Abstract
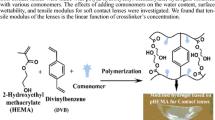
In this study, different ratios of poly (hydroxyethyl methacrylate-co-hydroxyethyl acrylate) hydrogels (HEMA-co-HEA), corresponding to (100/0, 70/30, 60/40, 50/50, 40/60, 30/70, and 0/100) were successfully prepared by free radical polymerization leading to tunable drug release materials for therapeutic soft contact lenses (SCLs) applications. The microstructures of the materials were investigated using Fourier transform infrared (FTIR) spectroscopy and scanning electron microscopy (SEM), and their thermal properties were examined with differential scanning calorimetry (DSC) and thermogravimetric (TGA) analyses. The results showed that the presence of HEA in the hydrogels matrix improved the materials’ microstructure and enhanced the swelling capacity in a 0.9% NaCl solution (pH = 5.5) at 37 °C. Furthermore, in vitro study of the loading and release of acetazolamide, as a model drug, showed a non-Fickian drug release behavior for all the studied hydrogels, whereas the highest drug release/loading capacity was observed for the hydrogel ratio HEMA/HEA of 40/60. Therefore, it can be concluded that the corresponding hydrogel is a promising curative SCLs material for a sustainable drug release application.












Similar content being viewed by others
References
Bounabi L et al (2016) Development of poly (2-hydroxyethyl methacrylate)/clay composites as drug delivery systems of paracetamol. J Drug Deliv Sci Technol 33:58–65
Cesteros LC, Isasi JR, Katime I (1993) Hydrogen bonding in poly (4-vinylpyridine)/poly (vinyl acetate-co-vinyl alcohol) blends. Infrared Study Macromol 26(26):7256–7262
Coleman MM et al (1988) Hydrogen bonding in polymer blends. 1. FTIR studies of urethane-ether blends. Macromolecules 21(1):59–65
ElMiloudi K et al (2005) FT-IR Spectroscopy and Hydrogen Bonding Interactions in poly (styrene-co-methacrylic acid)/poly (styrene-co-4-vinyl pyridine) Blends. Macromol Symp 230:39–50
Bouslah N, Hammachin R, Amrani F (1999) Study of the compatibility of poly [styrene-co-(cinnamic acid)]/poly [(ethyl methacrylate)-co-(2-dimethylaminoethyl methacrylate)] blends. Macromol Chem Phys 200(4):678–682
Haddadine-Rahmoun N et al (2008) Interpolymer complexation and thermal behaviour of poly (styrene-co-maleic acid)/poly (vinyl pyrrolidone) mixtures. Thermochim Acta 475(1–2):25–32
Meaurio E, Cesteros LC, Katime I (1997) FTIR study of hydrogen bonding of blends of poly (mono n-alkyl itaconates) with poly (N, N-dimethylacrylamide) and poly (ethyloxazoline). Macromolecules 30(16):4567–4573
Ferreira L, Vidal MM, Gil MH (2000) Evaluation of poly (2-hydroxyethyl methacrylate) gels as drug delivery systems at different pH values. Int J Pharm 194(2):169–180
Huang CF et al (2006) Syntheses and specific interactions of poly (hydroxyethyl methacrylate-b-vinyl pyrrolidone) diblock copolymers and comparisons with their corresponding miscible blend systems. Polymer 47(20):7060–7069
Faria MDG, Teixeira-Dias JJC, Fausto R (1991) Conformational stability for methyl acrylate: a vibrational spectroscopic and ab initio MO study. Vib Spectrosc 2(1):43–60
Khutoryanskaya OV et al (2008) Designing temperature-responsive biocompatible copolymers and hydrogels based on 2-hydroxyethyl (meth) acrylates. Biomacromol 9(12):3353–3361
Dursch TJ et al (2014) Water-soluble drug partitioning and adsorption in HEMA/MAA hydrogels. Biomaterials 35(2):620–629
Pradas MM et al (2001) Porous poly (2-hydroxyethyl acrylate) hydrogels. Polymer 42(10):4667–4674
Bevington JC, Melville HW, Taylor RP (1954) The termination reaction in radical polymerizations. Polymerizations of methyl methacrylate and styrene at 25°. J Polym Sci 12(1):449–459
Al-Musa S, Fara DA, Badwan A (1999) Evaluation of parameters involved in preparation and release of drug loaded in crosslinked matrices of alginate. J Control Release 57(3):223–232
Nobuhiko Y et al (1993) Regulated release of drug microspheres from inflammation responsive degradable matrices of crosslinked hyaluronic acid. J Control Release 25(1–2):133–143
Andrade-Vivero P et al (2007) Improving the loading and release of NSAIDs from pHEMA hydrogels by copolymerization with functionalized monomers. J Pharm Sci 96(4):802–813
Di Marco G, Lanza M, Pieruccini M (1994) Dynamical mechanical measurements in dry PHEMA and its hydrogels. Il Nuovo Cimento D 16(7):849–854
Andreopoulos AG (1989) Properties of poly (2-hydroxyethyl acrylate) networks. Biomaterials 10(2):101–104
Ritger PL, Peppas NA (1987) A simple equation for description of solute release I fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J Control Release 5(1):23–36
Peppas N et al (2000) Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm 50(1):27–46
Frisch H (1980) Sorption and transport in glassy polymers–a review. Polym Eng Sci 20(1):2–13
Peppas N (1985) Analysis of fickian and non-fickian drug release from polymers. Pharm Acta Helv 60(4):110–111
Peppas NA, Franson NM (1983) The swelling interface number as a criterion for prediction of diffusional solute release mechanisms in swellable polymers. J Polym Sci Polym Phys Edit 21(6):983–997
Lee PI (1985) Kinetics of drug release from hydrogel matrices. J Control Release 2:277–288
Jacques C, Hopfenberg H, Stannett V (1974) Permeability of plastic films and coatings. In: Hopfenberg HB (ed) Permeability of plastic films and coatings. Plenum, New York, p 73
Miao J, Tsige M, Taylor PL (2017) Generalized model for the diffusion of solvents in glassy polymers: from fickian to super case II. J Chem Phys 147(4):044904
Desai S, Simonelli A, Higuchi W (1965) Investigation of factors influencing release of solid drug dispersed in inert matrices. J Pharm Sci 54(10):1459–1464
Desai SJ et al (1966) Investigation of factors influencing release of solid drug dispersed in inert matrices III. Quantitative studies involving the polyethylene plastic matrix. J Pharm Sci 55(11):1230–1234
George KA et al (2004) Investigation into the diffusion of water into HEMA-co-MOEP Hydrogels. Biomacromol 5(4):1194–1199
Tomić SL et al (2007) Swelling and drug release behavior of poly (2-hydroxyethyl methacrylate/itaconic acid) copolymeric hydrogels obtained by gamma irradiation. Radiat Phys Chem 76(5):801–810
Katime I et al (1999) Theophylline release from poly (acrylic acid-co-acrylamide) hydrogels. Polym Test 18(7):559–566
Katime I, Sáez V, Hernáez E (2005) Nafcillin release from poly (acrylic acid-co-methyl methacrylate) hydrogels. Polym Bull 55(6):403–409
Chapman JM, Cheeks L, Green K (1992) Drug interaction with intraocular lenses of different materials. J Cataract Refract Surg 18(5):456–459
Grass GM, Cobby J, Makoid MC (1984) Ocular delivery of pilocarpine from erodible matrices. J Pharm Sci 73(5):618–621
Saettone MF et al (1984) Vehicle effects in ophthalmic bioavailability: An evaluation of polymeric inserts containing pilocarpine 2. J Pharm Pharmacol 36(4):229–234
Marles RJ et al (2011) United states pharmacopeia safety evaluation of spirulina. Crit Rev Food Sci Nutr 51(7):593–604
Lowman AM (2004) Biomaterials in drug delivery. In: Shi D (ed) Biomedical devices and their applications. Springer, Berlin, Heidelberg, pp 1–31
Flynn GL, Yalkowsky SH, Roseman TJ (1974) Mass transport phenomena and models: theoretical concepts. J Pharm Sci 63(4):479–510
Hackl EV et al (2015) Evaluation of water properties in HEA–HEMA hydrogels swollen in aqueous-PEG solutions using thermoanalytical techniques. J Therm Anal Calorim 121(1):335–345
Gupta P, Vermani K, Garg S (2002) Hydrogels: from controlled release to pH-responsive drug delivery. Drug Discov Today 7(10):569–579
Korsmeyer RW, Peppas NA (1984) Solute and penetrant diffusion in swellable polymers. III. Drug release from glassy poly (HEMA-co-NVP) copolymers. J Control Release 1(2):89–98
Acknowledgements
This work was supported by a special grant from the DGRSDT, Direction Générale de la Recherche Scientifique et du développement Technologique (Algeria) which is gratefully acknowledged for the financial support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oucif, A., Haddadine, N., Zakia, D. et al. Poly (hydroxyethyl methacrylate-co-hydroxyethyl acrylate) soft contact lenses for acetazolamide release. Polym. Bull. 79, 1535–1554 (2022). https://doi.org/10.1007/s00289-021-03573-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03573-5