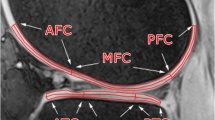
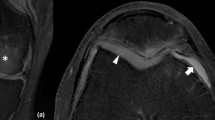
Abstract
Objective
To validate a radial imaging spin-echo diffusion tensor (RAISED) sequence for high-resolution diffusion tensor imaging (DTI) of articular cartilage at 3 T.
Methods
The RAISED sequence implementation is described, including the used non-linear motion correction algorithm. The robustness to eddy currents was tested on phantoms, and accuracy of measurement was assessed with measurements of temperature-dependent diffusion of free water. Motion correction was validated by comparing RAISED with single-shot diffusion-weighted echo-planar imaging (EPI) measures. DTI was acquired in asymptomatic subjects (n = 6) and subjects with doubtful (Kellgren-Lawrence [KL] grade 1, n = 9) and mild (KL = 2, n = 9) symptomatic knee osteoarthritis (OA). MD and FA values without correction, and after all corrections, were calculated. A test-retest evaluation of the DTI acquisition on three asymptomatic and three OA subjects was also performed.
Results
The root mean squared coefficient of variation of the global test-restest reproducibility was 3.54% for MD and 5.34% for FA. MD was significantly increased in both femoral condyles (7–9%) of KL 1 and in the medial (11–17%) and lateral (10–12%) compartments of KL 2 subjects. Averaged FA presented a trend of lower values with increasing KL grade, which was significant for the medial femoral condyle (-11%) of KL 1 and all three compartments in KL 2 subjects (-18 to -11%). Group differences in MD and FA were only significant after motion correction.
Conclusion
The RAISED sequence with the proposed reconstruction framework provides reproducible assessment of DTI parameters in vivo at 3 T and potentially the early stages of the disease in large regions of interest.
Key Points
• DTI of articular cartilage is feasible at 3T with a multi-shot RAISED sequence with non-linear motion correction.
• RAISED sequence allows estimation of the diffusion indices MD and FA with test-retest errors below 4% (MD) and 6% (FA).
• RAISED-based measurement of DTI of articular cartilage with non-linear motion correction holds potential to differentiate healthy from OA subjects.






Similar content being viewed by others
Abbreviations
- CV:
-
Coefficient of variation
- DTI:
-
Diffusion tensor imaging
- EPI:
-
Echo-planar imaging
- FA:
-
Fractional anisotropy
- FT:
-
Femoral trochlea
- KL:
-
Kellgren-Lawrence score
- LFC:
-
Lateral femoral condyle
- LT:
-
Lateral tibia
- MD:
-
Mean diffusivity
- MFC:
-
Medial femoral condyle
- MRI:
-
Magnetic resonance imaging
- MT:
-
Medial tibia
- OA:
-
Osteoarthritis
- P:
-
Patella
- PG:
-
Proteoglycan
- RAISED:
-
Radial imaging spin-echo diffusion tensor
- SE:
-
Spin echo
- SNR:
-
Signal-to-noise ratio
References
Filidoro L, Dietrich O, Weber J et al (2005) High-resolution diffusion tensor imaging of human patellar cartilage: feasibility and preliminary findings. Magn Reson Med 53:993–998
Meder R, de Visser SK, Bowden JC, Bostrom T, Pope JM (2006) Diffusion tensor imaging of articular cartilage as a measure of tissue microstructure. Osteoarthritis Cartilage 14:875–881
de Visser SK, Bowden JC, Wentrup-Byrne E et al (2008) Anisotropy of collagen fibre alignment in bovine cartilage: comparison of polarised light microscopy and spatially resolved diffusion-tensor measurements. Osteoarthritis Cartilage 16:689–697
Raya JG, Melkus G, Adam-Neumair S et al (2011) Change of diffusion tensor imaging parameters in articular cartilage with progressive proteoglycan extraction. Invest Radiol 46:401–409
Raya JG, Melkus G, Adam-Neumair S et al (2013) Diffusion-tensor imaging of human articular cartilage specimens with early signs of cartilage damage. Radiology 266:831–841
Deng X, Farley M, Nieminen MT, Gray M, Burstein D (2007) Diffusion tensor imaging of native and degenerated human articular cartilage. Magn Reson Imaging 25:168–171
Ferizi U, Rossi I, Lee Y et al (2017) Diffusion tensor imaging of articular cartilage at 3T correlates with histology and biomechanics in a mechanical injury model. Magn Reson Med 78:69–78
Guha A, Wyatt C, Karampinos DC, Nardo L, Link TM, Majumdar S (2015) Spatial variations in magnetic resonance-based diffusion of articular cartilage in knee osteoarthritis. Magn Reson Imaging 33:1051–1058
Raya JG, Dettmann E, Notohamiprodjo M, Krasnokutsky S, Abramson S, Glaser C (2014) Feasibility of in vivo diffusion tensor imaging of articular cartilage with coverage of all cartilage regions. Eur Radiol 24(7):1700–1706
Raya JG, Horng A, Dietrich O et al (2012) Articular cartilage: in vivo diffusion-tensor imaging. Radiology 262:550–559
Berstein MA, King KF, Zhou XJ (2004) Chapter 14 − Basic pulse sequences. In: Berstein MA, King KF, Zhou XJ (eds) Handbook of MRI pulse sequences, 1st edition
Miller KL, Pauly JM (2003) Nonlinear phase correction for navigated diffusion imaging. Magn Reson Med 50:343–353
Skare S, Hedehus M, Moseley ME, Li TQ (2000) Condition number as a measure of noise performance of diffusion tensor data acquisition schemes with MRI. J Magn Reson 147:340–352
Mills R (1973) Self-diffusion in normal and heavy water. J Phys Chem 77:685–688
Tofts PS, Lloyd D, Clark CA et al (2000) Test liquids for quantitative MRI measurements of self-diffusion coefficients in vivo. Magn Reson Med 43:368–374
Gillen KT, Douglass DC, Hoch MJR (1972) Self-diffusion in liquid water to -31°C. J Chem Phys 57:5117–5119
Harris KR, Woolf LA (1980) Pressure and temperature dependence of the self diffusion coefficient of water and oxygen-18 water. J Chem Soc Faraday Trans 76:377–385
Dietrich O (2018) Diffusion coefficients of water. Available via http://dtrxde/od/diff/. Accessed 2018-07-08 2018
Bodammer N, Kaufmann J, Kanowski M, Tempelmann C (2004) Eddy current correction in diffusion-weighted imaging using pairs of images acquired with opposite diffusion gradient polarity. Magn Reson Med 51:188–193
Neeman M, Freyer JP, Sillerud LO (1991) A simple method for obtaining cross-term-free images for diffusion anisotropy studies in NMR microimaging. Magn Reson Med 21:138–143
König L, Groher M, Keil A, Glaser C, Reiser M, Navab N (2007) Semi-automatic segmentation of the patellar cartilage in MRI. Bildverarbeitung für die Medizin 17:404–408
Altman R, Asch E, Bloch D et al (1986) Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 29:1039–1049
Guizar-Sicairos M (2008) Efficient subpixel image registration by cross-correlation https://www.mathworks.com/matlabcentral/fileexchange/18401-efficient-subpixel-imageregistration-by-cross-correlation?requestedDomain=www.mathworks.com. Accessed 1 Jan 2016
Guizar-Sicairos M, Thurman ST, Fienup JR (2008) Efficient subpixel image registration algorithms. Opt Lett 33:156–158
Ferizi U, Ruiz A, Rossi I, Bencardino J, Raya JG (2018) A robust diffusion tensor model for clinical applications of MRI to cartilage. Magn Reson Med 79:1157–1164
Constantinides CD, Atalar E, McVeigh ER (1997) Signal-to-noise measurements in magnitude images from NMR phased arrays. Magn Reson Med 38:852–857
Lin LI (1989) A concordance correlation coefficient to evaluate reproducibility. Biometrics 45:255–268
Miller KL, Hargreaves BA, Gold GE, Pauly JM (2004) Steady-state diffusion-weighted imaging of in vivo knee cartilage. Magn Reson Med 5:394–398
Bieri O, Ganter C, Scheffler K (2012) Quantitative in vivo diffusion imaging of cartilage using double echo steady-state free precession. Magn Reson Med 68:720–729
Heule R, Ganter C, Bieri O (2014) Rapid estimation of cartilage T with reduced T sensitivity using double echo steady state imaging. Magn Reson Med 71(3):1137–1143. https://doi.org/10.1002/mrm.24748
Staroswiecki E, Granlund KL, Alley MT, Gold GE, Hargreaves BA (2012) Simultaneous estimation of T(2) and apparent diffusion coefficient in human articular cartilage in vivo with a modified three-dimensional double echo steady state (DESS) sequence at 3 T. Magn Reson Med 67:1086–1096
Raya JG (2015) Techniques and applications of in vivo diffusion imaging of articular cartilage. J Magn Reson Imaging 41:1487–1504
Azuma T, Nakai R, Takizawa O, Tsutsumi S (2009) In vivo structural analysis of articular cartilage using diffusion tensor magnetic resonance imaging. Magn Reson Imaging 27:1242–1248
Glaser C, Mendlik T, Dinges J et al (2006) Global and regional reproducibility of T2 relaxation time measurements in human patellar cartilage. Magn Reson Med 56:527–534
Raya JG, Horng A, Dietrich O et al (2009) Voxel-based reproducibility of T2 relaxation time in patellar cartilage at 1.5 T with a new validated 3D rigid registration algorithm. MAGMA 22:229–239
Madelin G, Babb JS, Xia D, Chang G, Jerschow A, Regatte RR (2012) Reproducibility and repeatability of quantitative sodium magnetic resonance imaging in vivo in articular cartilage at 3 T and 7 T. Magn Reson Med 68:841–849
Multanen J, Rauvala E, Lammentausta E et al (2009) Reproducibility of imaging human knee cartilage by delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) at 1.5 Tesla. Osteoarthritis Cartilage 17:559–564
Jordan CD, McWalter EJ, Monu UD et al (2014) Variability of CubeQuant T1rho, quantitative DESS T2, and cones sodium MRI in knee cartilage. Osteoarthritis Cartilage 22:1559–1567
Gupta R, Virayavanich W, Kuo D et al (2014) MR T(1)rho quantification of cartilage focal lesions in acutely injured knees: correlation with arthroscopic evaluation. Magn Reson Imaging 32:1290–1296
Xu J, Xie G, Di Y, Bai M, Zhao X (2011) Value of T2-mapping and DWI in the diagnosis of early knee cartilage injury. J Radiol Case Rep 5:13–18
Funding
This study has received funding from the (US) National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institute of Health (NIH), Grant/Award Number R01AR067789.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Guarantor
The scientific guarantor of this publication is José G Raya, PhD.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• case-control study
• performed at one institution
Rights and permissions
About this article
Cite this article
Duarte, A., Ruiz, A., Ferizi, U. et al. Diffusion tensor imaging of articular cartilage using a navigated radial imaging spin-echo diffusion (RAISED) sequence. Eur Radiol 29, 2598–2607 (2019). https://doi.org/10.1007/s00330-018-5780-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5780-9