Abstract
Currently, the health benefits of probiotic bacteria are well known, and this has taken up a great deal of space in food science and health, both research and operational. On the other hand, anti-biofilm properties on food pathogens in the food and pharmaceutical industries have created an attractive challenge. This study aimed to describe the inhibitory activity of cell-free supernatants (CFS), planktonic cells, and biofilm form of lactobacilus strains (L. rhamnosus and L. plantarum) against food pathogens such as Pseudomonas aeruginosa and Listeria monocytogenes. Anti-bacterial activities of the CFS of lactobacillus strains were assessed by the microplate method and via violet staining. Evaluation of the antagonistic activity of planktonic cells and biofilm of LAB were performed by the spread plate method. The results showed the incubation time of 48 h was the best time to produce biofilm. Although the planktonic states reduce the pathogens bacterial about 1 –1.5 log, but in biofilm forms, decreased L. monocytogenes about 4.5 log compared to the control, and in the case of P. aeruginosa, a growth reduction of about 2.13 log was observed. Furthermore, biofilm formation of L. monocytogenes in the presence of L. rhamnosus cell-free supernatant was more weakly than L. plantarum CFS, but their CFS effect on reducing the bacterial population of P. aeruginosa was the same. According to the study, biofilm produced by probiotic strains can be considered a new approach for biological control. Also, cell-free supernatant can be used as postbiotic in the food and pharmaceutical industries.
Similar content being viewed by others
Introduction
Food safety is one of the most important issues for food producers and consumers. As food supply has become increasingly global, food safety issues need more attention (Moradi et al. 2020). For this purpose, a new approach of researchers and industries in recent years has been using biological policies in the face of the challenge of foodborne pathogens. Among the wide range of strategies currently being used or proposed, biocontrol based on organisms or their antimicrobial products has increased because of their popularity, no side effects, low processing costs, and low dependence on new technologies (Gálvez et al. 2010; Collazo Cordero et al. 2017). In the initial research, studies have focused on planktonic cells’ antimicrobial properties and their mode of action (McIntyre et al. 2007). However, recently, the use of free cells supernatant and biofilms of probiotics in biological control and development of intelligent antimicrobial surfaces are at the forefront of explorations (Guerrieri et al. 2009; Vuotto et al. 2014; Kaur et al. 2018). Biofilm is a membrane structure comprising a polysaccharide matrix, vitamins, proteins, and other components that surround microorganisms and have a complex internal structure and channels for transporting nutrients across the network. Nowadays, by discovering probiotics’ biofilm formation ability, this phenomenon has been introduced as a controller for pathogenic biofilms (Liu et al. 2015; Muhsin et al. 2015; Gómez et al. 2016; Speranza et al. 2020). Since this phenomenon is straightforward and inherent in bacteria itself, it can be used as an intelligent technique to control food pathogens (Balaure and Grumezescu 2020). Lactobacillus strains, which have the ability to auto-aggregation and co-aggregation, have antibiofilm properties. L. rhamnosus and L. plantarum are probiotic strains that have biofilm formation capacity and produced more robust biofilms than other species (Lebeer et al. 2007a, b; Simoes et al. 2009; Bujňáková and Kmeť 2012; Léonard et al. 2014).
Another version of probiotics is a cell-free supernatant, which in recent studies has been named post-biotic. This cell extract contains biosurfactant and antimicrobial agents produced during Lactobacillus growth and fermentation in complex growth conditions and which has been proposed as a method for biological control of pathogens (Abdelhamid et al. 2018; Chappell and Nair 2020). Since the Listeria monocytogenes (Leverentz et al. 2006; Warke et al. 2017; Kyere et al. 2020) and Pseudomonas aeruginosa are two food pathogens that have caused many problems in the food industry by producing biofilm (Rasamiravaka et al. 2015; Kumar 2016), therefore, in this study, antagonistic properties of three forms of planktonic, biofilm and cell-free supernatant of L. plantarum and L. rhamnusus were compared on these pathogens until to provides a better understanding of different forms of probiotic bacteria’s potency for their multifunctional use cluding food preservative agent, antibacterial and antibiofilm agent under different conditions.
Materials and methods
Lactobacillus and food patogene strains and culture conditions
Lyophilized culture of Lacticaseibacillus rhamnosus (PTCC1712) and Lactiplantibacillus plantarum (PTCC1745) isolated from pickled cabbage was obtained from the Iranian Research Organization for Science and Technology. The microbial culture was activated according to the company’s instructions. The activated bacteria were transferred into De Man, Rogosa, and Sharpe (MRS) broth or agar (Oxoid, Milan, Italy) and incubated under anaerobic conditions (Anaerobic conditions were achieved by the use of anaerobic jars with using Gas-Pack C.) at 37 °C for 72 h Kalantarmahdavi et al. (2021). Listeria monocytogenes (ATCC 7644) from American Type Culture Collection and Pseudomonas aeruginosa (PTCC 1074) were selected as food pathogens and cultured in Tryptic Soy broth (TSB, Oxoid).
Probiotic biofilm formation assay
One milliliter of culture medium containing 1.5 × 108 CFU mL−1 from each strain was poured into each well and incubated for 48 h at 30 °C. After incubation, the culture medium was drained from the wells and washed twice with 0.5 mL of 150 mM NaCl solution. The microplate was then stained for 45 min with 1 mL of 0.05% (v/v) of crystalline violet solution and washed twice. One mL of 96% ethanol (v/v) was added to each well, and the optical density was determined at 430 and 595 nm (Chen et al. 2017). To determine the best incubation time and in order to create a stronger biofilm, biofilm production was examined at intervals of 6, 12, 24, 36, 48, and 72 h of incubation.
Antagonistic activity
The antagonistic activity of probiotic bacteria on food pathogens was investigated in three models: planktonic form, cell-free supernatant, and biofilm.
Antagonistic activity of planktonic form
One milliliter of BHI (brain heart infusion) broth inoculated with 1.5 × 108 CFU/mL of each pathogen strain was dispensed per well in a microplate. Then, One milliliter of fresh MRS broth inoculated with 1.5 × 108 CFU/mL of lactobacillus strains was added. The microplate was incubated for 48 h at 30 °C. After incubation, the medium was removed from each well, and the microplates were washed twice with 500 mL of 150 mM NaCl solution. Evaluation of microorganisms was performed by the spread plate method. For each test, 1 mL of the samples was mixed with 9 mL of sterile peptone water. After sequential dilutions, appropriate dilutions were plated on set Oxford-Listeria-Selective-Agar (Base (Merck)) for L. monocytogenes and Pseudomonas agar base (Merck) for P. aeruginosa. Then, they were incubated at 37 °C for 72 h. The total counts of the viable bacteria were reported as logarithmic colony forming units per gram (log CFU/g). All the experiments were performed in triplicate, which means that each experiment was repeated at least three times.
Antagonistic activity of LAB biofilms
Biofilm of LAB was formed in a microplate; then, one milliliter of fresh BHI broth inoculated with 1.5 × 108 CFU/mL of L. monocytognes and P. aeruginosa was poured into wells that contained biofilm and incubated for 48 h at 30 °C. The number of L.monocytogenes and P. aeruginosa were counted by the spread plate method in selective media (Oxford-Listeria-Selective-Agar (Base) and Pseudomonas agar base, respectively). The control sample biofilm of the pathogen was formed similar to Lactobacillus biofilm (Zhang et al. 2013).
Antibiofilm activity of the cell-free supernatant
To break down the membrane of a cell, 1.5 × 108 CFU / mL of each lactic acid bacteria were subjected to sonication at 60 HZ for 5 min. Then, it was centrifuged (6000 g, 10 min, 4 °C), and supernatants were collected. 1.5 × 108 CFU / mL of each pathogenic bacteria were inoculated into BHI broth, and 0.9 ml of it was poured into each well of a 24-well microplate, and 0.1 ml of supernatant was added to each well. Then, the microplates were incubated for 48 h at 37 °C, and to determine pathogen biofilm formation, OD value was measured at 430 and 595 nm (Kubota et al. 2008; Satpute et al. 2016). Adhesion rate was set to be B and can be calculated as followings: (Zhang et al. 2013; Chen et al. 2017)
ODB refer to the optical density value in the Blank.
No biofilm producer = B<0.1;
Weak biofilm producer = 0.1≤ B <0.5;
Moderate biofilm producer = 0.1≤ B <1;
Strong biofilm producer = B ≥1.
Investigation of biofilm microstructure by SEM
Biofilm was fixed in 2.5% glutardialdehyde solution in 10 Mm sodium cacodylate buffer for 24 h at 4 °C. It was then washed thrice for 15 min in 10 mM sodium cacodylate buffer by gentle mixing at room temperature, dehydrated in a graded ethanol series (50, 70, 80, 90, 95, and 100%). The samples were air-dried, placed on SEM stub, coated with gold/palladium by Sputter Coater device Model SC7620 (England), and investigated by a LEO1450VP scanning electron microscope (Germany) with resolution 2.5 nm and maximum voltage 35kv (Stefania et al. 2017).
Statistical analysis
Data were analyzed by the one-way analysis of variance (ANOVA). If Onaway ANOVA was significant, the HolmeSidak test was used to determine significant differences (P < 0.05).
Result
The ability of biofilm formation by LAB over time
The results showed that incubation time has a significant effect on biofilm formation. Figure 1 shows the process of biofilm formation over time; with increasing the incubation time, the rate of biofilm formation increased initially and reached its maximum value after 48 h. However, as incubation time continued, a decrease in biofilm formation rates in the strains was observed after 72 h. Notably, L. rhamnosus had a higher biofilm formation rate than L. plantarum.
Antagonistic activity
Planktonic form
As shown in Table 1, the planktonic form of probiotics has reduced the growth of pathogens. Compared to the control sample, it has reduced pathogens’ growth by 1.1–1.5 log CFU/ml, but there is no significant difference in antagonistic properties between probiotic strains.
Biofilm
The results demonstrated that the presence of L. rhamnosus, and L. plantarum biofilm, decreased L. monocytogenes by about 4.5 log compared to the control. In the case of P. aeruginosa, a growth reduction of about 2.13 log was observed. The obtained data show that the biofilm has more antagonistic power than the planktonic state; therefore, it has decreased 1.5 and 1 log CFU/ml in the case of L. monocytogenes and P. aeruginosa, respectively (Table 1).
Cell-free supernatant antibiofilm activity
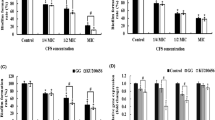
The results showed that the cell-free supernatants of probiotic bacteria had an effect on the biofilm formation of food pathogens and reduced their biofilm formation strength (Figs. 2 and 3). Meanwhile, in the presence of L. rhamnosus cell-free supernatant (CFS), the biofilm of L. monocytogenes formation was weaker than L. plantarum CFS, but their CFS effect reduces the bacterial population of P. aeruginosa was the same. Another point is that CFS L. rhamnosus had a more substantial inhibitory effect on the formation of P. aeruginosa biofilm. In general, the biofilms formed in the presence of CFS were much weaker than the control.
Microstructure of biofilm
As can be seen from the comparison of the images (Figs. 4 and 5), the L. rhamnosus biofilm has a more uniform and impermeable surface than the L. plantarum and has a higher cell density, in general, it has formed a stronger biofilm, which is directly related to the biofilm formation power.
Discussion
In this study, two strains of lactic acid bacteria were examined, and their potential for biofilm production was measured. Both strains were able to grow in the microplate and mature biofilm formation, and there was a slight difference in the biofilm density of the strains (Figs. 2, 3). In recent years, several studies have investigated the ability of L. plantarum and L. rhamnosus biofilm formation ability and their antagonistic activity in different forms separately. Kaur et al. (2018) and Léonard et al. (2014) survey Planktonic cell of Lactobacillus strain on Vibrio and L. monocytogenes respectively. Also, Speranza et al. (2020) and Gómez et al. (2016) examinated the biofilm of probiotics on pathogens (Lebeer et al. 2007a, b; Guerrieri et al. 2009; Kubota et al. 2009; Bujňáková and Kmeť 2012; Vuotto et al. 2014; Muhsin et al. 2015). However, in most cases, a simultaneous comparison has not been performed. The results of this study showed that probiotic bacteria in all forms could have antimicrobial effects on pathogens, but this effect is more severe in biofilm form, which can be due to the nature of the biofilm and the number of bacteria and more antimicrobial compounds. Aoudia et al. (2016) showed that the biofilm supernatants of lactobacillus strains had more substantial effects than the supernatant produced by planktonic cells (Cotter et al. 2013). In a study by Satpute et al. (2016) the lactic acid bacteria showed high antimicrobial effects on the L. monocytogenes, which could be related to the presence of bacteriocin and biosurfactants compounds. Bacteriocins are antagonistic compounds that metabolic end-products are bactericidal proteins and substances similar to antibiotics (Stefania et al. 2017; Balaure and Grumezescu 2020). Other studies have shown that different strains of Lactobacillus isolated from dairy products were able to produce strong biofilms that can prevent the growth of food pathogens such as Salmonella and E. coli (Abdelhamid et al. 2018). Kubota et al. (2009) conducted studies on L. plantarum, examined bacterial resistance in both biofilm and planktonic states, and concluded that biofilm was effective in increasing bacterial resistance. Probiotics can produce bacteriocins, even in the face of intestinal infections (Cotter et al. 2013). Today, numerous studies have been performed in the field of bacterial therapy with probiotics, especially L. plantarum in human and animal models, which is not unrelated to these antagonistic compounds (Cotter et al. 2013; Argenta et al. 2016). Jones et al. (2012) reported that the polysaccharide compounds in biofilms act as TNF, a limiting factor, and exert their antagonistic effect (Jones et al. 2012). Since the bacterial population’s biofilm state is higher than the planktonic state, it has higher antagonistic potency. The investigation of the effects of time on biofilm formation showed that 48-h incubation time is the best time for strong and coherent biofilm formation. One of the most important achievements of the present study is the comprehensive investigation of probiotic bacteria’s antagonistic effects in their various forms. Analysis of the present study results showed that probiotics in three forms of planktonic, cell-free supernatant, and biofilm weaken pathogens’ growth. However, the bacterial antagonist’s simultaneous effect and the bacteriocin compounds produced and other antimicrobial compounds in the biofilm form are stronger and greater. Due to the fact that increasing resistance of foodborne pathogens compared to industrial disinfectants has created a serious challenge in the food and pharmaceutical industries and the environment. The biofilm of these bacteria at different surfaces and joints has created suitable growing conditions for them, endanger safety, quality, and stability. Therefore, researchers in recent years have investigated various competitive applications by probiotic bacteria, including natural antimicrobial products (Fijan et al. 2019; El-Mokhtar et al. 2020), bio factors and biofilm have been studied as a new way to control pathogenic bacteria (Jeong et al. 2018) and prevent food contamination. The second significant achievement of the present study is that if it was not possible to use probiotics as live bacteria, we could use their cell extracts as a natural preservative. in other applications can be used by forming biofilms on different surfaces of the industry to reduce the problem of biofilm formation of pathogens as a good idea to produce smart antimicrobial surfaces. Also, cell-free supernatant produced by probiotic strains which has recently been named Postbiotic can be considered a new generation of biological control agents and create a new approach in the food and pharmaceutical industries (Jiang et al. 2016; Tahmourespour et al. 2019; He et al. 2021).
Availability of data and materials
The corresponding author could provide all experimental data on a valid request.
References
Abdelhamid AG, Esaam A, Hazaa MM (2018) Cell free preparations of probiotics exerted antibacterial and antibiofilm activities against multidrug resistant E. coli.. Saudi Pharm J 26(5):603–607
Aoudia N, Rieu A, Briandet R, Deschamps J, Chluba J, Jego G, Garrido C, Guzzo J (2016) Biofilms of Lactobacillus plantarum and Lactobacillus fermentum: effect on stress responses, antagonistic effects on pathogen growth and immunomodulatory properties. Food Microbiol 53:51–59
Argenta A, Satish L, Gallo P, Liu F, Kathju S (2016) Local application of probiotic bacteria prophylaxes against sepsis and death resulting from burn wound infection. PLoS One 11(10):e0165294
Balaure PC, Grumezescu AM (2020) Recent advances in surface nanoengineering for biofilm prevention and control. Part II: active, combined active and passive, and smart bacteria-responsive antibiofilm nanocoatings. Nanomaterials 10(8): 1527
Bujňáková D, Kmeť V (2012) Functional properties of Lactobacillus strains isolated from dairy products. Folia Microbiol 57(4):263–267
Chappell TC, Nair NU (2020) Engineered lactobacilli display anti-biofilm and growth suppressing activities against Pseudomonas aeruginosa. NPJ Biofilms Microbiomes 6(1): 1–10
Chen Q, Sa R, Jia J, Xu R (2017) Research on biofilm formation ability of lactic acid bacteria under different conditions. Adv J Food Sci Technol 13(2):77–82
Collazo Cordero C, Abadias i Sero M, Aguiló-Aguayo I, Alegre Vilas I, Chenoll E, Viñas Almenar I (2017) Studies on the biocontrol mechanisms of Pseudomonas graminis strain CPA-7 against food-borne pathogens in vitro and on fresh-cut melon. LWT-Food Sci Technol 2017, 85(Part B): 301–308
Cotter PD, Ross RP, Hill C (2013) Bacteriocins—a viable alternative to antibiotics? Nat Rev Microbiol 11(2):95–105
El-Mokhtar MA, Hassanein KM, Ahmed AS, Gad GF, Amin MM, Hassanein OF (2020) Antagonistic activities of cell-free supernatants of lactobacilli against extended-spectrum β-lactamase producing Klebsiella pneumoniae and Pseudomonas aeruginosa. Infect Drug Resist 13:543
Fijan S, Frauwallner A, Langerholc T, Krebs B, ter Haar née Younes JA, Heschl A, Mičetić Turk D, Rogelj A (2019) Efficacy of using probiotics with antagonistic activity against pathogens of wound infections: an integrative review of literature. BioMed Res Int 2019:1–21
Gálvez A, Abriouel H, Benomar N, Lucas R (2010) Microbial antagonists to food-borne pathogens and biocontrol. Curr Opin Biotechnol 21(2):142–148
Gómez NC, Ramiro JM, Quecan BX, de Melo Franco BD (2016) Use of potential probiotic lactic acid bacteria (LAB) biofilms for the control of Listeria monocytogenes, Salmonella Typhimurium, and Escherichia coli O157: H7 biofilms formation. Front Microbiol 7:863
Guerrieri E, de Niederhäusern S, Messi P, Sabia C, Iseppi R, Anacarso I, Bondi M (2009) Use of lactic acid bacteria (LAB) biofilms for the control of Listeria monocytogenes in a small-scale model. Food Control 20(9):861–865
He Y, Na R, Niu X, Xiao B, Yang H (2021) Lactobacillus rhamnosus and Lactobacillus casei affect various stages of Gardnerella species biofilm formation. Front Cell Infect Microbiol 11:568178
Jeong D, Kim D-H, Song K-Y, Seo K-H (2018) Antimicrobial and anti-biofilm activities of Lactobacillus kefiranofaciens DD2 against oral pathogens. J Oral Microbiol 10(1):1472985
Jiang Q, Stamatova I, Kainulainen V, Korpela R, Meurman JH (2016) Interactions between Lactobacillus rhamnosus GG and oral micro-organisms in an in vitro biofilm model. BMC Microbiol 16(1):1–11
Jones M, Martoni C, Prakash S (2012) Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: a randomized controlled trial. Eur J Clin Nutr 66(11):1234–1241
Kalantarmahdavi M, Khanzadi S, Salari A (2021) Edible films incorporating with Lactobacillus plantarum based on sourdough, wheat flour, and gelatin: films characterization and cell viability during storage and simulated gastrointestinal condition. Starch-Stärke: 2000268:1–10
Kaur S, Sharma P, Kalia N, Singh J, Kaur S (2018) Anti-biofilm properties of the fecal probiotic lactobacilli against Vibrio spp. Front Cell Infect Microbiol 8:120
Kubota H, Senda S, Nomura N, Tokuda H, Uchiyama H (2008) Biofilm formation by lactic acid bacteria and resistance to environmental stress. J Biosci Bioeng 106(4):381–386
Kubota H, Senda S, Tokuda H, Uchiyama H, Nomura N (2009) Stress resistance of biofilm and planktonic Lactobacillus plantarum subsp. plantarum JCM 1149. Food Microbiol 26(6):592–597
Kumar M (2016) Beneficial biofilm works as a “probiotic” to control membrane biofouling. Membr Technol 2016(11):8
Kyere EO, Foong G, Palmer J, Wargent JJ, Fletcher GC, Flint S (2020) Biofilm formation of Listeria monocytogenes in hydroponic and soil grown lettuce leaf extracts on stainless steel coupons. LWT 126: 109114
Lebeer S, Verhoeven TLA, Vélez MP, Vanderleyden J, De Keersmaecker SC (2007a) Impact of environmental and genetic factors on biofilm formation by the probiotic strain Lactobacillus rhamnosus GG. Appl Environ Microbiol 73(21):6768–6775
Lebeer S, De Keersmaecker SCJ, Verhoeven TLA, Fadda AA, Marchal K, Vanderleyden J (2007b) Functional analysis of luxS in the probiotic strain Lactobacillus rhamnosus GG reveals a central metabolic role important for growth and biofilm formation. J Bacteriol 189:860–871
Léonard L, Degraeve P, Gharsallaoui A, Saurel R, Oulahal N (2014) Design of biopolymeric matrices entrapping bioprotective lactic acid bacteria to control Listeria monocytogenes growth: comparison of alginate and alginate-caseinate matrices entrapping Lactococcus lactis subsp. lactis cells. Food Control 37:200–209
Leverentz B, Conway WS, Janisiewicz W, Abadias M, Kurtzman CP, Camp MJ (2006) Biocontrol of the food-borne pathogens Listeria monocytogenes and Salmonella enterica serovar Poona on fresh-cut apples with naturally occurring bacterial and yeast antagonists. Appl Environ Microbiol 72(2):1135–1140
Liu J, Prindle A, Humphries J, Gabalda-Sagarra M, Asally M, Dong-yeon DL, Garcia-Ojalvo J, Süel GM (2015) Metabolic co-dependence gives rise to collective oscillations within biofilms. Nature 523(7562):550–554
McIntyre L, Hudson J, Billington C, Withers H (2007) Biocontrol of foodborne bacteria: past, present and future strategies. Food New Zealand 7(5):25
Moradi M, Kousheh SA, Almasi H, Alizadeh A, Guimarães JT, Yılmaz N, Lotfi A (2020) Postbiotics produced by lactic acid bacteria: the next frontier in food safety. Compr Rev Food Sci Food Saf 19(6):3390–3415
Muhsin J, Ufaq T, Tahir H, Saadia A (2015) Bacterial biofilm: its composition, formation and role in human infections. J Microbiol Biotechnol 4:1–14
Rasamiravaka T, Vandeputte OM, Pottier L, Huet J, Rabemanantsoa C, Kiendrebeogo M, Andriantsimahavandy A, Rasamindrakotroka A, Stévigny C, Duez P (2015) Pseudomonas aeruginosa biofilm formation and persistence, along with the production of quorum sensing-dependent virulence factors, are disrupted by a triterpenoid coumarate ester isolated from Dalbergia trichocarpa, a tropical legume. PLoS One 10(7):e0132791
Satpute SK, Kulkarni GR, Banpurkar AG, Banat IM, Mone NS, Patil RH, Cameotra SS (2016) Biosurfactant/s from Lactobacilli species: Properties, challenges and potential biomedical applications. J Basic Microbiol 56(11):1140–1158
Simoes M, Bennett RN, Rosa EA (2009) Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Nat Prod Rep 26(6):746–757
Speranza B, Liso A, Russo V, Corbo MR (2020) Evaluation of the potential of biofilm formation of Bifidobacterium longum subsp. infantis and Lactobacillus reuteri as competitive biocontrol agents against pathogenic and food spoilage bacteria. Microorganisms 8(2):177
Stefania D, Miranda P, Diana M, Claudia Z, Rita P, Donatella P (2017) Antibiofilm and antiadhesive activities of different synbiotics. J Probiot Health 5(3):182–191
Tahmourespour A, Kasra-Kermanshahi R, Salehi R (2019) Lactobacillus rhamnosus biosurfactant inhibits biofilm formation and gene expression of caries-inducing Streptococcus mutans. Dent Res J 16(2):87
Vuotto C, Longo F, Donelli G (2014) Probiotics to counteract biofilm-associated infections: promising and conflicting data. Int J Oral Sci 6(4):189–194
Warke S, Ingle V, Kurkure N, Tembhurne P, Prasad M, Chaudhari S, Barbuddhe S (2017) Biofilm formation and associated genes in Listeria monocytogenes. Indian J Vet Sci Biotechnol 12(03):07–12
Zhang H, Xie L, Zhang W, Zhou W, Su J, Liu J (2013) The association of biofilm formation with antibiotic resistance in lactic acid bacteria from fermented foods. J Food Saf 33(2):114–120
Acknowledgements
This research results from a Ph.D. thesis with code 44721, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran. The authors express their gratitude. The authors would like to thank the Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, for providing raw materials, the facilities, and the financial support that make this project possible.
Funding
This research is the result of a Ph.D. thesis with code 44721, and this work was supported by the Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran.
Author information
Authors and Affiliations
Contributions
Z.R. A. S and S.Kh. contributed equally to this study. A. S. Created the original idea. Z.R. and S.Kh. expanded the idea. Z.R. carried out the experiments, and A. S and S. Kh. directed the project. All authors analyzed and interpreted the data and contributed to the writing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rezaei, Z., Khanzadi, S. & Salari, A. Biofilm formation and antagonistic activity of Lacticaseibacillus rhamnosus (PTCC1712) and Lactiplantibacillus plantarum (PTCC1745). AMB Expr 11, 156 (2021). https://doi.org/10.1186/s13568-021-01320-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13568-021-01320-7