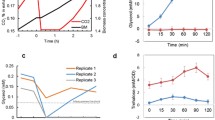
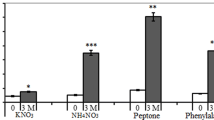
Abstract
Whereas osmotic stress response induced by solutes has been well-characterized in fungi, less is known about the other activities of environmentally ubiquitous substances. The latest methodologies to define, identify and quantify chaotropicity, i.e. substance-induced destabilization of macromolecular systems, now enable new insights into microbial stress biology (Cray et al. in Curr Opin Biotechnol 33:228–259, 2015a, doi:10.1016/j.copbio.2015.02.010; Ball and Hallsworth in Phys Chem Chem Phys 17:8297–8305, 2015, doi:10.1039/C4CP04564E; Cray et al. in Environ Microbiol 15:287–296, 2013a, doi:10.1111/1462-2920.12018). We used Aspergillus wentii, a paradigm for extreme solute-tolerant fungal xerophiles, alongside yeast cell and enzyme models (Saccharomyces cerevisiae and glucose-6-phosphate dehydrogenase) and an agar-gelation assay, to determine growth-rate inhibition, intracellular compatible solutes, cell turgor, inhibition of enzyme activity, substrate water activity, and stressor chaotropicity for 12 chemically diverse solutes. These stressors were found to be: (i) osmotically active (and typically macromolecule-stabilizing kosmotropes), including NaCl and sorbitol; (ii) weakly to moderately chaotropic and non-osmotic, these were ethanol, urea, ethylene glycol; (iii) highly chaotropic and osmotically active, i.e. NH4NO3, MgCl2, guanidine hydrochloride, and CaCl2; or (iv) inhibitory due primarily to low water activity, i.e. glycerol. At ≤0.974 water activity, Aspergillus cultured on osmotically active stressors accumulated low-M r polyols to ≥100 mg g dry weight−1. Lower-M r polyols (i.e. glycerol, erythritol and arabitol) were shown to be more effective for osmotic adjustment; for higher-M r polyols such as mannitol, and the disaccharide trehalose, water-activity values for saturated solutions are too high to be effective; i.e. 0.978 and 0.970 (25 ºC). The highly chaotropic, osmotically active substances exhibited a stressful level of chaotropicity at physiologically relevant concentrations (20.0–85.7 kJ kg−1). We hypothesized that the kosmotropicity of compatible solutes can neutralize chaotropicity, and tested this via in-vitro agar-gelation assays for the model chaotropes urea, NH4NO3, phenol and MgCl2. Of the kosmotropic compatible solutes, the most-effective protectants were trimethylamine oxide and betaine; but proline, dimethyl sulfoxide, sorbitol, and trehalose were also effective, depending on the chaotrope. Glycerol, by contrast (a chaotropic compatible solute used as a negative control) was relatively ineffective. The kosmotropic activity of compatible solutes is discussed as one mechanism by which these substances can mitigate the activities of chaotropic stressors in vivo. Collectively, these data demonstrate that some substances concomitantly induce chaotropicity-mediated and osmotic stresses, and that compatible solutes ultimately define the biotic window for fungal growth and metabolism. The findings have implications for the validity of ecophysiological classifications such as ‘halophile’ and ‘polyextremophile’; potential contamination of life-support systems used for space exploration; and control of mycotoxigenic fungi in the food-supply chain.








Similar content being viewed by others
References
Albers E, Larsson C, Lidén G, Niklasson C, Gustafsson L (1996) Influence of the nitrogen source on Saccharomyces cerevisiae anaerobic growth and product formation. Appl Environ Microbiol 62:3187–3195
Albertyn J, Hohmann S, Thevelein JM, Prior BA (1994) GPD1, which encodes glycerol-3-phosphate dehydrogenase, is essential for growth under osmotic stress in Saccharomyces cerevisiae, and its expression is regulated by the high-osmolarity glycerol response pathway. Mol Cell Biol 14:4135–4144
Ansell R, Granath K, Hohmann S, Thevelein JM, Adler L (1997) The two isozymes for yeast NAD+-dependent glycerol 3-phosphate dehydrogenase encoded by GPD1 and GPD2 have distinct roles in osmoadaptation and redox regulation. EMBO J 16:2179–2187
Ball P, Hallsworth JE (2015) Water structure and chaotropicity: their uses, abuses, and implications for biology. Phys Chem Chem Phys 17:8297–8305. doi:10.1039/C4CP04564E
Bell AN, Magill E, Hallsworth JE, Timson DJ (2013) Effects of alcohols and compatible solutes on the activity of β-galactosidase. Appl Biochem Biotechnol 169:786–794
Bhaganna P, Volkers RJM, Bell ANW, Kluge K, Timson DJ, McGrath JW, Ruijssenaars HJ, Hallsworth JE (2010) Hydrophobic substances induce water stress in microbial cells. Microb Biotechnol 3:701–716
Biederbeck VO, Curtin D, Bouman OT, Campbell CA, Ukrainetz H (1996) Soil microbial and biochemical properties after ten years of fertilization with urea and anhydrous ammonia. Can J Soil Sci 76:7–14
Borowitzka LJ, Brown AD (1974) The salt relations of marine and halophilic species of the unicellular green alga, Dunaliella. Arch Microbiol 96:37–52
Bräse S, Encinas A, Keck J, Nising CF (2009) Chemistry and biology of mycotoxins and related fungal metabolites. Chem Rev 109:3903–3990
Brown AD (1990) Microbial water stress physiology. Principles and perspectives. Wiley, Chichester
Bruinenberg PM, Van Dijken JP, Scheffers WA (1983) An enzymic analysis of NADPH production and consumption in Candida utilis. J Gen Microbiol 129:965–971
Carisse O, Philion V, Rolland D, Bernier J (2000) Effect of fall application of fungal antagonists on spring ascospore production of the apple scab pathogen, Venturia inaequalis. Phytopathology 90:31–37
Chin JP, Megaw J, Magill CL, Nowotarski K, Williams JP, Bhaganna P, Linton M, Patterson MF, Underwood GJC, Mswaka AY, Hallsworth JE (2010) Solutes determine the temperature windows for microbial survival and growth. Proc Natl Acad Sci USA 107:7835–7840
Chirife J, Favetto G, Fontán CF (1984) Microbial growth at reduced water activities: some physicochemical properties of compatible solutes. J Appl Bacteriol 56:259–268
Cray JA, Russell JT, Timson DJ, Singhal RS, Hallsworth JE (2013a) A universal measure of chaotropicity and kosmotropicity. Environ Microbiol 15:287–296. doi:10.1111/1462-2920.12018
Cray JA, Bell ANW, Bhaganna P, Mswaka AY, Timson DJ, Hallsworth JE (2013b) The biology of habitat dominance; can microbes behave as weeds? Microb Biotechnol 6:453–492
Cray JA, Bhaganna P, Singhal RS, Patil SV, Saha D, Chakraborty R, Iwaguchi S, Timson DJ, Hallsworth JE (2014) Chaotropic and hydrophobic stress mechanisms of antifungal substances. In: Dehne HW, Deising HB, Fraaije B, Gisi U, Hermann D, Mehl A, Oerke EC, Russell PE, Stammler G, Kuck KH, Lyr H (eds) Modern fungicides and antifungal compounds, vol VII. Deutsche Phytomedizinische Gesellschaft, Braunschweig. ISBN: 978-3-941261-13-6
Cray JA, Stevenson A, Ball P, Bankar SB, Eleutherio ECA, Ezeji TC, Singhal RS, Thevelein JM, Timson DJ, Hallsworth JE (2015a) Chaotropicity: a key factor in product tolerance of biofuel-producing microorganisms. Curr Opin Biotechnol 33:228–259. doi:10.1016/j.copbio.2015.02.010
Cray JA, Houghton JD, Cooke LR, Hallsworth JE (2015b) A simple inhibition coefficient for quantifying potency of biocontrol agents against plant-pathogenic fungi. Biol Control 81:93–100
Daffonchio D, Borin S, Brusa T, Brusetti L, van der Wielen PWJJ, Bolhuis H, Yakimov MM, D’Auria G, Giuliano L, Marty D, Tamburini C, McGenity TJ, Hallsworth JE, Sass AM, Timmis KN, Tselepides A, de Lange GJ, Hübner A, Thomson J, Varnavas SP, Gasparoni F, Gerber HW, Malinverno E, Corselli C, Garcin J, McKew B, Golyshin PN, Lampadariou N, Polumenakou P, Calore D, Cenedese S, Zanon F, Hoog S (2006) Stratified prokaryote network in the oxic-anoxic transition of a deep-sea halocline. Nature 440:203–207
Dhillon GS, Brar SK, Verma M, Tyagi RD (2011) Recent advances in citric acid bio-production and recovery. Food Bioprocess Technol 4:505–529
Duda VI, Danilevich VN, Suzina NE, Shorokhova AP, Dmitriev VV, Mokhova ON, Akimov VN (2004) Changes in the fine structure of microbial cells induced by chaotropic salts. Mikrobiologiya 73:341–349
Eleutherio ECA, Araujo PS, Panek AD (1993) Role of the trehalose carrier in dehydration resistance of Saccharomyces cerevisiae. Biochim Biophys Acta 1156:263–266
Ferreira C, van Voorst F, Martins A, Neves L, Oliveira R, Kielland-Brandt MC, Lucas C, Brandt A (2005) A member of the sugar transporter family, Stl1p is the glycerol/H+ symporter in Saccharomyces cerevisiae. Mol Biol Cell 16:2068–2076
Furukawa K, Hoshi Y, Maeda T, Nakajima T, Abe K (2005) Aspergillus nidulans HOG pathway is activated only by two-component signalling pathway in response to osmotic stress. Mol Microbiol 56:1246–1261
Ghose TK, Panda T, Bisaria VS (1985) Effect of culture phasing and mannanase on production of cellulase and hemicellulase by mixed culture of Trichoderma reesei D 1–6 and Aspergillus wentii pt 2804. Biotechnol Bioeng 27:1353–1361
Hagiwara D, Takahashi-Nakaguchi A, Toyotome T, Yoshimi A, Abe K, Kamei K, Gonoi T, Kawamoto S (2013) NikA/TcsC histidine kinase is involved in condition, hyphal morphology, and responses to osmotic stress and antifungal chemicals in Aspergillus fumigatus. PLoS ONE 8:e80881
Hallsworth JE (1995) Manipulation of intracellular glycerol and erythritol for successful biological control. PhD thesis, Cranfield University
Hallsworth JE (1998) Ethanol-induced water stress in yeast. J Ferment Bioeng 85:125–137
Hallsworth JE, Magan N (1994a) Effects of KCl concentration on accumulation of acyclic sugar alcohols and trehalose in conidia of three entomopathogenic fungi. Lett Appl Microbiol 18:8–11
Hallsworth JE, Magan N (1994b) Effect of carbohydrate type and concentration on polyols and trehalose in conidia of three entomopathogenic fungi. Microbiol SGM 140:2705–2713
Hallsworth JE, Magan N (1994c) Improved biological control by changing polyols/trehalose in conidia of entomopathogens. In: Brighton crop protection conference 1994 - pests and diseases, vol 3. BCPC Publications, Farnham, pp 1091–1096
Hallsworth JE, Magan N (1995) Manipulation of intracellular glycerol and erythritol enhances germination of conidia at low water availability. Microbiol SGM 29:7–13
Hallsworth JE, Magan N (1996) Culture age, temperature, and pH affect the polyol and trehalose contents of fungal propagules. Appl Environ Microbiol 62:2435–2442
Hallsworth JE, Magan M (1997) A rapid HPLC protocol for detection of polyols and trehalose. J Microbiol Methods 29:7–13
Hallsworth JE, Magan N (1999) Water and temperature relations of growth of three entomogenous fungi Beauveria bassiana, Metarhizium anisopliae and Paecilomyces farinosus. J Invertebr Pathol 74:261–266
Hallsworth JE, Nomura Y (1999) A simple method to determine the water activity of ethanol-containing samples. Biotechnol Bioeng 62:242–245
Hallsworth JE, Nomura Y, Iwahara M (1998) Ethanol-induced water stress and fungal growth. J Ferment Bioeng 86:451–456
Hallsworth JE, Heim S, Timmis KN (2003a) Chaotropic solutes cause water stress in Pseudomonas putida. Environ Microbiol 5:1270–1280
Hallsworth JE, Prior BA, Nomura Y, Iwahara M, Timmis KN (2003b) Compatible solutes protect against chaotrope (ethanol)-induced, nonosmotic water stress. Appl Environ Microb 69:7032–7034. doi:10.1128/aem.69.12.7032-7034.2003
Hallsworth JE, Yakimov MM, Golyshin PN, Gillion JLM, D’Auria G, Alves FL, La Cono V, Genovese M, McKew BA, Harris G, Guiliano L, Timmis KN, McGenity TJ (2007) Limits of life in MgCl2-containing environments: chaotropicity defines the window. Environ Microbiol 9:803–813
Harrison JP, Gheeraert N, Tsigelnitskiy D, Cockell CS (2013) The limits for life under multiple extremes. Trends Microbiol 21:204–212
Harrison JP, Hallsworth JE, Cockell CS (2015) Reduction of the temperature sensitivity of Halomonas hydrothermalis by iron starvation combined with microaerobic conditions. Appl Environ Microbiol 81:2156–2162
Hohmann S (2002) Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol Rev 66:300–372
Huang MR, Li SX, Dong ZQ, Feng W, Wang XY, Gu SY, Wu YH, Huang XA (2002) Oxygen enrichment from air through multilayer thin low-density polyethylene films. J Appl Polym Sci 83:3013–3021
Kar JR, Hallsworth JE, Singhal RS (2015) Fermentative production of glycine betaine and trehalose from acid whey using Actinopolyspora halophila (MTCC 263). Environ Technol Innov 3:68–76
Kiyosawa K (1991) Volumetric properties of polyols (ethylene glycol, glycerol, meso-erythritol, xylitol and mannitol) in relation to their membrane permeability: group additivity and estimation of the maximum radius of their molecules. Biochim Biophys Acta 1064:251–255
Kiyosawa K (1993) Permeability of the Chara cell membrane for ethylene glycol, glycerol, meso-erythritol, xylitol and mannitol. Physiol Plant 88:366–371
Kroll K, Paehtz V, Kniemeyer O (2014) Elucidating the fungal stress response by proteomics. J Proteomics 97:151–163
Laguna MF, Guzman J, Riande E (2001) Transport of carbon dioxide in linear low-density polyethylene determined by permeation measurements and NMR spectroscopy. Polymer 42:4321–4327
Lievens B, Hallsworth JE, Pozo MI, Belgacem ZB, Stevenson A, Willems KA, Jacquemyn H (2014) Microbiology of sugar-rich environments: diversity, ecology, and system constraints. Environ Microbiol 17:278–298
Lilley TH, Sutton RL (1991) The prediction of water activities in multicomponent systems. In: Levine H, Slade L (eds) Water relationships in foods. Springer, New York, pp 291–304
Luyten K, Albertyn J, Skibbe WF, Prior BA, Ramos J, Thevelein JM, Hohmann S (1995) Fps1, a yeast member of the MIP family of channel proteins, is a facilitator for glycerol uptake and efflux and is inactive under osmotic stress. EMBO J 14:1360–1371
Magan N, Olsen M (2004) Mycotoxins in food: detection and control, vol 103. Woodhead Publishing, Cambridge
Mansure JJC, Panek AD, Crowe LM, Crowe JH (1994) Trehalose inhibits ethanol effects on intact yeast cells and liposomes. Biochim Biophys Acta 1191:309–316
McCammick EM, Gomase VS, Timson DJ, McGenity TJ, Hallsworth JE (2010) Water-hydrophobic compound interactions with the microbial cell. In: Timmis KN (ed) Handbook of hydrocarbon and lipid microbiology—hydrocarbons, oils and lipids: diversity, properties and formation, vol 2. Springer, New York, pp 1451–1466
Medina A, Schmidt-Heydt M, Rodríguez A, Parra R, Geisen R, Magan N (2015) Impacts of environmental stress on growth, secondary metabolite biosynthetic gene clusters and metabolite production of xerotolerant/xerophilic fungi. Curr Genet. doi:10.1007/s00294-014-0455-9
Miermont A, Waharte F, Hu S, McClean MN, Bottani S, Léon S, Hersen P (2013) Severe osmotic compression triggers a slowdown of intracellular signaling, which can be explained by molecular crowding. Proc Natl Acad Sci USA 110:5725–5730
Miles AA, Misra SS (1938) The estimation of the bactericidal power of the blood. J Hyg (Lond) 38:732–749
Ninni L, Camargo MS, Meirelles AJ (2000) Water activity in polyol systems. J Chem Eng Data 45:654–660
Novikova N, De Boever P, Poddubko S, Deshevaya E, Polikarpov N, Rakova N, Coninx I, Mergeay M (2006) Survey of environmental biocontamination on board the International Space Station. Res Microbiol 157:5–12
Oren A, Hallsworth JE (2014) Microbial weeds in saline habitats: the enigma of the weed-like Haloferax mediterranei. FEMS Microbiol Lett 359:134–142
Pais TM, Foulquié-Moreno MR, Hubmann G, Duitama J, Swinnen S, Goovaerts A, Yang Y, Dumortier F, Thevelein JM (2013) Comparative polygenic analysis of maximal ethanol accumulation capacity and tolerance to high ethanol levels of cell proliferation in yeast. PLoS Genet 9:e1003548
Parry BR, Surovtsev IV, Cabeen MT, O’Hern CS, Dufresne ER, Jacobs-Wagner C (2014) The bacterial cytoplasm has glass-like properties and is fluidized by metabolic activity. Cell 156:183–194
Pitt JI, Christian JHB (1968) Water relations of xerophilic fungi isolated from prunes. Appl Microbiol 16:1853–1858
Ramirez ML, Chulze SN, Magan N (2004) Impact of osmotic and matric water stress on germination, growth, mycelial water potentials and endogenous accumulation of sugars and sugar alcohols in Fusarium graminearum. Mycologia 96:470–478
Ramos AJ, Magan N, Sanchis V (1999) Osmotic and matric potential effects on growth, sclerotial production and partitioning of polyols and sugars in colonies and spores of Aspergillus ochraceus. Mycol Res 103:141–147
Rangel DEN, Braga GUL, Fernandes ÉKK, Keyser CA, Hallsworth JE, Roberts DW (2015a) Stress tolerance and virulence of insect-pathogenic fungi are determined by environmental conditions during conidial formation. Curr Genet. doi:10.1007/s00294-015-0477-y
Rangel DEN, Alder-Rangel A, Dadachova E, Finlay R, Kupiec M, Dijksterhuis J, Braga GUL, Corrochano LM, Hallsworth JE (2015b) Fungal stress biology: a preface to the Fungal Stress Responses special edition. Curr Genet. doi:10.1007/s00294-015-0500-3
Rummel JD, Beaty DW, Jones MA, Bakermans C, Barlow NG, Boston PJ, Chevrier VF, Clark BC, de Vera JPP, Gough RV, Hallsworth JE, Head JW, Hipkin VJ, Kieft TL, McEwen AS, Mellon MT, Mikucki JA, Nicholson WL, Omelon CR, Peterson R, Roden EE, Sherwood Lollar B, Tanaka KL, Viola D, Wray JJ (2014) A new analysis of Mars “Special Regions”: findings of the second MEPAG Special Regions Science Analysis Group (SR-SAG2). Astrobiology 14:887–968
Safdar N, Crnich CJ, Maki DG (2005) The pathogenesis of ventilator-associated pneumonia: its relevance to developing effective strategies for prevention. Respir Care 50:725–741
Sanchis V, Magan N (2004) Environmental conditions affecting mycotoxins. In: Magan N, Olsen M (eds) Mycotoxins in food: detection and control, vol 103. Woodhead Publishing, Cambridge, pp 174–189
Santos R, Stevenson A, de Carvalho CCCR, Grant IR, Hallsworth JE (2015) Extraordinary solute stress tolerance contributes to the environmental tenacity of mycobacteria. Environ Microbiol Rep (in press)
Sauer T, Galinski EA (1998) Bacterial milking: a novel bioprocess for production of compatible solutes. Biotechnol Bioeng 57:306–313
Sautour M, Sixt N, Dalle F, L’Ollivier C, Calinon C, Fourquenet V, Thibaut C, Jury H, Lafon I, Aho S, Couillault G, Vagner O, Cuisenier B, Besancenot JP, Caillot D, Bonnin A (2007) Prospective survey of indoor fungal contamination in hospital during a period of building construction. J Hosp Infect 67:367–373
Schmidt-Heydt M, Magan N, Geisen R (2008) Stress induction of mycotoxin biosynthesis genes by abiotic factors. FEMS Microbiol Lett 284:142–149
Slater SJ, Ho C, Taddeo FJ, Kelly MB, Stubbs CD (1993) Contribution of hydrogen-bonding to lipid lipid interactions in membranes and the role of lipid order—effects of cholesterol, increased phospholipid unsaturation, and ethanol. Biochem 32:3714–3721
Stevenson A, Hallsworth JE (2014) Water and temperature relations of soil Actinobacteria. Environ Microbiol Rep 6:744–755
Stevenson A, Cray JA, Williams JP, Santos R, Sahay R, Neuenkirchen N, McClure CD, Grant IR, Houghton JDR, Quinn JP, Timson DJ, Patil SV, Singhal RS, Anton J, Dijksterhuis J, Hocking AD, Lievens B, Rangel DEN, Voytek MA, Gunde-Cimerman N, Oren A, Timmis KN, McGenity TJ, Hallsworth JE (2015a) Is there a common water-activity limit for the three domains of life? ISME J 9:1333–1351. doi:10.1038/ismej.2014.219
Stevenson A, Burkhardt J, Cockell CS, Cray JA, Dijksterhuis J, Fox-Powell M, Kee TP, Kminek G, McGenity TJ, Timmis KN, Timson DJ, Voytek MA, Westall F, Yakimov MM, Hallsworth JE (2015b) Multiplication of microbes below 0.690 water activity: implications for terrestrial and extraterrestrial life. Environ Microbiol 17:257–277
Suryawanshi RK, Patil CD, Borase HP, Narkhede CP, Stevenson A, Hallsworth JE, Patil SV (2015) Towards an understanding of bacterial metabolites prodigiosin and violacein and their potential for use in commercial sunscreens. Int J Cosmet Sci 37:98–107
Sutherland FC, Lages F, Lucas C, Luyten K, Albertyn J, Hohmann S, Prior BA, Kilian SG (1997) Characteristics of Fps1-dependent and -independent glycerol transport in Saccharomyces cerevisiae. J Bacteriol 179:7790–7795
Toh TH, Kayingo G, van der Merwe J, Kilian SG, Hallsworth JE, Hohmann S, Prior BA (2001) Implications of FPS1 deletion and membrane ergosterol content for glycerol efflux from Saccharomyces cerevisiae. FEMS Yeast Res 1:205–211
Treberg JR, Speers-Roesch B, Piermarini PM, Ip YK, Ballantyne JS, Driedzic WR (2006) The accumulation of methylamine counteracting solutes in elasmobranchs with differing levels of urea: a comparison of marine and freshwater species. J Exp Biol 209:860–870
Tregoning GS, Kempher ML, Jung DO, Samarkin VA, Joye SB, Madigan MT (2015) A halophilic bacterium inhabiting the warm, CaCl2-rich brine of the perennially ice-covered Lake Vanda, McMurdo Dry Valleys, Antarctica. Appl Environ Microbiol 81:1988–1995
Trevisol ET, Panek AD, Mannarino SC, Eleutherio ECA (2011) The effect of trehalose on the fermentation performance of aged cells of Saccharomyces cerevisiae. Appl Microbiol Biotechnol 90:697–704
Washabaugh MW, Collins KD (1986) The systematic characterization by aqueous column chromatography of solutes which affect protein stability. J Biol Chem 261:12477–12485
Wheeler KA, Hocking AD, Pitt JI (1988) Water relations of some Aspergillus species isolated from dried fish. Trans Br Mycol Soc 91:631–637
Wheeler KA, Hurdman BF, Pitt JI (1991) Influence of pH on the growth of some toxigenic species of Aspergillus, Penicillium and Fusarium. Int J Food Microbiol 12:141–149
Williams JP, Hallsworth JE (2009) Limits of life in hostile environments; no limits to biosphere function? Environ Microbiol 11:3292–3308
Winston PW, Bates PS (1960) Saturated salt solutions for the control of humidity in biological research. Ecology 41:232–237
Wyatt TT, Golovina EA, van Leeuwen MR, Hallsworth JE, Wösten HAB, Dijksterhuis J (2015a) A decrease in bulk water and mannitol and accumulation of trehalose and trehalose-based oligosaccharides define a two-stage maturation process towards extreme stress resistance in ascospores of Neosartorya fischeri (Aspergillus fischeri). Environ Microbiol 17:283–294
Wyatt TT, van Leeuwen MR, Gerwig GJ, Golovina EA, Hoekstra FA, Kuenstner EJ, Palumbo EA, Snyder NL, Visagie C, Verkennis A, Hallsworth JE, Kamerling JP, Wösten HAB, Dijksterhuis J (2015b) Functionality and prevalence of trehalose-based oligosaccharides as novel compatible solutes in ascospores of Neosartorya fischeri (Aspergillus fischeri) and other fungi. Environ Microbiol 17:395–411
Yakimov MM, Lo Cono V, La Spada G, Bortoluzzi G, Messina E, Smedile F, Werner J, Teeling H, Borghini M, Ferrer M, Cray JA, Hallsworth JE, Golyshin PN, Giuliano L (2015) Microbial community of seawater-brine interface of the deep-sea brine Lake Kryos as revealed by recovery of mRNA are active below the chaotropicity limit of life. Environ Microbiol 17:364–382
Yancey PH, Burg MB (1990) Counteracting effects of urea and betaine in mammalian cells in culture. Am J Physiol 258:R198–R204
Zhang Z, Liew CW, Handy DE, Zhang Y, Leopold JA, Hu J, Guo L, Kulkarni RN, Loscalzo J, Stanton RC (2010) High glucose inhibits glucose-6-phosphate dehydrogenase, leading to increased oxidative stress and β-cell apoptosis. FASEB J 24:1497–1505
Zou Q, Bennion BJ, Daggett V, Murphy KP (2002) The molecular mechanism of stabilization of proteins by TMAO and its ability to counteract the effects of urea. J Am Chem Soc 124:1192–1202
Acknowledgments
The authors wish to thank Philip Ball for scientifically stimulating discussions. Funding was supplied by the University of Stellenbosch (South Africa) and EU grants MIFRIEND and BIODEEP who supported JE Hallsworth and FL Alves; the Department of Agriculture and Rural Development (Northern Ireland) who supported A Stevenson; and grants from the São Paulo Research Foundation (FAPESP) of Brazil (#2010/06374-1, #2013/50518-6 and #2014/01229-4) and the Brazilian National Council for Scientific and Technological Development (CNPq) (#PQ2 302312/2011-0 and #PQ1D 308436/2014-8) which supported DEN Rangel.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. E. N. Rangel.
This article is part of the Special Issue “Fungal Stress Responses”.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Lima Alves, F., Stevenson, A., Baxter, E. et al. Concomitant osmotic and chaotropicity-induced stresses in Aspergillus wentii: compatible solutes determine the biotic window. Curr Genet 61, 457–477 (2015). https://doi.org/10.1007/s00294-015-0496-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-015-0496-8