p < 0.01). To date, to our knowledge, this is the first evidence-based study to evaluate the DRs of 18F-labeled and 68Ga-labeled PSMA. Recently, there have been multiple meta-analyses showing that the summary DR of 18F-labeled PSMA in patients with BCR was approximately 49% for PSA < 0.5 ng/mL [1, 78, 85], which is slightly better than the 44.9% detection rate of 68Ga-PSMA in a recent prospective study [73]. Compared to 18F-PSMA, 68Ga-PSMA ligands have a short half-life (68 min), and thus are inconvenient for transport [86]. Moreover, they are characterized by a lower signal-to-noise ratio for images [87], limiting its clinical application in detecting occult or metastatic lesions in the prostate bed. However, 18F-PSMA analogs seemed to be more favorable due to their longer half-life and a higher physical spatial resolution, and [18F]PSMA-1007, as a second-generation 18F-labeled PSMA tracer, demonstrated high labeling yields, better tumor uptake, and hepatobiliary excretion, making it an ideal PSMA-target tracer for diagnostic imaging in patients with BCR. Accordingly, this might explain why some authors considered [18F]DCFPyL to be a good replacement for recurrent PCa.
Our meta-analysis has several limitations. First, significant heterogeneity was observed in all cohorts. Second, because the sample size was limited, retrospective, single-institutional studies accounted for a large amount, which might be one of the reasons for the selection bias. Additionally, the different PET/CT scanners, radiotracers, patient populations, and various treatment modes increased the risk of bias and significant heterogeneity.
In conclusion, our meta-analysis revealed that PSMA-radiotracers demonstrate a potentially promising DR with low PSA levels in biochemically recurrent PCa. PSMA has a relatively higher DR than fluciclovine and choline in PCa patients with BCR and with PSA < 2.0 ng/mL. Additionally, 18F-labeled PSMA achieved a higher DR than 68Ga-labeled PSMA.
Abbreviations
- BCR:
-
Biochemical recurrence
- CT:
-
Computed tomography
- DR:
-
Detection rate
- MRI:
-
Magnetic resonance imaging
- PCa:
-
Prostate cancer
- PSA:
-
Prostate-specific antigen
- PSMA:
-
Prostate-specific membrane antigen
References
Pan KH, Wang JF, Wang CY et al (2020) Evaluation of 18F-DCFPyL PSMA PET/CT for prostate cancer: a meta-analysis. Front Oncol 10:597422. https://doi.org/10.3389/fonc.2020.597422
Sathianathen NJ, Butaney M, Konety BR (2019) The utility of PET-based imaging for prostate cancer biochemical recurrence: a systematic review and meta-analysis. World J Urol 37(7):1239–1249
Hull GW, Rabbani F, Abbas F, Wheeler TM, Kattan MW, Scardino PT (2002) Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol 167(2 Pt 1):528–534
Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ (2004) Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol 172(3):910–914
Paller CJ, Antonarakis ES (2013) Management of biochemically recurrent prostate cancer after local therapy: evolving standards of care and new directions. Clin Adv Hematol Oncol 11(1):14–23
Wang R, Shen G, Huang M, Tian R (2021) The diagnostic role of (18)F-choline, (18)F-fluciclovine and (18)F-PSMA PET/CT in the detection of prostate cancer with biochemical recurrence: a meta-analysis. Front Oncol 11:684629. https://doi.org/10.3389/fonc.2021.684629
Tan N, Oyoyo U, Bavadian N et al (2020) PSMA-targeted radiotracers versus (18)F fluciclovine for the detection of prostate cancer biochemical recurrence after definitive therapy: a systematic review and meta-analysis. Radiology 296(1):44–55
Domachevsky L, Bernstine H, Goldberg N, Nidam M, Catalano OA, Groshar D (2020) Comparison between pelvic PSMA-PET/MR and whole-body PSMA-PET/CT for the initial evaluation of prostate cancer: a proof of concept study. Eur Radiol 30(1):328–336
Evans JD, Jethwa KR, Ost P et al (2017) Prostate cancer-specific PET radiotracers: a review on the clinical utility in recurrent disease. Pract Radiat Oncol 8(1):28–39
Hoffmann MA, Buchholz HG, Wieler HJ et al (2019) The positivity rate of 68Gallium-PSMA-11 ligand PET/CT depends on the serum PSA-value in patients with biochemical recurrence of prostate cancer. Oncotarget 10(58):6124–6137
Kabasakal L, Demirci E, Nematyazar J et al (2017) The role of PSMA PET/CT imaging in restaging of prostate cancer patients with low prostate-specific antigen levels. Nucl Med Commun 38(2):149–155
Czarniecki M, Mena E, Lindenberg L et al (2018) Keeping up with the prostate-specific membrane antigens (PSMAs): an introduction to a new class of positron emission tomography (PET) imaging agents. Transl Androl Urol 7(5):831–843
Eiber M, Fendler WP, Rowe SP et al (2017) Prostate-specific membrane antigen ligands for imaging and therapy. J Nucl Med 58(Suppl 2):67s–76s
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6(7):e1000100. https://doi.org/10.1371/journal.pmed.1000100
Whiting PF, Rutjes AW, Westwood ME et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155(8):529–536
Bluemel C, Krebs M, Polat B et al (2016) 68Ga-PSMA-PET/CT in patients with biochemical prostate cancer recurrence and negative 18F-choline-PET/CT. Clin Nucl Med 41(7):515–521
Cantiello F, Crocerossa F, Russo GI et al (2018) Comparison between (64)Cu-PSMA-617 PET/CT and (18)F-choline PET/CT imaging in early diagnosis of prostate cancer biochemical recurrence. Clin Genitourin Cancer 16(5):385–391
Nanni C, Zanoni L, Pultrone C et al (2016) (18)F-FACBC (anti1-amino-3-(18)F-fluorocyclobutane-1-carboxylic acid) versus (11)C-choline PET/CT in prostate cancer relapse: results of a prospective trial. Eur J Nucl Med Mol Imaging 43(9):1601–1610
Odewole OA, Tade FI, Nieh PT et al (2016) Recurrent prostate cancer detection with anti-3-[(18)F]FACBC PET/CT: comparison with CT. Eur J Nucl Med Mol Imaging 43(10):1773–1783
Akin-Akintayo OO, Jani AB, Odewole O et al (2017) Change in salvage radiotherapy management based on guidance with FACBC (fluciclovine) PET/CT in postprostatectomy recurrent prostate cancer. Clin Nucl Med 42(1):e22–e28
Bach-Gansmo T, Nanni C, Nieh PT et al (2017) Multisite experience of the safety, detection rate and diagnostic performance of fluciclovine ((18)F) positron emission tomography/computerized tomography imaging in the staging of biochemically recurrent prostate cancer. J Urol 197(3 Pt 1):676–683. https://doi.org/10.1016/j.juro.2016.09.117
England JR, Paluch J, Ballas LK, Jadvar H (2019) 18F-fluciclovine PET/CT detection of recurrent prostate carcinoma in patients with serum PSA ≤ 1 ng/mL after definitive primary treatment. Clin Nucl Med 44(3):e128–e132
Andriole GL, Kostakoglu L, Chau A et al (2019) The impact of positron emission tomography with 18F-fluciclovine on the treatment of biochemical recurrence of prostate cancer: results from the LOCATE trial. J Urol 201(2):322–331
Michael J, Khandani AH Basak Ret al(2021). Patterns of recurrence, detection rates, and impact of 18-F fluciclovine PET/CT on the management of men with recurrent prostate cancer. Urology. https://doi.org/10.1016/j.urology.2021.01.038
Kwee SA, Coel MN, Lim J (2012) Detection of recurrent prostate cancer with 18F-fluorocholine PET/CT in relation to PSA level at the time of imaging. Ann Nucl Med 26(6):501–507
Schillaci O, Calabria F, Tavolozza M et al (2012) Influence of PSA, PSA velocity and PSA doubling time on contrast-enhanced 18F-choline PET/CT detection rate in patients with rising PSA after radical prostatectomy. Eur J Nucl Med Mol Imaging 39(4):589–596
Marzola MC, Chondrogiannis S, Ferretti A et al (2013) Role of 18F-choline PET/CT in biochemically relapsed prostate cancer after radical prostatectomy: correlation with trigger PSA, PSA velocity, PSA doubling time, and metastatic distribution. Clin Nucl Med 38(1):e26–e32
Rybalov M, Breeuwsma AJ, Leliveld AM, Pruim J, Dierckx RA, de Jong IJ (2013) Impact of total PSA, PSA doubling time and PSA velocity on detection rates of 11C-choline positron emission tomography in recurrent prostate cancer. World J Urol 31(2):319–323
Mitchell CR, Lowe VJ, Rangel LJ, Hung JC, Kwon ED, Karnes RJ (2013) Operational characteristics of 11C-choline positron emission tomography/computerized tomography for prostate cancer with biochemical recurrence after initial treatment. J Nucl Med 189(4):1308–1313
Rodado-Marina S, Coronado-Poggio M, García-Vicente AM et al (2015) Clinical utility of (18)F-fluorocholine positron-emission tomography/computed tomography (PET/CT) in biochemical relapse of prostate cancer after radical treatment: results of a multicentre study. BJU Int 115(6):874–883
Van Leeuwen P, Stricker P, Morigi JJ, Nguyen Q, Kneebone A, Emmett L (2015) Prospective comparison of the detection rate of 18Ffluoromethylcholine and 68Ga-PSMA-HBED PET/CT in men with prostate cancer with rising PSA post curative treatment, being considered for targeted therapy. BJU Int 116:29. https://doi.org/10.1111/bju.13196
Cimitan M, Evangelista L, Hodolič M et al (2015) Gleason score at diagnosis predicts the rate of detection of 18F-choline PET/CT performed when biochemical evidence indicates recurrence of prostate cancer: experience with 1,000 patients. J Nucl Med 56(2):209–215
Michaud L, Touijer KA, Mauguen A et al (2020) (11)C-choline PET/CT in recurrent prostate cancer: retrospective analysis in a large U.S. patient series. J Nucl Med 61(6):827–833
Eiber M, Maurer T, Souvatzoglou M et al (2015) Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med 56(5):668–674
Verburg FA, Pfister D, Heidenreich A et al (2016) Extent of disease in recurrent prostate cancer determined by [(68)Ga]PSMA-HBED-CC PET/CT in relation to PSA levels, PSA doubling time and Gleason score. Eur J Nucl Med Mol Imaging 43(3):397–403
van Leeuwen PJ, Stricker P, Hruby G et al (2016) (68) Ga-PSMA has a high detection rate of prostate cancer recurrence outside the prostatic fossa in patients being considered for salvage radiation treatment. BJU Int 117(5):732–739
Sachpekidis C, Eder M, Kopka K et al (2016) (68)Ga-PSMA-11 dynamic PET/CT imaging in biochemical relapse of prostate cancer. Eur J Nucl Med Mol Imaging 43(7):1288–1299
Berliner C, Tienken M, Frenzel T et al (2017) Detection rate of PET/CT in patients with biochemical relapse of prostate cancer using [(68)Ga]PSMA I&T and comparison with published data of [(68)Ga]PSMA HBED-CC. Eur J Nucl Med Mol Imaging 44(4):670–677
Meredith G, Wong D, Yaxley J et al (2016) The use of (68 ) Ga-PSMA PET CT in men with biochemical recurrence after definitive treatment of acinar prostate cancer. BJU Int 118(Suppl 3):49–55. https://doi.org/10.1111/bju.13616
Mena E, Lindenberg ML, Shih JH et al (2018) Clinical impact of PSMA-based (18)F-DCFBC PET/CT imaging in patients with biochemically recurrent prostate cancer after primary local therapy. Eur J Nucl Med Mol Imaging 45(1):4–11
Kranzbühler B, Nagel H, Becker AS et al (2018) Clinical performance of (68)Ga-PSMA-11 PET/MRI for the detection of recurrent prostate cancer following radical prostatectomy. Eur J Nucl Med Mol Imaging 45(1):20–30
Schmuck S, Nordlohne S, Klot CV et al (2017) Comparison of standard and delayed imaging to improve the detection rate of [68Ga]PSMA I&T PET/CT in patients with biochemical recurrence or prostate-specific antigen persistence after primary therapy for prostate cancer. Eur J Nucl Med Mol Imaging 44(6):960–968
Afshar-Oromieh A, Holland-Letz T, Giesel FL et al (2017) Diagnostic performance of (68)Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging 44(8):1258–1268
Hope TA, Aggarwal R, Chee B, Tao D, Carroll PR (2017) Impact of Ga-68 PSMA-11 PET on management in patients with biochemically recurrent prostate cancer. J Nucl Med 58(12):1956–1961
Dietlein F, Stockter S, Dietlein M et al (2017) PSA-stratified performance of 18F- and 68Ga-PSMA PET in patients with biochemical recurrence of prostate cancer. J Nucl Med 58(6):947–952
Gupta SK, Watson T, Denham J et al (2017) PSMA PET-CT for prostate cancer: distribution of disease and implications for radiotherapy planning. Int J Radiat Oncol Biol Phys 99(3):701–709
Sanli Y, Kuyumcu S, Sanli O et al (2017) Relationships between serum PSA levels, Gleason scores and results of 68Ga-PSMAPET/CT in patients with recurrent prostate cancer. Ann Nucl Med 31(9):709–717
Emmett L, van Leeuwen PJ, Nandurkar R et al (2017) Treatment outcomes from (68)Ga-PSMA PET/CT-informed salvage radiation treatment in men with rising PSA after radical prostatectomy: prognostic value of a negative PSMA PET. J Nucl Med 58(12):1972–1976
Habl G, Sauter K, Schiller K et al (2017) 68 Ga-PSMA-PET for radiation treatment planning in prostate cancer recurrences after surgery: individualized medicine or new standard in salvage treatment. Prostate 77(8):920–927
Medina-Ornelas Sevastián S, García-Pérez Francisco O, Hernández-Pedro Norma Y, Arellano-Zarate Angélica E, Abúndiz-López Blanca L (2018) Correlation between molecular tumor volume evaluated with (68)Ga-PSMA PET/CT and prostatic specific antigen levels. Rev Esp Med Nucl Imagen Mol 37(4):223–228
Zacho HD, Nielsen JB, Dettmann K et al (2018) 68Ga-PSMA PET/CT in patients with biochemical recurrence of prostate cancer: a prospective, 2-center study. Clin Nucl Med 43(8):579–585
Caroli P, Sandler I, Matteucci F et al (2018) (68)Ga-PSMA PET/CT in patients with recurrent prostate cancer after radical treatment: prospective results in 314 patients. Eur J Nucl Med Mol Imaging 45(12):2035–2044
Calais J, Czernin J, Cao M et al (2018) (68)Ga-PSMA-11 PET/CT mapping of prostate cancer biochemical recurrence after radical prostatectomy in 270 patients with a PSA level of less than 1.0 ng/mL: impact on salvage radiotherapy planning. J Nucl Med 59(2):230–237
Lengana T, van de Wiele C, Lawal I et al (2018) 68Ga-PSMA-HBED-CC PET/CT imaging in Black versus White South African patients with prostate carcinoma presenting with a low volume, androgen-dependent biochemical recurrence: a prospective study. Nucl Med Commun 39(2):179–185
Müller J, Ferraro DA, Muehlematter UJ et al (2019) Clinical impact of (68)Ga-PSMA-11 PET on patient management and outcome, including all patients referred for an increase in PSA level during the first year after its clinical introduction. Eur J Nucl Med Mol Imaging 46(4):889–900
De Bari B, Mazzola R, Aiello D et al (2018) Could 68-Ga PSMA PET/CT become a new tool in the decision-making strategy of prostate cancer patients with biochemical recurrence of PSA after radical prostatectomy? A preliminary, monocentric series. Radiol Med 123(9):719–725
Giesel FL, Knorr K, Spohn F et al (2019) Detection efficacy of (18)F-PSMA-1007 PET/CT in 251 patients with biochemical recurrence of prostate cancer after radical prostatectomy. J Nucl Med 60(3):362–368
Kambiz R, Ali AO, Robert S et al (2018) Diagnostic performance of 18F-PSMA-1007 PET/CT in patients with biochemical recurrent prostate cancer. Eur J Nucl Med Mol Imaging 45(12):2055–2061
Rauscher I, Düwel C, Haller B et al (2018) Efficacy, predictive factors, and prediction nomograms for (68)Ga-labeled prostate-specific membrane antigen-ligand positron-emission tomography/computed tomography in early biochemical recurrent prostate cancer after radical prostatectomy. Eur Urol 73(5):656–661
Mattiolli AB, Santos A, Vicente A et al (2018) Impact of 68GA-PSMA PET / CT on treatment of patients with recurrent / metastatic high risk prostate cancer - a multicenter study. Int Braz J Urol 44(5):892–899
Prado Júnior LM, Marino FM, Barra R, do Prado LFM, Barra Sobrinho A (2018) One-year experience with (68)Ga-PSMA PET/CT: applications and results in biochemical recurrence of prostate cancer. Radiol Bras 51(3):151–155
Derlin T, Schmuck S, Juhl C et al (2018) PSA-stratified detection rates for [ 68 Ga]THP-PSMA, a novel probe for rapid kit-based 68 Ga-labeling and PET imaging, in patients with biochemical recurrence after primary therapy for prostate cancer. Eur J Nucl Med Mol Imaging 45(6):913–922
Ringheim A, Campos Neto GC, Martins KM, Vitor T, da Cunha ML, Baroni RH (2018) Reproducibility of standardized uptake values of same-day randomized (68)Ga-PSMA-11 PET/CT and PET/MR scans in recurrent prostate cancer patients. Ann Nucl Med 32(8):523–531
Gutiérrez-Cardo AL, Pérez Duarte A, García-Argüello SF, López Lorenzo B, Lillo García ME, Valdivielso P (2019) Assessment of (68)Ga-PSMA-11 PET positivity predictive factors in prostate cancer. Rev Esp Med Nucl Imagen Mol (Engl Ed) 38(1):22–28
Eiber M, Krnke M, Wurzer A et al (2020) 18F-rhPSMA-7PET for the detection of biochemical recurrence of prostate cancer after radical prostatectomy. J Nucl Med 61(5):696–701
Neslihan AE, Murat T, Fadıl A et al (2019) 68 Ga-labelled PSMA ligand HBED-CC PET/CT imaging in patients with recurrent prostate cancer. World J Urol 37:813–821
Hamed MAG, Basha MAA, Ahmed H, Obaya AA, Afifi AHM, Abdelbary EH (2019) (68)Ga-PSMA PET/CT in patients with rising prostatic-specific antigen after definitive treatment of prostate cancer: detection efficacy and diagnostic accuracy. Acad Radiol 26(4):450–460
Farolfi A, Ceci F, Castellucci P et al (2019) (68)Ga-PSMA-11 PET/CT in prostate cancer patients with biochemical recurrence after radical prostatectomy and PSA <0.5 ng/ml. Efficacy and impact on treatment strategy. Eur J Nucl Med Mol Imaging 46(1):11–19
Ceci F, Castellucci P, Graziani T et al (2019) (68)Ga-PSMA-11 PET/CT in recurrent prostate cancer: efficacy in different clinical stages of PSA failure after radical therapy. Eur J Nucl Med Mol Imaging 46(1):31–39
Wondergem M, Jansen BHE, van der Zant FM et al (2019) Early lesion detection with (18)F-DCFPyL PET/CT in 248 patients with biochemically recurrent prostate cancer. Eur J Nucl Med Mol Imaging 46(9):1911–1918
Asokendaran ME, Meyrick DP, Skelly LA, Lenzo NP, Henderson A (2019) Gallium-68 prostate-specific membrane antigen positron emission tomography/computed tomography compared with diagnostic computed tomography in relapsed prostate cancer. World J Nucl Med 18(3):232–237
Bashir U, Tree A, Mayer E et al (2019) Impact of Ga-68-PSMA PET/CT on management in prostate cancer patients with very early biochemical recurrence after radical prostatectomy. Eur J Nucl Med Mol Imaging 46(4):901–907
Beheshti M, Manafi-Farid R, Geinitz H et al (2020) Multiphasic (68)Ga-PSMA PET/CT in the detection of early recurrence in prostate cancer patients with a PSA level of less than 1 ng/mL: a prospective study of 135 patients. J Nucl Med 61(10):1484–1490
Song H, Harrison C, Duan H et al (2020) Prospective evaluation of (18)F-DCFPyL PET/CT in biochemically recurrent prostate cancer in an academic center: a focus on disease localization and changes in management. J Nucl Med 61(4):546–551
Kulkarni M, Hughes S, Mallia A et al (2020) The management impact of (68)gallium-tris(hydroxypyridinone) prostate-specific membrane antigen ((68)Ga-THP-PSMA) PET-CT imaging for high-risk and biochemically recurrent prostate cancer. Eur J Nucl Med Mol Imaging 47(3):674–686
Seniaray N, Verma R, Khanna S, Belho E, Pruthi A, Mahajan H (2020) Localization and restaging of carcinoma prostate by (68)Gallium prostate-specific membrane antigen positron emission tomography computed tomography in patients with biochemical recurrence. Indian J Urol 36(3):191–199. https://doi.org/10.4103/iju.IJU_275_19
Perry E, Talwar A, Taubman K et al (2021) [(18)F]DCFPyL PET/CT in detection and localization of recurrent prostate cancer following prostatectomy including low PSA < 0.5 ng/mL. Eur J Nucl Med Mol Imaging 48(6):2038–2046
Sun J, Lin Y, Wei X, Ouyang J, Huang Y, Ling Z (2021) Performance of 18F-DCFPyL PET/CT imaging in early detection of biochemically recurrent prostate cancer: a systematic review and meta-analysis. Front Oncol 11:649171. https://doi.org/10.3389/fonc.2021.649171
Tan N, Bavadian N, Calais J et al (2019) Imaging of prostate specific membrane antigen targeted radiotracers for the detection of prostate cancer biochemical recurrence after definitive therapy: a systematic review and meta-analysis. J Urol 202(2):231–240
Umbehr MH, Müntener M, Hany T, Sulser T, Bachmann LM (2013) The role of 11C-choline and 18F-fluorocholine positron emission tomography (PET) and PET/CT in prostate cancer: a systematic review and meta-analysis. Eur Urol 64(1):106–117
Castellucci P, Picchio M (2013) 11C-choline PET/CT and PSA kinetics. Eur J Nucl MedMol Imaging 40(Suppl 1):S36–S40. https://doi.org/10.1007/s00259-013-2377-z
Castellucci P, Fuccio C, Rubello D et al (2011) Is there a role for 11C-choline PET/CT in the early detection of metastatic disease in surgically treated prostate cancer patients with a mild PSA increase <1.5 ng/ml? Eur J Nucl Med Mol Imaging 38(1):55–63
Evangelista L, Guttilla A, Zattoni F, Muzzio PC, ZattoniF (2013) Utility of choline positron emission tomography/computed tomography for lymph node involvement identification in intermediate- to high-risk prostate cancer: a systematic literature review and meta-analysis. Eur Urol 63(6):1040–1048
Alberts I, Hünermund JN, Sachpekidis C et al (2021) The influence of digital PET/CT on diagnostic certainty and interrater reliability in [(68)Ga]Ga-PSMA-11 PET/CT for recurrent prostate cancer. Eur Radiol 31(10):8030–8039
Treglia G, Annunziata S, Pizzuto DA, Giovanella L, Prior JO, Ceriani L (2019) Detection rate of (18)F-labeled PSMA PET/CT in biochemical recurrent prostate cancer: a systematic review and a meta-analysis. Cancers 2019:11 (5)
Lindenberg L, Choyke P, Dahut W (2016) Prostate cancer imaging with novel PET tracers. Curr Urol Rep 17(3):18
Dietlein F, Kobe C, Neubauer S et al (2017) PSA-stratified performance of (18)F- and (68)Ga-PSMA PET in patients with biochemical recurrence of prostate cancer. J Nucl Med 58(6):947–952
Funding
Key Laboratory of Functional Molecular Imaging of Tumor and Interventional Diagnosis and Treatment of Shaoxing City.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Jiwei Mao.
Conflict of interest
The authors declare no competing interests.
Statistics and biometry
One of the authors performed statistical analysis for this paper.
Informed consent
Written informed consent was not required for this study because this study was designed as a systematic review with meta-analysis.
Ethical approval
Institutional Review Board approval was not required because this study was designed as a systematic review with meta-analysis.
Methodology
• systematic review
• meta-analysis
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ma, W., Mao, J., Yang, J. et al. Comparing the diagnostic performance of radiotracers in prostate cancer biochemical recurrence: a systematic review and meta-analysis. Eur Radiol 32, 7374–7385 (2022). https://doi.org/10.1007/s00330-022-08802-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08802-7