Abstract
Purpose
The primary aim of this retrospective, single-centre analysis was to assess the performance of 68Ga-PSMA-11 PET/CT in prostate cancer (PCa) patients in early PSA failure after radical prostatectomy (RP). The secondary aim was to assess the potential impact of 68Ga-PSMA-11 PET/CT on treatment strategy.
Methods
68Ga-PSMA-11 PET/CT is performed in our institution within an investigational new drug (IND) trial in PCa patients with biochemical recurrence (BCR). The records of all patients enrolled between March 2016 and July 2017 were evaluated. These records were retrospectively analysed according to the following inclusion criteria: (a) RP as primary therapy, (b) proven BCR, ©) PSA levels in the range 0.2–0.5 ng/ml at the time of the 68Ga-PSMA-11 PET/CT investigation, and (d) no salvage radiotherapy (S-RT) performed after recurrence. The performance of 68Ga-PSMA-11 PET/CT was evaluated in terms of detection rate on a per-patient and a per-region basis (local vs. distant lesions). We further performed an intention-to-treat (ITT) analysis. The patient cohort was grouped into three subpopulations, blinded to the 68Ga-PSMA-11 PET/CT results, according to the patients’ characteristics and different patterns of treatment: (1) S-RT (with or without systemic treatment), (2) stereotactic body radiotherapy (SBRT) (with or without systemic treatment), and (3) systemic treatment. The treatment strategy was re-evaluated for each patient taking into consideration the 68Ga-PSMA-11 PET/CT images.
Results
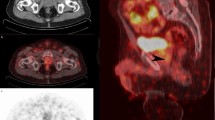
We enrolled 119 PCa patients (mean age 66 years, range 44–78 years) with a mean PSA level at the time of 68Ga-PSMA-11 PET/CT of 0.34 ng/ml (median 0.32 ng/ml, SD ±0.09, range 0.20–0.50 ng/ml). 68Ga-PSMA-1 1 PET/CT was positive in 41 of the 119 patients, resulting in an overall detection rate of 34.4%. 68Ga-PSMA-11 uptake was observed in the prostate bed (3 patients, 2.5%), in the pelvic lymph nodes (21, 17.6%), in the retroperitoneal lymph nodes (4, 3.4%) and in the skeleton (21, 17.6%). Regarding ITT, 81 patients (68.1%) were considered possible candidates for S-RT only in the prostate bed and none of the patients (0%) for SBRT. According to the 68Ga-PSMA-11 PET/CT results, the intended treatment was changed in 36 patients (30.2%). According to the PET/CT results, S-RT was recommended in 70 patients (58.8%), only to the prostate bed in 58 (48.7%) and SBRT in 29 (24.4%). The intended RT planning was modified in 36 (87.8%) of 41 patients with a positive 68Ga-PSMA-11 PET/CT result.
Conclusion
In our patient series with PSA levels <0.5 ng/ml, 68Ga-PSMA-11 PET/CT had a detection rate of 34.4%. In the ITT analysis, 30.2% of patients had a change in the intended treatment. These data support the hypothesis that 68Ga-PSMA-11 PET/CT is a useful procedure in the management of PCa patients showing early recurrence after RP, and should be implemented in routine clinical practice.




Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. https://doi.org/10.3322/caac.20107.
Rosenbaum E, Partin A, Eisenberger MA. Biochemical relapse after primary treatment for prostate cancer: studies on natural history and therapeutic considerations. J Natl Compr Cancer Netw. 2004;2:249–56.
Simmons MN, Stephenson AJ, Klein EA. Natural history of biochemical recurrence after radical prostatectomy: risk assessment for secondary therapy. Eur Urol. 2007;51:1175–84. https://doi.org/10.1016/j.eururo.2007.01.015.
Punnen S, Cooperberg MR, D'Amico AV, Karakiewicz PI, Moul JW, Scher HI, et al. Management of biochemical recurrence after primary treatment of prostate cancer: a systematic review of the literature. Eur Urol. 2013;64:905–15. https://doi.org/10.1016/j.eururo.2013.05.025.
Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–42. https://doi.org/10.1016/j.eururo.2016.08.002.
Amling CL, Bergstralh EJ, Blute ML, Slezak JM, Zincke H. Defining prostate specific antigen progression after radical prostatectomy: what is the most appropriate cut point? J Urol. 2001;165:1146–51.
Stephenson AJ, Scardino PT, Kattan MW, Pisansky TM, Slawin KM, Klein EA, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25:2035–41. https://doi.org/10.1200/jco.2006.08.9607.
Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, Bruycker AD, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36:446–53. https://doi.org/10.1200/jco.2017.75.4853.
Umbehr MH, Müntener M, Hany T, Sulser T, Bachmann LM. The role of 11C-choline and 18F-fluorocholine positron emission tomography (PET) and PET/CT in prostate cancer: a systematic review and meta-analysis. Eur Urol. 2013;64:106–17. https://doi.org/10.1016/j.eururo.2013.04.019.
Yu CY, Desai B, Ji L, Groshen S, Jadvar H. Comparative performance of PET tracers in biochemical recurrence of prostate cancer: a critical analysis of literature. Am J Nucl Med Mol Imaging. 2014;4:580–601.
Castellucci P, Ceci F, Graziani T, Schiavina R, Brunocilla E, Mazzarotto R, et al. Early biochemical relapse after radical prostatectomy: which prostate cancer patients may benefit from a restaging 11C-choline PET/CT scan before salvage radiation therapy? J Nucl Med. 2014;55:1424–9. https://doi.org/10.2967/jnumed.114.138313.
Afshar-Oromieh A, Haberkorn U, Eder M, Eisenhut M, Zechmann CM. [68Ga]Gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: comparison with 18F-FECH. Eur J Nucl Med Mol Imaging. 2012;39:1085–6. https://doi.org/10.1007/s00259-012-2069-0.
Ceci F, Uprimny C, Nilica B, Geraldo L, Kendler D, Kroiss A, et al. 68Ga-PSMA PET/CT for restaging recurrent prostate cancer: which factors are associated with PET/CT detection rate? Eur J Nucl Med Mol Imaging. 2015;42:1284–94. https://doi.org/10.1007/s00259-015-3078-6.
Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998;52:637–40.
Rauscher I, Maurer T, Beer AJ, Graner FP, Haller B, Weirich G, et al. Value of 68Ga-PSMA HBED-CC PET for the assessment of lymph node metastases in prostate cancer patients with biochemical recurrence: comparison with histopathology after salvage lymphadenectomy. J Nucl Med. 2016;57:1713–9. https://doi.org/10.2967/jnumed.116.173492.
von Eyben FE, Picchio M, von Eyben R, Rhee H, Bauman G. 68Ga-labeled prostate-specific membrane antigen ligand positron emission tomography/computed tomography for prostate cancer: a systematic review and meta-analysis. Eur Urol Focus. 2016. https://doi.org/10.1016/j.euf.2016.11.002.
Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–74. https://doi.org/10.2967/jnumed.115.154153.
Calais J, Fendler WP, Herrmann K, Eiber M, Ceci F. Head-to-head comparison of 68Ga-PSMA-11 PET/CT and 18F-Fluciclovine PET/CT in a case series of 10 patients with prostate cancer recurrence. J Nucl Med. 2018;59:789–94. https://doi.org/10.2967/jnumed.117.203257.
Gasch C, Düwel C, Kopka K, Kratochwil C, Vinsensia M, Eiber M, et al. Significance of PSMA imaging in prostate cancer. Urologe A. 2017;56:3–12. https://doi.org/10.1007/s00120-016-0293-0.
Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, et al. Sensitivity, specificity, and predictors of positive (68)Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70:926–37. https://doi.org/10.1016/j.eururo.2016.06.021.
Morigi JJ, Stricker PD, van Leeuwen PJ, Tang R, Ho B, Nguyen Q, et al. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med. 2015;56:1185–90. https://doi.org/10.2967/jnumed.115.160382.
Giesel FL, Will L, Kesch C, Freitag M, Kremer C, Merkle J, et al. Biochemical recurrence of prostate cancer: initial results with [18F]PSMA-1007 PET/CT. J Nucl Med. 2018;59:632–5. https://doi.org/10.2967/jnumed.117.196329.
Sridharan S, Steigler A, Spry NA, Joseph D, Lamb DS, Matthews JH, et al. Oligometastatic bone disease in prostate cancer patients treated on the TROG 03.04 RADAR trial. Radiother Oncol. 2016;121:98–102. https://doi.org/10.1016/j.radonc.2016.07.021.
Ingrosso G, Trippa F, Maranzano E, Carosi A, Ponti E, Arcidiacono F, et al. Stereotactic body radiotherapy in oligometastatic prostate cancer patients with isolated lymph nodes involvement: a two-institution experience. World J Urol. 2017;35:45–9. https://doi.org/10.1007/s00345-016-1860-0.
Ost P, Jereczek-Fossa BA, Van As N, Zilli T, Tree A, Henderson D, et al. Pattern of progression after stereotactic body radiotherapy for oligometastatic prostate cancer nodal recurrences. Clin Oncol (R Coll Radiol). 2016;28:e115–20. https://doi.org/10.1016/j.clon.2016.04.040.
Saluja R, Cheung P, Zukotynski K, Emmenegger U. Disease volume and distribution as drivers of treatment decisions in metastatic prostate cancer: from chemohormonal therapy to stereotactic ablative radiotherapy of oligometastases. Urol Oncol. 2016;34:225–32. https://doi.org/10.1016/j.urolonc.2016.02.016.
Ost P, Jereczek-Fossa BA, As NV, Zilli T, Muacevic A, Olivier K, et al. Progression-free survival following stereotactic body radiotherapy for oligometastatic prostate cancer treatment-naive recurrence: a multi-institutional analysis. Eur Urol. 2016;69:9–12. https://doi.org/10.1016/j.eururo.2015.07.004.
Yao HH, Hong MK, Corcoran NM, Siva S, Foroudi F. Advances in local and ablative treatment of oligometastasis in prostate cancer. Asia Pac J Clin Oncol. 2014;10:308–21. https://doi.org/10.1111/ajco.12256.
Eder M, Neels O, Müller M, Bauder-Wüst U, Remde Y, Schäfer M, et al. Novel preclinical and radiopharmaceutical aspects of [(68)Ga]Ga-PSMA-HBED-CC: a new PET tracer for imaging of prostate cancer. Pharmaceuticals. 2014;7:779–96. https://doi.org/10.3390/ph7070779.
Rauscher I, Maurer T, Fendler WP, Sommer WH, Schwaiger M, Eiber M. (68)Ga-PSMA ligand PET/CT in patients with prostate cancer: how we review and report. Cancer Imaging. 2016;16:14. https://doi.org/10.1186/s40644-016-0072-6.
Fanti S, Minozzi S, Morigi JJ, Giesel F, Ceci F, Uprimny C, et al. Development of standardized image interpretation for 68Ga-PSMA PET/CT to detect prostate cancer recurrent lesions. Eur J Nucl Med Mol Imaging. 2017;44:1622–35. https://doi.org/10.1007/s00259-017-3725-1.
Fendler WP, Eiber M, Beheshti M, Bomanji J, Ceci F, Cho S, et al. 68Ga-PSMA PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2017;44:1014–24. https://doi.org/10.1007/s00259-017-3670-z.
Khan MA, Carter HB, Epstein JI, Miller MC, Landis P, Walsh PW, et al. Can prostate specific antigen derivatives and pathological parameters predict significant change in expectant management criteria for prostate cancer? J Urol. 2003;170:2274–8. https://doi.org/10.1097/01.ju.0000097124.21878.6b.
Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:197–209. https://doi.org/10.1007/s00259-014-2949-6.
Schwenck J, Rempp H, Reischl G, Kruck S, Stenzl A, Nikolaou K, et al. Comparison of 68Ga-labelled PSMA-11 and 11C-choline in the detection of prostate cancer metastases by PET/CT. Eur J Nucl Med Mol Imaging. 2017;44:92–101. https://doi.org/10.1007/s00259-016-3490-6.
Calais J, Czernin J, Cao M, Kishan AU, Hegde JV, Shaverdian N, et al. 68Ga-PSMA-11 PET/CT mapping of prostate cancer biochemical recurrence after radical prostatectomy in 270 patients with a PSA level of less than 1.0 ng/ml: impact on salvage radiotherapy planning. J Nucl Med. 2018;59:230–7. https://doi.org/10.2967/jnumed.117.201749.
Afshar-Oromieh A, Holland-Letz T, Giesel FL, Kratochwil C, Mier W, Haufe S, et al. Diagnostic performance of (68)Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017;44:1258–68. https://doi.org/10.1007/s00259-017-3711-7.
Kranzbühler B, Nagel H, Becker AS, Müller J, Huellner M, Stolzmann P, et al. Clinical performance of (68)Ga-PSMA-11 PET/MRI for the detection of recurrent prostate cancer following radical prostatectomy. Eur J Nucl Med Mol Imaging. 2018;45:20–30. https://doi.org/10.1007/s00259-017-3850-x.
Emmett L, van Leeuwen PJ, Nandurkar R, Scheltema MJ, Cusick T, Hruby G, et al. Treatment outcomes from 68Ga-PSMA PET/CT-informed salvage radiation treatment in men with rising PSA after radical prostatectomy: prognostic value of a negative PSMA PET. J Nucl Med. 2017;58:1972–6. https://doi.org/10.2967/jnumed.117.196683.
Rauscher I, Düwel C, Haller B, Rischpler C, Heck MM, Gschwend JE, et al. Efficacy, predictive factors, and prediction nomograms for 68Ga-labeled prostate-specific membrane antigen–ligand positron-emission tomography/computed tomography in early biochemical recurrent prostate cancer after radical prostatectomy. Eur Urol. 2018;73:656–61 https://doi.org/10.1016/j.eururo.2018.01.006.
Mamede M, Ceci F, Castellucci P, Schiavina R, Fuccio C, Nanni C, et al. The role of 11C-choline PET imaging in the early detection of recurrence in surgically treated prostate cancer patients with very low PSA level <0.5 ng/mL. Clin Nucl Med. 2013;38:e342–e5. https://doi.org/10.1097/RLU.0b013e31829af913.
Afaq A, Alahmed S, Chen SH, Lengana T, Haroon A, Payne H, et al. Impact of 68Ga-prostate-specific membrane antigen PET/CT on prostate cancer management. J Nucl Med. 2018;59:89–92. https://doi.org/10.2967/jnumed.117.192625.
Albisinni S, Artigas C, Aoun F, Biaou I, Grosman J, Gil T, et al. Clinical impact of 68Ga-prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) in patients with prostate cancer with rising prostate-specific antigen after treatment with curative intent: preliminary analysis of a multidisciplinary approach. BJU Int. 2017;120:197–203. https://doi.org/10.1111/bju.13739.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Farolfi, A., Ceci, F., Castellucci, P. et al. 68Ga-PSMA-11 PET/CT in prostate cancer patients with biochemical recurrence after radical prostatectomy and PSA <0.5 ng/ml. Efficacy and impact on treatment strategy. Eur J Nucl Med Mol Imaging 46, 11–19 (2019). https://doi.org/10.1007/s00259-018-4066-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4066-4