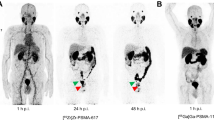
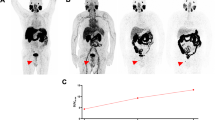
Abstract
Purpose
[68Ga]Tris(hydroxypyridinone)(THP)-PSMA is a novel radiopharmaceutical for one-step kit-based radiolabelling, based on direct chelation of 68Ga3+ at low concentration, room temperature and over a wide pH range, using direct elution from a 68Ge/68Ga-generator. We evaluated the clinical detection rates of [68Ga]THP-PSMA PET/CT in patients with biochemically recurrent prostate cancer after prostatectomy.
Methods
Consecutive patients (n=99) referred for evaluation of biochemical relapse of prostate cancer by [68Ga]THP-PSMA PET/CT were analyzed retrospectively. Patients underwent a standard whole-body PET/CT (1 h p.i.), followed by delayed (3 h p.i.) imaging of the abdomen. PSA-stratified cohorts of positive PET/CT results, standardized uptake values (SUVs) and target-to-background ratios (TBRs) were analyzed, and compared between standard and delayed imaging.
Results
At least one lesion suggestive of recurrent or metastatic prostate cancer was identified on PET images in 52 patients (52.5%). Detection rates of [68Ga]THP-PSMA PET/CT increased with increasing PSA level: 94.1% for a PSA value of ≥10 ng/mL, 77.3% for a PSA value of 2 to <10 ng/mL, 54.5% for a PSA value of 1 to <2 ng/mL, 14.3% for a PSA value of 0.5 to <1 ng/mL, 20.0% for a PSA value of >0.2 to <0.5, and 22.2% for a PSA value of 0.01 to 0.2 ng/mL. [68Ga]THP-PSMA uptake (SUVs) in metastases decreased over time, whereas TBRs improved. Delayed imaging at 3 h p.i. exclusively identified pathologic findings in 2% of [68Ga]THP-PSMA PET/CT scans. Detection rate was higher in patients with a Gleason score ≥8 (P=0.02) and in patients receiving androgen deprivation therapy (P=0.003).
Conclusions
In this study, [68Ga]THP-PSMA PET/CT showed suitable detection rates in patients with biochemical recurrence of prostate cancer and PSA levels ≥ 2 ng /mL. Detections rates were lower than in previous studies evaluating other PSMA ligands, though prospective direct radiotracer comparison studies are mandatory particularly in patients with low PSA levels to evaluate the relative performance of different PSMA ligands.




Similar content being viewed by others
References
Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:197–209.
Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of Hybrid 68Ga-PSMA Ligand PET/CT in 248 Patients with Biochemical Recurrence After Radical Prostatectomy. J Nucl Med. 2015;56:668–74.
Schmuck S, Mamach M, Wilke F, von Klot CA, Henkenberens C, Thackeray JT, et al. Multiple Time-Point 68Ga-PSMA I&T PET/CT for Characterization of Primary Prostate Cancer: Value of Early Dynamic and Delayed Imaging. Clin Nucl Med. 2017;42:e286–93.
Eiber M, Weirich G, Holzapfel K, Souvatzoglou M, Haller B, Rauscher I, et al. Simultaneous 68Ga-PSMA HBED-CC PET/MRI Improves the Localization of Primary Prostate Cancer. Eur Urol. 2016;70:829–36.
Schmuck S, Nordlohne S, von Klot CA, Henkenberens C, Sohns JM, Christiansen H, et al. Comparison of standard and delayed imaging to improve the detection rate of [68Ga]PSMA I&T PET/CT in patients with biochemical recurrence or prostate-specific antigen persistence after primary therapy for prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44:960–8.
Afshar-Oromieh A, Hetzheim H, Kratochwil C, Benesova M, Eder M, Neels OC, et al. The Theranostic PSMA Ligand PSMA-617 in the Diagnosis of Prostate Cancer by PET/CT: Biodistribution in Humans, Radiation Dosimetry, and First Evaluation of Tumor Lesions. J Nucl Med. 2015;56:1697–705.
Dietlein F, Kobe C, Neubauer S, Schmidt M, Stockter S, Fischer T, et al. PSA-stratified performance of 18F- and 68Ga-labeled tracers in PSMA-PET imaging of patients with biochemical recurrence of prostate cancer. J Nucl Med. 2017;58:947–52.
Schmuck S, von Klot CA, Henkenberens C, Sohns JM, Christiansen H, Wester HJ, et al. Initial Experience with Volumetric 68Ga-PSMA I&T PET/CT for Assessment of Whole-body Tumor Burden as a Quantitative Imaging Biomarker in Patients with Prostate Cancer. J Nucl Med. 2017;58:1962–68.
Berliner C, Tienken M, Frenzel T, Kobayashi Y, Helberg A, Kirchner U, et al. Detection rate of PET/CT in patients with biochemical relapse of prostate cancer using [68Ga]PSMA I&T and comparison with published data of [68Ga]PSMA HBED-CC. Eur J Nucl Med Mol Imaging. 2017;44:670–7.
Ceci F, Uprimny C, Nilica B, Geraldo L, Kendler D, Kroiss A, et al. (68)Ga-PSMA PET/CT for restaging recurrent prostate cancer: which factors are associated with PET/CT detection rate? Eur J Nucl Med Mol Imaging. 2015;42:1284–94.
Afshar-Oromieh A, Holland-Letz T, Giesel FL, et al. Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017;44:1258–68.
Pyka T, Okamoto S, Dahlbender M, Tauber R, Retz M, Heck M, et al. Comparison of bone scintigraphy and 68Ga-PSMA PET for skeletal staging in prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:2114–21.
Ma MT, Cullinane C, Imberti C, Baguña Torres J, Terry SY, Roselt P, et al. New Tris(hydroxypyridinone) Bifunctional Chelators Containing Isothiocyanate Groups Provide a Versatile Platform for Rapid One-Step Labeling and PET Imaging with (68)Ga(3.). Bioconjug Chem. 2016;27:309–18.
Ma MT, Cullinane C, Waldeck K, Roselt P, Hicks RJ, Blower PJ. Rapid kit-based (68)Ga-labelling and PET imaging with THP-Tyr(3)-octreotate: a preliminary comparison with DOTA-Tyr(3)-octreotate. EJNMMI Res. 2015;5:52.
Young JD, Abbate V, Imberti C, Meszaros LK, Ma MT, Terry SY, et al. 68Ga-THP-PSMA: a PET imaging agent for prostate cancer offering rapid, room temperature, one-step kit-based radiolabeling. J Nucl Med. 2017;58:1270–7.
Paller CJ, Antonarakis ES. Management of biochemically recurrent prostate cancer after local therapy: evolving standards of care and new directions. Clin Adv Hematol Oncol. 2013;11:14–23.
Rauscher I, Maurer T, Fendler WP, Sommer WH, Schwaiger M, Eiber M. (68)Ga-PSMA ligand PET/CT in patients with prostate cancer: How we review and report. Cancer Imaging. 2016;16:14.
Sahlmann CO, Meller B, Bouter C, Ritter CO, Ströbel P, Lotz J, et al. Biphasic 68Ga-PSMA-HBED-CC-PET/CT in patients with recurrent and high-risk prostate carcinoma. Eur J Nucl Med Mol Imaging. 2016;43:898–905.
Hope TA, Aggarwal R, Chee B, Tao D, Greene KL, Cooperberg M, et al. Impact of Ga-68 PSMA-11 PET on Management in Patients with Biochemically Recurrent Prostate Cancer. J Nucl Med. 2017;58:1956–61.
Afaq A, Alahmed S, Chen SH, Lengana T, Haroon A, Payne H, et al. 68Ga-PSMA PET/CT impact on prostate cancer management. J Nucl Med. 2018;59:89–92.
Derlin T, Weiberg D, von Klot C, Wester HJ, Henkenberens C, Ross TL, et al. 68Ga-PSMA I&T PET/CT for assessment of prostate cancer: evaluation of image quality after forced diuresis and delayed imaging. Eur Radiol. 2016;26:4345–53.
Evans MJ, Smith-Jones PM, Wongvipat J, Navarro V, Kim S, Bander NH, et al. Noninvasive measurement of androgen receptor signaling with a positron-emitting radiopharmaceutical that targets prostate-specific membrane antigen. Proc Natl Acad Sci U S A. 2011;108:9578–82.
Liu T, LY W, Fulton MD, Johnson JM, Berkman CE. Prolonged androgen deprivation leads to downregulation of androgen receptor and prostate-specific membrane antigen in prostate cancer cells. Int J Oncol. 2012;41:2087–92.
Hope TA, Truillet C, Ehman EC, Afshar-Oromieh A, Aggarwal R, Ryan CJ, et al. 68Ga-PSMA-11 PET Imaging of Response to Androgen Receptor Inhibition: First Human Experience. J Nucl Med. 2017;58:81–4.
Giesel FL, Hadaschik B, Cardinale J, Radtke J, Vinsensia M, Lehnert W, et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2017;44:678–88.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
[68Ga]THP-PSMA was provided by ROTOP Pharmaka GmbH under an evaluation agreement. Cathleen Juhl and Johanna Zörgiebel are employees of ROTOP Pharmaka GmbH. Thorsten Derlin and Tobias L. Ross received travel expenses from ROTOP Pharmaka GmbH. All other authors have nothing to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Derlin, T., Schmuck, S., Juhl, C. et al. PSA-stratified detection rates for [68Ga]THP-PSMA, a novel probe for rapid kit-based 68Ga-labeling and PET imaging, in patients with biochemical recurrence after primary therapy for prostate cancer. Eur J Nucl Med Mol Imaging 45, 913–922 (2018). https://doi.org/10.1007/s00259-017-3924-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3924-9