Abstract
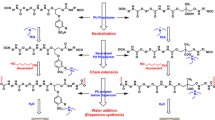
An easily peelable coating was prepared using silane-terminated polyurethane dispersions (SPUDs) and UiO-66 catalyst (a zirconium(IV)-based metal–organic framework), to capture and decompose the nerve agent simulant, methyl paraoxon (MPO), at room temperature. SPUDs were used as the binder. The peel strength of the SPUD film containing UiO-66 decreased with increasing UiO-66 content, and the film with 40 wt% UiO-66 could not be easily peeled off. In contrast, the SPUD/PVB/UiO-66 peelable coating film could be easily peeled off. With increasing UiO-66 content, the Young’s moduli of the SPUD/UiO-66 and SPUD/PVB/UiO-66 coating films gradually increased, while the elongation decreased. The increase in the glass transition temperature was less than approximately 5%, depending on the UiO-66 content of the SPUD/UiO-66 film. Two peaks of tan δ appeared for the SPUD/PVB/UiO-66 coating film. As the UiO-66 content increased, the second peak shifted to the right. This could be attributed to the bond strength between the mixed polymeric binder and the nanoparticles. Furthermore, MPO decomposition by the SPUD/PVB/UiO-66 coating film increased with increasing UiO-66 content. These findings suggest the possibility of the development of a peelable coating film for the capture and decomposition of MPO.













Similar content being viewed by others
Abbreviations
- GPC:
-
Gel permeation chromatography
- Tg:
-
Glass transition temperature
- MOF:
-
Metal–organic framework
- MPO:
-
Methyl paraoxon
- PVB:
-
Poly(vinyl butyral)
- SPUD:
-
Silane-terminated polyurethane dispersion
References
Kreno LE, Leong K, Farha OK et al (2012) Metal–organic framework materials as chemical sensors. Chem Rev 112:1105–1125. https://doi.org/10.1021/cr200324t
Lee J, Farha OK, Roberts J et al (2009) Metal–organic framework materials as catalysts. Chem Soc Rev 38:1450–1459. https://doi.org/10.1039/B807080F
Liu J, Chen L, Cui H et al (2014) Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chem Soc Rev 43:6011–6061. https://doi.org/10.1039/C4CS00094C
Li J-R, Sculley J, Zhou H-C (2012) Metal–organic frameworks for separations. Chem Rev 112:869–932. https://doi.org/10.1021/cr200190s
Mason JA, Veenstra M, Long JR (2014) Evaluating metal–organic frameworks for natural gas storage. Chem Sci 5:32–51. https://doi.org/10.1039/C3SC52633J
Della Rocca J, Liu D, Lin W (2011) Nanoscale metal–organic frameworks for biomedical imaging and drug delivery. Acc Chem Res 44:957–968. https://doi.org/10.1021/ar200028a
Moghadam PZ, Fairen-Jimenez D, Snurr RQ (2016) Efficient identification of hydrophobic MOFs: application in the capture of toxic industrial chemicals. J Mater Chem A 4:529–536. https://doi.org/10.1039/C5TA06472D
Cavka JH, Jakobsen S, Olsbye U et al (2008) A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J Am Chem Soc 130:13850–13851. https://doi.org/10.1021/ja8057953
Schaate A, Roy P, Godt A et al (2011) Modulated synthesis of Zr-based metal–organic frameworks: from nano to single crystals. Chem Eur J 17:6643–6651. https://doi.org/10.1002/chem.201003211
Liu X, Shen Z-Q, Xiong H-H et al (2015) Hierarchical porous materials based on nanoscale metal–organic frameworks dominated with permanent interparticle porosity. Microporous Mesoporous Mater 204:25–33. https://doi.org/10.1016/j.micromeso.2014.11.005
Katz MJ, Brown ZJ, Colón YJ et al (2013) A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem Commun 49:9449. https://doi.org/10.1039/c3cc46105j
Cho KY, Seo JY, Kim H-J et al (2019) Facile control of defect site density and particle size of UiO-66 for enhanced hydrolysis rates: insights into feasibility of Zr(IV)-based metal–organic framework (MOF) catalysts. Appl Catal B Environ 245:635–647. https://doi.org/10.1016/j.apcatb.2019.01.033
Natali I, Carretti E, Angelova L et al (2011) Structural and mechanical properties of “peelable” organoaqueous dispersions with partially hydrolyzed poly(vinyl acetate)-borate networks: applications to cleaning painted surfaces. Langmuir 27:13226–13235. https://doi.org/10.1021/la2015786
Lewandowski K, Krepski LR, Mickus DE (2004) Dry-peelable temporary protective coatings from waterborne self-crosslinkable sulfourethane-silanol dispersions. J Appl Polym Sci 91:1443–1449. https://doi.org/10.1002/app.13316
Shirai M, Bamba T, Hayashi K et al (2000) Sheet for protecting paint film
Ozeki K, Wada T, Ito K et al (1982) Peelable film-forming urethane/isocyanate paints
Rahman MM, Kim H-D, Lee W-K (2009) Properties of waterborne polyurethane adhesives: effect of chain extender and polyol content. J Adhes Sci Technol 23:177–193. https://doi.org/10.1163/156856108X344667
Lei L, Zhong L, Lin X et al (2014) Synthesis and characterization of waterborne polyurethane dispersions with different chain extenders for potential application in waterborne ink. Chem Eng J 253:518–525. https://doi.org/10.1016/j.cej.2014.05.044
Li S, Kong X, Feng S (2015) Preparation of uniform poly(urea-siloxane) microspheres through precipitation polymerization. RSC Adv 5:90313–90320. https://doi.org/10.1039/C5RA18140B
Hou Z, Qu W, Kan C (2015) Synthesis and properties of triethoxysilane-terminated anionic polyurethane and its waterborne dispersions. J Polym Res 22:111. https://doi.org/10.1007/s10965-015-0757-8
Kim K, Seo JY, Baek K-Y et al (2019) Metal–organic framework (UiO-66)-dispersed polyurethane composite films for the decontamination of methyl paraoxon. Polym Int. https://doi.org/10.1002/pi.5856
Mondloch JE, Katz MJ, Isley WC III et al (2015) Destruction of chemical warfare agents using metal–organic frameworks. Nat Mater 14:512–516. https://doi.org/10.1038/nmat4238
Katz MJ, Mondloch JE, Totten RK et al (2014) Simple and compelling biomimetic metal–organic framework catalyst for the degradation of nerve agent simulants. Angew Chem 126:507–511. https://doi.org/10.1002/ange.201307520
Katz MJ, Moon S-Y, Mondloch JE et al (2015) Exploiting parameter space in MOFs: a 20-fold enhancement of phosphate-ester hydrolysis with UiO-66-NH 2. Chem Sci 6:2286–2291. https://doi.org/10.1039/C4SC03613A
Mirmohseni A, Akbari M, Najjar R, Hosseini M (2019) Self-healing waterborne polyurethane coating by pH-dependent triggered-release mechanism. J Appl Polym Sci 136:47082. https://doi.org/10.1002/app.47082
Feng L, Iroh JO (2013) Novel polyimide-b-polyurea supramacromolecule with remarkable thermomechanical and dielectric properties. Eur Polym J 49:1811–1822. https://doi.org/10.1016/j.eurpolymj.2013.04.007
Maya-Visuet E, Gao T, Soucek M, Castaneda H (2015) The effect of TiO2 as a pigment in a polyurethane/polysiloxane hybrid coating/aluminum interface based on damage evolution. Prog Org Coat 83:36–46. https://doi.org/10.1016/j.porgcoat.2015.02.001
Wu G, An J, Sun D et al (2014) Robust microcapsules with polyurea/silica hybrid shell for one-part self-healing anticorrosion coatings. J Mater Chem A 2:11614–11620. https://doi.org/10.1039/C4TA01312C
Moghaddam ZS, Kaykhaii M, Khajeh M, Oveisi AR (2018) Synthesis of UiO-66-OH zirconium metal–organic framework and its application for selective extraction and trace determination of thorium in water samples by spectrophotometry. Spectrochim Acta A Mol Biomol Spectrosc 194:76–82. https://doi.org/10.1016/j.saa.2018.01.010
Yuan Y, Zhang Y, Fu X et al (2016) Silane-terminated polyurethane applied to a moisture-curable pressure-sensitive adhesive using triethoxysilane. RSC Adv 6:83688–83696. https://doi.org/10.1039/C6RA19883J
Blaine SJ, Wilson KK (1996) Protective solvent free liquid masking compounds and related method. US Patent 5,494,702, 27 Feb, 1996
Muller H-P, Gruttmann H, Casselmann H, et al (1999) Cosolvent-free aqueous, anionic polyurethane dispersions and their use as peelable coatings. US Patent 5,965,195, 12 Oct, 1999
Salamon PA 54) Temporary protective coatings for precision surfaces. 14
Polymeric peel-off coating compositions and methods of use thereof. Google Patents US6124044A. https://patents.google.com/patent/US6124044A/en. Accessed 24 May 2019
Adhesion performance of PSA–clay nano-composites by the in situ polymerization and mechanical blending. ScienceDirect. https://www.sciencedirect.com/science/article/pii/S0143749613001395. Accessed 2 July 2019
Mahdi EM, Tan J-C (2016) Dynamic molecular interactions between polyurethane and ZIF-8 in a polymer-MOF nanocomposite: microstructural, thermo-mechanical and viscoelastic effects. Polymer 97:31–43. https://doi.org/10.1016/j.polymer.2016.05.012
Li P, Klet RC, Moon S-Y et al (2015) Synthesis of nanocrystals of Zr-based metal–organic frameworks with csq-net: significant enhancement in the degradation of a nerve agent simulant. Chem Commun 51:10925–10928. https://doi.org/10.1039/C5CC03398E
Long NH, Park H, Chae G et al (2019) Preparation of peelable coating films with a metal organic framework (UiO-66) and self-crosslinkable polyurethane for the decomposition of methyl paraoxon. Polymers 11:1298. https://doi.org/10.3390/polym11081298
Acknowledgements
This work was supported by the National Research Council of Science and Technology (NST) grant by the Korean government (MSIP) (No. CMP-16-04-KITECH).
Author information
Authors and Affiliations
Contributions
K-M Kim, H-J Kim, and S Shin designed the experiments. H-W Park, G-S Shim, and G Chae carried out the measurements. S-W Jang and J-H Lee analyzed the data. K-M Kim wrote the manuscript.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, KM., Park, HW., Shim, GS. et al. Mechanical properties and decomposition performance of peelable coating containing UiO-66 catalyst and waterborne silane-terminated polyurethane dispersions. J Mater Sci 55, 2604–2617 (2020). https://doi.org/10.1007/s10853-019-04184-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-04184-2