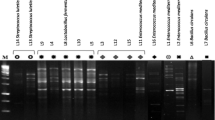
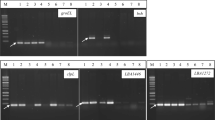
Abstract
Fermented foods have been recognized as a source of probiotic bacteria which can have a positive effect when administered to humans and animals. Discovering new probiotics in fermented food products poses a global economic and health importance. In this study, we investigated the antimicrobial and probiotic potential of lactobacilli isolated from fermented beverages produced traditionally by ethnic groups in Northeast India. Out of thirty Lactobacilli, fifteen exhibited strong antimicrobial activity against Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter aerogenes with significant anti-biofilm and anti-quorum sensing activity. These isolates also showed characteristics associated with probiotic properties, such as tolerance to low pH and bile salts, survival in the gastric tract, auto-aggregation, and hydrophobicity without exhibiting hemolysis formation or resistance to certain antibiotics. The isolates were identified using gram staining, biochemical tests, and 16S rDNA sequencing. They exhibited probiotic potential, broad-spectrum of antibacterial activity, promising anti-biofilm, anti-quorum sensing activity, non-hemolytic, and tolerance to acidic pH and bile salts. Overall, four specific Lactobacillus isolates, Lactiplantibacillus plantarum BRD3A and Lacticaseibacillus paracasei RB10OW from fermented rice-based beverage, and Lactiplantibacillus plantarum RB30Y and Lacticaseibacillus paracasei MP11A from traditional local curd demonstrated potent antimicrobial and probiotic properties. These findings suggest that these lactobacilli isolates from fermented beverages have the potential to be used as probiotics with therapeutic benefits, highlighting the importance of traditional fermented foods for promoting gut health and infectious disease management.





Similar content being viewed by others
References
Makarova K, Slesarev A, Wolf Y, Sorokin A, Mirki B, Koonin E, Pavlov A, Pavlova N, Karamychev V, Polouchine N, Shakhova V (2006) Comparative genomics of the lactic acid bacteria. Proc Natl Acad Sci USA 103:15611–15616. https://doi.org/10.1073/pnas.060711710
O’Toole PW, Marchesi JR, Hill C (2017) Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat Microbiol 2(5):1–6
Zheng J, Wittouckm S, Salvetti E, Franz CM, Harris H, Mattarelli P, O’Toole PW, Pot B, Vandamme P, Walter J, Watanabe K (2020) A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol 70:2782–2858. https://doi.org/10.1099/ijsem.0.004107
Chen CC, Lai CC, Huang HL, Huang WY, Toh HS, Weng TC, Chuang YC, Lu YC, Tang HJ (2019) Antimicrobial activity of Lactobacillus species against carbapenem-resistant Enterobacteriaceae. Front Microbiol 10:789. https://doi.org/10.3389/fmicb.2019.00789
Brunel AS, Guery B (2017) Multidrug resistant (or antimicrobial-resistant) pathogens-alternatives to new antibiotics? Swiss Med Wkly. https://doi.org/10.4414/smw.2017.14553
Fuqua C, Greenberg EP (1998) Self-perception in bacteria: quorum sensing with acylated homoserine lactones. Curr Opin Microbiol 1(2):183–189. https://doi.org/10.1016/S1369-5274(98)80009-X
Ivanova A, Ivanova K, Tzanov T (2018) Inhibition of quorum-sensing: a new paradigm in controlling bacterial virulence and biofilm formation. Biotechnol Appl Quor Sens Inhib 3:21. https://doi.org/10.1007/978-981-10-9026-4_1
Camele I, Elshafie HS, Caputo L, De Feo V (2019) Anti-quorum sensing and antimicrobial effect of Mediterranean plant essential oils against phytopathogenic bacteria. Front Microbiol 10:2619. https://doi.org/10.3389/fmicb.2019.02619
Rhee SJ, Lee JE, Lee CH (2011) Importance of lactic acid bacteria in Asian fermented foods. In: Microbial cell factories. BioMed Central, pp 1–13. https://doi.org/10.1186/1475-2859-10-S1-S5
Tamang JP (2022) “Ethno-microbiology” of ethnic Indian fermented foods and alcoholic beverages. J Appl Microbiol 133(1):145–161
Ray M, Ghosh K, Singh S, Mondal KC (2016) Folk to functional: an explorative overview of rice-based fermented foods and beverages in India. J Ethn Foods 3:5–18. https://doi.org/10.1016/j.jef.2016.02.002
Wahengbam R, Thangjam AS, Keisam S, Asem ID, Ningthoujam DS, Jeyaram K (2020) Ethnic fermented foods and alcoholic beverages of Manipur. In: Tamang JP (ed) Ethnic fermented foods and beverages of India: science history and culture. Springer, Singapore, pp 349–419
Di Cagno R, Coda R, De Angelis M, Gobbetti M (2013) The exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol 33:1–10. https://doi.org/10.1016/j.fm.2012.09.00
Romero-Luna HE, Hernández-Sánchez H, Dávila-Ortiz G (2017) Traditional fermented beverages from Mexico as a potential probiotic source. Ann Microbiol 67:577–586. https://doi.org/10.1007/s13213-017-1290-2
Sharpe ME (1979) Identification of the lactic acid bacteria. In: Skinner FA, Lovelock DW (eds) Identification methods for microbiologists. Academic Press, London, pp 233–259
Kim PI, Jung MY, Chang YH, KimS KSJ, Park YH (2007) Probiotic properties of Lactobacillus and Bifidobacterium strains isolated from porcine gastrointestinal tract. Appl Microbiol Biotechnol 74:1103–1111. https://doi.org/10.1007/s00253-006-0741
Hall TA (1999) Bio Edit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In: Nucleic acids symposium series, vol 41. pp 95–98
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Tagg JR, McGiven AR (1971) Assay system for bacteriocins. Appl Microbiol 21(5):943. https://doi.org/10.1128/am.21.5.943-943.1971
Overhage J, Campisano A, Bains M, Torfs EC, Rehm BH, Hancock RE (2008) Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect Immun 76:4176–4182. https://doi.org/10.1128/IAI.00318-08
Singh BN, Singh BR, Singh RL, Prakash D, Sarma BK, Singh HB (2009) Antioxidant and anti-quorum sensing activities of green pod of Acacia nilotica L. Food Chem Toxicol 47:778–786. https://doi.org/10.1016/j.fct.2009.01.009
Rajkumari J, Borkotoky S, Murali A, Suchiang K, Mohanty SK, Busi S (2018) Attenuation of quorum sensing controlled virulence factors and biofilm formation in Pseudomonas aeruginosa by pentacyclic triterpenes, betulin and betulinic acid. Microb Pathog 118:48–60. https://doi.org/10.1016/j.micpath.2018.03.012
Guo Z, Wang J, Yan L, Chen W, Liu XM, Zhang HP (2009) In vitro comparison of probiotic properties of Lactobacillus casei Zhang, a potential new probiotic, with selected probiotic strains. LWT 42(10):1640–1646. https://doi.org/10.1016/j.lwt.2009.05.025
Ramos CL, Thorsen L, Schwan RF, Jespersen L (2013) Strain-specific probiotics properties of Lactobacillus fermentum, Lactobacillus plantarum and Lactobacillus brevis isolates from Brazilian food products. Food Microbiol 36(1):22–29. https://doi.org/10.1016/j.fm.2013.03.010
Ilyazova A, Blazheva D, Slavchev A, Krastanov A (2002) In vitro simulation of the gastrointestinal tract environment and its interaction with probiotic lactobacilli. In: BIO web of conferences 2022 45:02003. EDP Sciences
Zommiti M, Connil N, Hamida JB, Ferchichi M (2017) Probiotic characteristics of Lactobacillus curvatus DN317, a strain isolated from chicken ceca. Probiot Antimicrob Proteins 9:415–424. https://doi.org/10.1007/s12602-017-9301-y
Lee H, Yoon H, Ji Y, Kim H, Park H, Lee J, Shin H, Holzapfel W (2011) Functional properties of Lactobacillus strains isolated from kimchi. Int J Food Microbiol 145:155–161. https://doi.org/10.1016/j.ijfoodmicro.2010.12.003
Sharma C, Gulati S, Thakur N, Singh BP, Gupta S, Kaur S, Mishra SK, Puniya AK, Gill JPS, Panwar H (2017) Antibiotic sensitivity pattern of indigenous lactobacilli isolated from curd and human milk samples. 3 Biotech 7(1):53. https://doi.org/10.1007/s13205-017-0682-0
CLSI (2016) CLSI supplement M100S. Performance standards for antimicrobial susceptibility testing, 26th edn. Clinical and Laboratory Standards Institute, Wayne
Mangia NP, Saliba L, Deiana P (2019) Functional and safety characterization of autochthonous Lactobacillus paracasei FS103 isolated from sheep cheese and its survival in sheep and cow fermented milk during cold storage. Ann Microbiol 69(2):161–170. https://doi.org/10.1007/s13213-018-1416-1
Álvarez-Cisneros YM, Ponce-Alquicira E (2018) Antibiotic resistance in lactic acid bacteria. In: Antimicrobial resistance—a global threat. Intech Open. https://doi.org/10.5772/intechopen.80624
Min JH, Kim YH, Kim JH, Choi SY, Lee JS, Kim HK (2012) Comparison of microbial diversity of Korean commercial Makgeolli showing high β-glucan content and high antihypertensive activity, respectively. Mycobiology 40:138–141. https://doi.org/10.5941/MYCO.2012.40.2.138
Akman PK, Ozulku G, Tornuk F, Yetim H (2021) Potential probiotic lactic acid bacteria isolated from fermented gilaburu and shalgam beverages. LWT 149:111705. https://doi.org/10.1016/j.lwt.2021.111705
Vasiee A, AlizadehBehbahani B, TabatabaeiYazdi F, Mortazavi SA, Noorbakhsh H (2018) Diversity and probiotic potential of lactic acid bacteria isolated from Horreh, a traditional Iranian fermented food. Probiot Antimicrob Proteins 10:258–268. https://doi.org/10.1007/s12602-017-9282-x
Osimani A, Garofalo C, Aquilanti L, Milanović V, Clementi F (2015) Unpasteurised commercial boza as a source of microbial diversity. Int J Food Microbiol 194:62–70. https://doi.org/10.1016/j.ijfoodmicro.2014.11.011
Poornachandra Rao K, Chennappa G, Suraj U, Nagaraja H, Charith Raj AP, Sreenivasa MY (2015) Probiotic potential of Lactobacillus strains isolated from sorghum-based traditional fermented food. Probiot Antimicrob Proteins 7:146–156. https://doi.org/10.1007/s12602-015-9186-6
Sagdic O, Ozturk I, Yapar N, Yetim H (2014) Diversity and probiotic potentials of lactic acid bacteria isolated from gilaburu, a traditional Turkish fermented European cranberrybush (Viburnum opulus L.) fruit drink. Food Res Int 64:537–545. https://doi.org/10.1016/j.foodres.2014.07.045
Girma A, Aemiro A (2021) Antibacterial activity of lactic acid bacteria isolated from fermented Ethiopian traditional dairy products against food spoilage and pathogenic bacterial strains. J Food Qual. https://doi.org/10.1155/2021/9978561
Zhang X, Esmail GA, Alzeer AF, Arasu MV, Vijayaraghavan P, Choi KC, Al-Dhabi NA (2020) Probiotic characteristics of Lactobacillus strains isolated from cheese and their antibacterial properties against gastrointestinal tract pathogens. Saudi J Biol Sci 27:3505–3513. https://doi.org/10.1016/j.sjbs.2020.10.022
Yan X, Gu S, Cui X, Shi Y, Wen S, Chen H, Ge J (2019) Antimicrobial, anti-adhesive and anti-biofilm potential of biosurfactants isolated from Pediococcus acidilactici and Lactobacillus plantarum against Staphylococcus aureus CMCC26003. Microb Pathog 127:12–20. https://doi.org/10.1016/j.micpath.2018.11.039
Rezaei Z, Khanzadi S, Salari A (2021) Biofilm formation and antagonistic activity of Lacticaseibacillus rhamnosus (PTCC1712) and Lactiplantibacillus plantarum (PTCC1745). AMB Expr 11:156. https://doi.org/10.1186/s13568-021-01320-7
Cui T, Bai F, Sun M, Lv X, Li X, Zhang D, Du H (2020) Lactobacillus crustorum ZHG 2-1 as novel quorum-quenching bacteria reducing virulence factors and biofilms formation of Pseudomonas aeruginosa. LWT 117:108696. https://doi.org/10.1016/j.lwt.2019.108696
Giri SS, Sen SS, Saha S, Sukumaran V, Park SC (2018) Use of a potential probiotic, Lactobacillus plantarum L7, for the preparation of a rice-based fermented beverage. Front Microbiol 9:473. https://doi.org/10.3389/fmicb.2018.00473
Handa S, Sharma N (2016) In vitro study of probiotic properties of Lactobacillus plantarum F22 isolated from chhang—a traditional fermented beverage of Himachal Pradesh, India. J Genet Eng Biotechnol 14:91–97. https://doi.org/10.1016/j.jgeb.2016.08.001
Salminen S, Von Wright A, Morelli L, Marteau P, Brassart D, de Vos WM, Fondén R, Saxelin M, Collins K, Mogensen G, Birkeland SE, Mattila-Sandholm T (1998) Demonstration of safety of probiotics—a review. Int J Food Microbiol 44(1–2):93–106. https://doi.org/10.1016/s0168-1605(98)00128-7
Vinderola CG, Reinheimer JA (2003) Lactic acid starter and probiotic bacteria: a comparative “in vitro” study of probiotic characteristics and biological barrier resistance. Food Res Int 36(9–10):895–904. https://doi.org/10.1016/S0963-9969(03)00098-X
Marchwińska K, Gwiazdowska D (2022) Isolation and probiotic potential of lactic acid bacteria from swine feces for feed additive composition. Arch Microbiol 204(1):61. https://doi.org/10.1007/s00203-021-02700-0
Adikari AMMU, Priyashantha H, Disanayaka JNK, Jayatileka DV, Kodithuwakku SP, Jayatilake JAMS, Vidanarachchi JK (2021) Isolation, identification and characterisation of Lactobacillus species diversity from Meekiri: traditional fermented buffalo milk gels in Sri Lanka. Heliyon 7:e08136. https://doi.org/10.1016/j.heliyon.2021.e08136
Kirtzalidou E, Pramateftaki P, Kotsou M, Kyriacou A (2011) Screening for lactobacilli with probiotic properties in the infant gut microbiota. Anaerobe 17(6):440–443. https://doi.org/10.1016/j.anaerobe.2011.05.007
Yadav R, Puniya AK, Shukla P (2016) Probiotic properties of Lactobacillus plantarum RYPR1 from an indigenous fermented beverage Raabadi. Front Microbiol 7:1683. https://doi.org/10.3389/fmicb.2016.01683
Acknowledgements
This work was fully supported by the Institute of Bioresources and Sustainable Development (IBSD Imphal, an autonomous institute under the Department of Biotechnology, Govt of India and the institute manuscript Communication Number No. IBSD/MS/2020/01/092. We would also like to thank the Department of Microbiology, Regional Institute of Medical Sciences Imphal for helping in procuring the clinical bacterial strain.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
The SH carried out the whole experiment, analyzed the data and SI helped in the overall supervision of the work helped in designing the experiment and PKM helped with the correction.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huidrom, S., Mukherjee, P.K. & Devi, S.I. Antimicrobial and Probiotic Potential of Lactobacilli Associated with Traditional Fermented Beverages. Curr Microbiol 81, 137 (2024). https://doi.org/10.1007/s00284-024-03656-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-024-03656-2