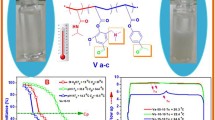
Abstract
New monomers from the acrylate group have been synthesized in facile methods. The first was fabricated to demonstrate the pH-responsive behavior and synthesized from p-cresol in two steps. The final product was named 2-((diethylamino) methyl)-4-methyl phenyl acrylate) (DEAMCA). The other was fabricated to achieve functionality; it was synthesized from vanillin as a sustainable material and named 4-formyl-2-methoxyphenylacrylate (VA). These monomers were used to perform a new series of thermo-pH functional terpolymers with N-isopropylacrylamide with different molar ratios of DEAMCA (5, 10, and 15%) 10 mol% of VA. Terpolymer with 10 mol% DEAMCA has been modified by the post-reaction with serine and valine. All compounds have been chemically evaluated using 1H, 13C NMR, and FTIR. Polymers were physically investigated by GPC, DSC, TGA, and contact angle. A study of the phase separation temperature (Tc) and the cloud point has been implemented via UV–vis spectroscopy; micro-DSC has also been used for recording the (Tc) of polymer solution at the onset point of the thermogram. The transition temperature has also been measured in Hofmeister anions (kosmotropic and chaotropic) using turbidimetric method by UV–vis spectroscopy. The new terpolymers have an interesting structure for bio-based polymers for bio-separation and drug delivery.
Graphical abstract

















Similar content being viewed by others
References
Liang H, Tong S, Yu W, Changhao F, Feng G, Michael JS (2021) Recent advances in stimuli-responsive polymers for sensing and actuation. J Macromol Sci A. https://doi.org/10.1080/10601325.2021.1960172
Shan L, Pratik SK, Muxuan Y, Naifu S, Linrui D, Yimin M, Weinan X (2020) Intimately bonded 2D materials and responsive polymer brushes for adaptive nanocomposites. Polymer 210:123033. https://doi.org/10.1016/j.polymer.2020.123033
Abdelaty MSA (2018) Preparation and characterization of new environmental functional polymers based on vanillin and N-isopropylacrylamide for post polymerization. J Polym Environ 26:636–646. https://doi.org/10.1007/s10924-017-0960-2
Abdelaty MSA (2018) Poly(N-isopropylacrylamide-co-2-((diethylamino)methyl)-4 formyl-6-methoxyphenylacrylate) environmental functional copolymers: synthesis, characterizations, and grafting with amino acids. Biomolecules 8:138. https://doi.org/10.3390/biom8040138
Abdelaty MSA, Kuckling D (2021) Altering of lower critical solution temperature of environmentally responsive poly (N-isopropylacrylamide-co-acrylic acid-co-vanillin acrylate) affected by acrylic acid, vanillin acrylate, and post-polymerization modification. Colloid Polym Sci 299:1617–1629. https://doi.org/10.1007/s00396-021-04882-x
Huang HJ, Tsai YL, Lin SH, Shan-Hui H (2019) Smart polymers for cell therapy and precision medicine. J Biomed Sci 26:73. https://doi.org/10.1186/s12929-019-0571-4
Jingcheng L, Reddy VS, Jayathilaka WADM, Chinnappan A, Ramakrishna S, Ghosh R (2021) Intelligent polymers, fibers and applications. Polymers 13:1427. https://doi.org/10.3390/polym13091427
Abdelaty MSA (2018) Environmental functional photo-cross-linked hydrogel bilayer thin films from vanillin (part 2): temperature responsive layer A, functional, temperature and pH layer B. Polym Bull 11:4837–4858. https://doi.org/10.1007/s00289-018-2297-y
HaqAsif A, Karnakar RR, Sreeharsha N et al (2021) pH and salt responsive hydrogel based on guar gum as a renewable material for delivery of curcumin: a natural anti-cancer drug. J Polym Environ 29:1978–1989. https://doi.org/10.1007/s10924-020-01934-1
Abdelaty MSA (2018) Preparation and characterization of environmental functional poly(styrene-Co-2-[(diethylamino)methyl]-4-formyl-6-methoxy-phenyl acrylate) copolymers for amino acid post polymerization. Open J Polym Chem 8:41–55. https://doi.org/10.4236/ojpchem.2018.83005
Zhang Y, Cao X, Liang T et al (2019) Acid/light dual-responsive biodegradable polymeric nanocarriers for efficient intracellular drug delivery. Polym Bull 76:1775–1792. https://doi.org/10.1007/s00289-018-2470-3
Roberto B, Federico A, Mattia PC (2019) Mechanics of innovative responsive polymers. Mech Res Commun 100:103403. https://doi.org/10.1016/j.mechrescom.2019.103403
Xinyi Z, Chen Y, Yinghao J, Hongbing D, Yumin D, Xiaowen S (2022) Ion-responsive chitosan hydrogel actuator inspired by carrot wood seedpod. Carbohydr Polym 276:118759. https://doi.org/10.1016/j.carbpol.2021.118759
Jun G, Evgenios N, Thomas JC, Ramin EB, Richard NZ (2012) Drug release from electric-field-responsive nanoparticles. ACS Nano 6:227–233. https://doi.org/10.1021/nn203430m
Theodore M, Maria V (2017) Field responsive materials: photo-, electro-, magnetic- and ultrasound-sensitive polymers. Polym Chem 8:74–96. https://doi.org/10.1039/C6PY01455K
Lanzalaco S, Armelin E (2017) Poly(N-isopropylacrylamide) and copolymers: a review on recent progresses in biomedical applications. Gels 3(4):36. https://doi.org/10.3390/gels3040036
Abdelaty MSA (2021) Poly(N-isopropylacrylamide-co-2-((diethylamino)methyl)-4-methylphenyl acrylate) thermo-ph responsive copolymer: trend in the lower critical solution temperature optimization of poly (N-isopropyylacrylamide). J Polym Res 28:213. https://doi.org/10.1007/s10965-021-02574-2
Bischofberger I, Trappe V (2015) New aspects in the phase behaviour of poly-N-isopropyl acrylamide: systematic temperature dependent shrinking of PNiPAM assemblies well beyond the LCST. Sci Rep 5:15520. https://doi.org/10.1038/srep15520
Abdelaty MSA (2019) Influence of vanillin acrylate and 4 acetylphenyl acrylate hydrophobic functional monomers on phase separation of N isopropylacrylamide environmental terpolymer: fabrication and characterization. Polym Bull. https://doi.org/10.1007/s00289-019-02890-0
Francoise MW, Ottaviani MF, Stefan HB, Wenseng P, Garcia-Garibay M, Nicholas JT (1993) Phase separation of poly(N-isopropylacrylamide) in water: a spectroscopic study of a polymer tagged with a fluorescent dye and a spin label. J Phys Chem 97(49):12998–13005. https://doi.org/10.1021/j100151a058
Abdelaty MSA (2021) Trends in the phase separation temperature optimization of a functional and thermo-pH responsive terpolymer of poly (N-isopropylacrylamide-co-N-(2-(dimethylamino)ethyl) acrylamide-co-vanillin acrylate). J Polym Environ. https://doi.org/10.1007/s10924-021-02096-4
Avraham H, Martin K, FranÅoise MW (2015) Poly(N-isopropylacrylamide) phase diagrams: fifty years of research. Angew Chem Int Ed 54:15342–15367. https://doi.org/10.1002/anie.201506663
Abdelaty MSA (2021) A facile method for the preparation of hydrophilic-hydrophobic functional thermo-pH responsive terpolymers based on poly(NIPAAm-co-DMAA-co-DMAMVA) and post-polymerization. J Polym Environ. https://doi.org/10.1007/s10924-021-02117-2
Wei T, Junqing W, Wolfgang JP, Omid CF, Jinjun S (2019) Nanobuffering of pH-responsive polymers: a known but sometimes overlooked phenomenon and its biological applications. ACS Nano 13(5):4876–4882. https://doi.org/10.1021/acsnano.9b01696
Fabrice O, Mohamad T, Noureddine L, Émilie G, Denis M, Abdelhamid E (2021) pH-sensitive polymers: classification and some fine potential applications. Polym Adv Technol 32(4):1455–1484. https://doi.org/10.1002/pat.5230
Kocak G, Tuncer C, Bütün V (2017) pH-responsive polymers. Polym Chem 8:144–176
M’Bareck C, S’Id EC, Kheribech A et al (2020) Synthesis of polyacrylonitrile-co-sodium methallyl sulfonate copolymer (AN69) and polyacrylic acid (PAA) membranes for the removal of methylene blue from water. Polym Bull 77:5451–5467. https://doi.org/10.1007/s00289-019-03024-2
Awadallah-F A, Sobhy A (2018) Dual function of cellulose triacetate–graft–polymethacrylic acid films for dyes removal and for high-dose radiation dosimetry. J Polym Environ 26:2758–2772. https://doi.org/10.1007/s10924-017-1163-6
Deirram N, Zhang C, Kermaniyan SS, Such GK (2019) (2019) pH-responsive polymer nanoparticles for drug delivery. Macromol Rapid Commun 40:1800917. https://doi.org/10.1002/marc.201800917
Tina C, Vinod KS (2014) Modified chitosan-based, pH-responsive membrane for protein separation. RSC Adv 4:53245–53252. https://doi.org/10.1039/C4RA05314A
da Silva LBJ, Oréfice RL (2014) Synthesis and electromechanical actuation of a temperature, pH, and electrically responsive hydrogel. J Polym Res 21:466. https://doi.org/10.1007/s10965-014-0466-8
Khan MI, Mukherjee K, Shoukat R et al (2017) A review on pH sensitive materials for sensors and detection methods. Microsyst Technol 23:4391–4404. https://doi.org/10.1007/s00542-017-3495-5
Tarabukina E, Harabagiu V, Fundueanu G et al (2021) Thermo and pH-responsive copolymer of N-isopropylacrylamide with acryloylvaline: synthesis and properties in aqueous solutions. J Polym Res 28:155. https://doi.org/10.1007/s10965-021-02515-z
Gao L, Sun Y, Zhang W et al (2015) Mechanical behavior of a terpolymer-based pH- and temperature-responsive hydrogel. J Polym Res 22:221. https://doi.org/10.1007/s10965-015-0858-4
Meléndez-Ortiz HI, Bucio E (2008) Radiation synthesis of a thermo-pH responsive binary graft copolymer (PP-g-DMAEMA)-g-NIPAAm by a two step method. Polym Bull 61:619–629. https://doi.org/10.1007/s00289-008-0982-y
Jin X, Wang Q, Sun J et al (2017) Dual temperature- and pH-responsive ibuprofen delivery from poly(N-isopropylacrylamide-co-acrylic acid) nanoparticles and their fractal features. Polym Bull 74:3619–3638. https://doi.org/10.1007/s00289-017-1915-4
Patil AS, Gadad AP, Hiremath RD et al (2018) Exploration of the effect of chitosan and crosslinking agent concentration on the properties of dual responsive chitosan-g-Poly (N-isopropylacrylamide) co-polymeric particles. J Polym Environ 26:596–606. https://doi.org/10.1007/s10924-017-0971-z
Yuting L, Hongxiang Z, Lei W, Hui H, Shuangfei W (2020) Biocompatible smart cellulose nanofibres for sustained drug release via pH and temperature dual-responsive mechanism. Carbohydr Polym 249:116876. https://doi.org/10.1016/j.carbpol.2020.116876
Abdelaty MSA, Kuckling D (2016) Synthesis and characterization of new functional photo cross-linkable smart polymers containing vanillin derivatives. Gels 2:1–13. https://doi.org/10.3390/gels2010003
Abdelaty MSA (2019) Layer by layer photo-cross-linked environmental functional hydrogel thin films based on vanillin: part 3. J Polym Environ. https://doi.org/10.1007/s10924-019-01421-2
Maxence F, Bernard B, Sylvain C (2015) Vanillin, a key-intermediate of biobased polymers. Eur Polym J 68:488–502. https://doi.org/10.1016/j.eurpolymj.2015.03.050
Abdelaty MSA (2018) Environmental functional photo-cross-linked hydrogel bilayer thin films from vanillin. J Polym Environ 26:2243–2256. https://doi.org/10.1007/s10924-017-1126-y
Robin K, Volkmar MS, Carsten C, Dominik M, Detlef S, Jens T (2021) Electrochemical synthesis of biobased polymers and polymer building blocks from vanillin. RSC Adv 11:8970–8985. https://doi.org/10.1039/D1RA00649E
Maxence F, Emilie D, Vincent B, Rémi A, Sylvain C, Bernard B (2014) Vanillin, a promising biobased building-block for monomer synthesis. Green Chem 16:1987–1998. https://doi.org/10.1039/C3GC42613K
Fiege H (2000) Cresols and Xylenols. In: Ullmann’s encyclopedia of industrial chemistry. Weinheim: Wiley-VCH. https://doi.org/10.1002/14356007.a08_025
Chutayothin P, Ishida H (2011) Polymerization of p-cresol, formaldehyde, and piperazine and structure of monofunctional benzoxazine-derived oligomers. Polymer 52:3897–3904. https://doi.org/10.1016/j.polymer.2011.07.006
Sangrama KS, Wei L, Lynne AS, Jayant K, Ashok LC (2002) Biocatalytic polymerization of p-cresol: an in-situ NMR approach to understand the coupling mechanism. Macromolecules 35(27):9990–9998. https://doi.org/10.1021/ma021142b
Geng C, Wang S, Wang H (2021) Recent advances in thermoresponsive OEGylated poly(amino acid)s. Polymers 13:1813. https://doi.org/10.3390/polym13111813
Edgar M, Hatice M, Patrick T (2021) Synthesis and post-polymerization modification of poly(N-(4-Vinylphenyl)sulfonamide)s. Macromol Rapid Commun 42:2100063. https://doi.org/10.1002/marc.202100063
Emma RL, Brisson ZX, George VF, Luke AC (2017) Versatile synthesis of amino acid functional polymers without protection group chemistry. Biomacromol 18(1):272–280. https://doi.org/10.1021/acs.biomac.6b01618
Hofmeister F (1888) Zur Lehre von der Wirkung der Salze. Archiv f experiment Pathol u Pharmakol 25:1–30. https://doi.org/10.1007/BF01838161
Von Hippel PH, Schleich T (1969) Ion effects on the solution structure of biological macromolecules. Acc Chem Res 2:257. https://doi.org/10.1021/ar50021a001
Zhang Y, Cremer PS (2006) Interactions between macromolecules and ions: the Hofmeister series. Curr Opin Chem Biology 10:658. https://doi.org/10.1016/j.cbpa.2006.09.020
Kunz W, Lo Nostro P, Ninham BW (2004) The present state of affairs with Hofmeister effects. Curr Opin Colloid Interface Sci 9:1–18. https://doi.org/10.1016/j.cocis.2004.05.004
Zhang Y, Furyk S, Sagle LB, Cho Y, Bergbreiter DE, Cremer PS (2007) Effects of Hofmeister anions on the LCST of PNIPAM as a function of molecular weight. J Phys Chem C 111:8916. https://doi.org/10.1021/jp0690603
Abdelaty MSA (2020) The effect hydrophilic/hydrophobic interaction of 2-((dimethylamino)methyl)-4-formyl-6 methoxyphenyl acrylate and 4-acetylphenyl acrylate monomers on the phase transition temperature of N-isopropylacrylamide terpolymers. J Polym Environ 28:2584–2598. https://doi.org/10.1007/s10924-020-01790-z
Feil H, Bae YH, Feijen J, Kim SW (1993) Effect of comonomer hydrophilicity and ionisation on the lower critical solution temperature of N-isopropylacrylamide copolymers. Macromolecules 26:2496–2500
Van Dyke JD, Kasperski KL (1993) Thermogravimetric study of polyacrylamide with evolved gas analysis. J Polym Sci Part A Polym Chem 31:1807
Law KY (2014) Definitions for hydrophilicity, hydrophobicity, and superhydrophobicity: getting the basics right. J Phys Chem Lett 20:686–688. https://doi.org/10.1021/jz402762h
Abdelaty MSA (2020) The Influence of vanillin acrylate derivative on the phase separation temperature of environmental photo-cross-linked N-isopropylacrylamide copolymer and hydrogel thin films. J Polym Environ 28:2599–2615. https://doi.org/10.1007/s10924-020-01793-w
Schild HG (1992) Poly(N-isopropylacrylamide): experiment, theory and application. Prog Polym Sci 17:163–249
Acknowledgements
The author would like to thank the University of Paderborn and DAAD Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The author declares that there are no conflicts of interest regarding this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abdelaty, M.S.A. Comprehensive study of the phase transition temperature of poly (NIPAAm-co-DEAMCA-co-VA) terpolymers, post-serine and valine: thermal/pH and Hofmeister anions. Polym. Bull. 80, 6051–6078 (2023). https://doi.org/10.1007/s00289-022-04337-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04337-5