Abstract
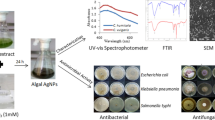
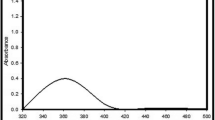
The biosynthesis of nanoparticles (NPs) has gained an overwhelming interest due to their biological applications. However, NPs synthesis by pigmented extreme halophiles remains underexplored. The NPs synthesis using pigmented halophiles is inexpensive and less toxic than other processes. In this study, pigmented halophilic microorganisms (n = 77) were screened to synthesize silver chloride nanoparticles (AgCl-NPs) with silver nitrate as metal precursors, and their biological applications were assessed. The synthesis of AgCl-NPs was possible using the crude extract from cellular lysis (CECL) of six extreme halophiles. Two of the AgCl-NPs viz. AK2-NPs and MY6-NPs synthesized by the CECL of Haloferax alexandrinus RK_AK2 and Haloferax lucentense RK_MY6, respectively, exhibited antimicrobial, antioxidative, and anti-inflammatory activities. The surface plasmon resonance of the AgCl-NPs was determined with UV spectroscopy. XRD analysis of AK2-NPs and MY6-NPs confirmed the presence of silver in the form of chlorargyrite (silver chloride) having a cubic structure. The crystallite size of AK2-NPs and MY6-NPs, estimated with the Scherrer formula, was 115.81 nm and 137.50 nm. FTIR analysis verified the presence of diverse functional groups. Dynamic light-scattering analysis confirmed that the average size distribution of NPs was 71.02 nm and 117.36 nm for AK2-NPs and MY6-NPs, respectively, with monodisperse nature. The functional group in 1623–1641 cm−1 indicated the presence of protein β-sheet structure and shifting of amino and hydroxyl groups from the pigmented CECL, which helps in capping and stabilizing nanoparticles. The study provides evidence that CECL of Haloferax species can rapidly synthesize NPs with unique characteristics and biological applications.





Similar content being viewed by others
Data Availability
The corresponding author will maintain the existing information supplied for the article.
References
Lach J, Jęcz P, Strapagiel D et al (2021) The methods of digging for “gold” within the salt: characterization of halophilic prokaryotes and identification of their valuable biological products using sequencing and genome mining tools. Genes (Basel). https://doi.org/10.3390/genes12111756
Giani M, Garbayo I, Vílchez C, Martínez-Espinosa RM (2019) Haloarchaeal carotenoids: healthy novel compounds from extreme environments. Mar Drugs 17:524. https://doi.org/10.3390/md17090524
Bonete MJ, Martínez-Espinosa RM (2011) Enzymes from halophilic archaea: open questions. Halophiles and hypersaline environments. Springer, Berlin, pp 359–371
Haque RU, Paradisi F, Allers T (2020) Haloferax volcanii for biotechnology applications: challenges, current state, and perspectives. Appl Microbiol Biotechnol 104:1371–1382. https://doi.org/10.1007/s00253-019-10314-2
Sreevalsan A, Moopantakath J, Kumavath R (2021) Extremophilic microbes. Microbiomes of extreme environments. CRC Press, Boca Raton, pp 218–229
Srivastava P, Bragança J, Ramanan SR, Kowshik M (2013) Synthesis of silver nanoparticles using haloarchaeal isolate Halococcus salifodinae BK3. Extremophiles 17:821–831. https://doi.org/10.1007/s00792-013-0563-3
Tag HM, Saddiq AA, Alkinani M, Hagagy N (2021) Biosynthesis of silver nanoparticles using Haloferax sp. NRS1: image analysis, characterization, in vitro thrombolysis, and cytotoxicity. AMB Express 11:75. https://doi.org/10.1186/s13568-021-01235-3
Moopantakath J, Imchen M, Kumavath R, Martínez-Espinosa RM (2021) Ubiquitousness of haloferax and carotenoid producing genes in Arabian Sea Coastal biosystems of India. Mar Drugs 19:442. https://doi.org/10.3390/md19080442
Imchen M, Vennapu RK, Ghosh P, Kumavath R (2019) Insights into antagonistic interactions of multidrug resistant bacteria in Mangrove sediments from the south Indian state of Kerala. Microorganisms 7:678. https://doi.org/10.3390/microorganisms7120678
Alam K, Farraj DAA, Mah-e-Fatima S et al (2020) Anti-biofilm activity of plant derived extracts against infectious pathogen-Pseudomonas aeruginosa PAO1. J Infect Public Health 13:1734–1741. https://doi.org/10.1016/J.JIPH.2020.07.007
Tatta ER, Kumavath R (2020) Rhodethrin and Rubrivivaxin as potential source of anti-biofilm agents against vancomycin resistant Enterococcus faecalis (ATCC 19443). Microb Pathog 148:104457. https://doi.org/10.1016/J.MICPATH.2020.104457
AAT Bioquest, Inc (2022, January 26) Quest Graph™ IC50 Calculator. AAT Bioquest. https://www.aatbio.com/tools/ic50-calculator
Lovecká P, Svobodová A, Macůrková A et al (2020) Decorative magnolia plants: a comparison of the content of their biologically active components showing antimicrobial effects. Plants 9:1–9. https://doi.org/10.3390/plants9070879
de Torre MP, Cavero RY, Calvo MI, Vizmanos JLW (2019) A simple and a reliable method to quantify antioxidant activity in vivo. Antioxidants 8:1–11. https://doi.org/10.3390/antiox8050142
Bailey-shaw YA, Williams LAD, Green CE et al (2017) In-vitro evaluation of the anti-inflammatory potential of selected Jamaican plant extracts using the Bovine Serum albumin protein denaturation assay. Int J Pharm Sci Rev Res 47:145–153
Buda DM, Bulzu PA, Barbu-Tudoran L et al (2019) Physiological response to silver toxicity in the extremely halophilic archaeon Halomicrobium mukohataei. FEMS Microbiol Lett 366:1–8. https://doi.org/10.1093/femsle/fnz231
Hammer Ø, Harper D, electronica PR-P, 2001 undefined (2001) PAST: paleontological statistics software package for education and data analysis. paleo.carleton.ca 4:178
Abd Alamer IS, Tomah AA, Ahmed T, Li B, Zhang J (2021) Biosynthesis of silver chloride nanoparticles by Rhizospheric bacteria and their Antibacterial Activity against phytopathogenic bacterium Ralstonia solanacearum. Molecules 27:224. https://doi.org/10.3390/molecules27010224
Bhatnagar S, Kobori T, Ganesh D et al (2019) Biosynthesis of silver nanoparticles mediated by extracellular pigment from talaromyces purpurogenus and their biomedical applications. Nanomaterials. https://doi.org/10.3390/nano9071042
Elamawi RM, Al-Harbi RE, Hendi AA (2018) Biosynthesis and characterization of silver nanoparticles using Trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egypt J Biol Pest Control 28:1–11. https://doi.org/10.1186/s41938-018-0028-1
Osorio-Echavarría J, Osorio-Echavarría J, Ossa-Orozco CP, Gómez-Vanegas NA (2021) Synthesis of silver nanoparticles using white-rot fungus Anamorphous Bjerkandera sp. R1: influence of silver nitrate concentration and fungus growth time. Sci Rep 11:1–14. https://doi.org/10.1038/s41598-021-82514-8
Imchen M, Moopantakath J, Kumavath R et al (2020) Current trends in experimental and computational approaches to combat antimicrobial resistance. Front Genet 11:1144. https://doi.org/10.3389/fgene.2020.563975
Patil S, Fernandes J, Tangasali R, Furtado I (2014) Exploitation of haloferax alexandrinus for biogenic synthesis of silver nanoparticles antagonistic to human and lower mammalian pathogens. J Clust Sci 25:423–433. https://doi.org/10.1007/s10876-013-0621-0
Abdollahnia M, Makhdoumi A, Mashreghi M, Eshghi H (2020) Exploring the potentials of halophilic prokaryotes from a solar saltern for synthesizing nanoparticles: the case of silver and selenium. PLoS ONE 15:e0229886. https://doi.org/10.1371/JOURNAL.PONE.0229886
Adiguzel AO, Adiguzel SK, Mazmanci B et al (2018) Silver nanoparticle biosynthesis from newly isolated streptomyces genus from soil. Mater Res Express 5:045402. https://doi.org/10.1088/2053-1591/aab861
Sivasankar P, Seedevi P, Poongodi S et al (2018) Characterization, antimicrobial and antioxidant property of exopolysaccharide mediated silver nanoparticles synthesized by Streptomyces violaceus MM72. Carbohydr Polym 181:752–759. https://doi.org/10.1016/J.CARBPOL.2017.11.082
Das P, Ghosal K, Jana NK et al (2019) Green synthesis and characterization of silver nanoparticles using belladonna mother tincture and its efficacy as a potential antibacterial and anti-inflammatory agent. Mater Chem Phys 228:310–317. https://doi.org/10.1016/J.MATCHEMPHYS.2019.02.064
Kedi PBE, Meva FE, Kotsedi L et al (2018) Eco-friendly synthesis, characterization, in vitro and in vivo anti-inflammatory activity of silver nanoparticle-mediated Selaginella myosurus aqueous extract. Int J Nanomedicine 13:8537–8548. https://doi.org/10.2147/IJN.S174530
Zewde B, Ambaye A, Stubbs J (2016) A review of stabilized silver nanoparticles: synthesis, biological properties, characterization, and potential areas of applications. Nanomedicine 4(1043):1–14
Maheshwaran G, Malai Selvi M, Selva Muneeswari R et al (2021) Green synthesis of lanthanum oxide nanoparticles using Moringa oleifera leaves extract and its biological activities. Adv Powder Technol 32:1963–1971. https://doi.org/10.1016/J.APT.2021.04.004
Ramamurthy CH, Padma M et al (2013) The extra cellular synthesis of gold and silver nanoparticles and their free radical scavenging and antibacterial properties. Coll Surf B Biointerfaces 102:808–815. https://doi.org/10.1016/J.COLSURFB.2012.09.025
Rodríguez-León E, Iñiguez-Palomares R, Navarro RE et al (2013) Synthesis of silver nanoparticles using reducing agents obtained from natural sources (Rumex hymenosepalus extracts). Nanoscale Res Lett 8:318. https://doi.org/10.1186/1556-276X-8-318
Rodrigo-Baños M, Garbayo I, Vílchez C et al (2015) Carotenoids from Haloarchaea and their potential in biotechnology. Mar Drugs 13:5508–5532. https://doi.org/10.3390/md13095508
Yu D, Yam VWW (2005) Hydrothermal-induced assembly of colloidal silver spheres into various nanoparticles on the basis of HTAB-modified silver mirror reaction. J Phys Chem B 109:5497–5503. https://doi.org/10.1021/jp0448346
Iravani S, Korbekandi H, Mirmohammadi SV, Zolfaghari B (2014) Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci 9:385
Martínez-Espinosa R (2001) Assimilatory nitrate reductase from the haloarchaeon Haloferax mediterranei: purification and characterization. FEMS Microbiol Lett 204:381–385. https://doi.org/10.1016/S0378-1097(01)00431-1
Kilic V, Kilic GA, Kutlu HM, Martínez-Espinosa RM (2017) Nitrate reduction in Haloferax alexandrinus: the case of assimilatory nitrate reductase. Extremophiles 21:551–561. https://doi.org/10.1007/s00792-017-0924-4
Miralles-Robledillo JM, Torregrosa-Crespo J, Martínez-Espinosa RM, Pire C (2019) DMSO reductase family: phylogenetics and applications of extremophiles. Int J Mol Sci 20:3349. https://doi.org/10.3390/ijms20133349
Martínez-Espinosa RM (2020) Microorganisms and their metabolic capabilities in the context of the biogeochemical nitrogen cycle at extreme environments. Int J Mol Sci 21:4228. https://doi.org/10.3390/ijms21124228
Chattopadhyay MK, Jagannadham MV, Vairamani M, Shivaji S (1997) Carotenoid pigments of an antarctic psychrotrophic bacterium Micrococcus roseus: temperature dependent biosynthesis, structure, and interaction with synthetic membranes. Biochem Biophys Res Commun 239:85–90. https://doi.org/10.1006/bbrc.1997.7433
Bankar A, Joshi B, Kumar AR, Zinjarde S (2010) Banana peel extract mediated novel route for the synthesis of silver nanoparticles. Coll Surf A Physicochem Eng Asp 368:58–63. https://doi.org/10.1016/j.colsurfa.2010.07.024
Vitolina S, Shulga G, Neiberte B, Jaunslavietis J, Verovkins A, Betkers T (2022) Characteristics of the waste wood biomass and its effect on the properties of wood sanding dust/recycled pp composite. Polymers (Basel) 14:468. https://doi.org/10.3390/polym14030468
Singh H, Du J, Singh P, Yi TH (2018) Extracellular synthesis of silver nanoparticles by Pseudomonas sp. THG-LS1.4 and their antimicrobial application. J Pharm Anal 8:258–264. https://doi.org/10.1016/J.JPHA.2018.04.004
Delfino I, Portaccio M, Della VB et al (2013) Enzyme distribution and secondary structure of sol-gel immobilized glucose oxidase by micro-attenuated total reflection FT-IR spectroscopy. Mater Sci Eng C 33:304–310. https://doi.org/10.1016/j.msec.2012.08.044
Kota S, Dumpala P, Anantha RK et al (2017) Evaluation of therapeutic potential of the silver/silver chloride nanoparticles synthesized with the aqueous leaf extract of Rumex acetosa. Sci Rep 7:11566. https://doi.org/10.1038/s41598-017-11853-2
Zhou X, Zhao Q, Liu G, Cai W (2019) 4-Mercaptophenylboronic acid modified Au nanosheets-built hollow sub-microcubes for active capture and ultrasensitive SERS-based detection of hexachlorocyclohexane pesticides. Sens Actuators B Chem 293:63–70. https://doi.org/10.1016/J.SNB.2019.04.153
Dar P, Dar A, Dar AM, Waqas U (2017) Synthesis of silver nanoparticles of mesocarp of Lagenaria siceraria fruit by using oleylamine and evaluation of its antimicrobial potential against clinically used pathogens. IJMBR 5:42–55
Vanaja M, Annadurai G (2013) Coleus aromaticus leaf extract mediated synthesis of silver nanoparticles and its bactericidal activity. Appl Nanosci 3:217–223. https://doi.org/10.1007/s13204-012-0121-9
Danaei M, Dehghankhold M, Ataei S et al (2018) Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 10:1–17. https://doi.org/10.3390/pharmaceutics10020057
Acknowledgements
The authors would like to express their gratitude for the research facilities provided by the Central University of Kerala lab. JM acknowledges the ICMR-SRF (45/41/2019-BIO/BMS) fellowship from the Government of India. The IM acknowledges the DBT-RA in Biotechnology & Life Sciences, Government of India.
Funding
This work was the partial financial support from DST-EMR (2016/003715/BBM), SERB-EMEQ/051/2014, and EEQ/2018/001085.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: RK, JM, IM. Analyzed the data: RK, JM, IM. Contributed reagents/materials/analysis tools: RK, BS. Wrote the paper: JM, RK, IM, RMME.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
Not applicable.
Consent for Publication
The authors have approved its submission for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moopantakath, J., Imchen, M., Sreevalsan, A. et al. Biosynthesis of Silver Chloride Nanoparticles (AgCl-NPs) from Extreme Halophiles and Evaluation of Their Biological Applications. Curr Microbiol 79, 266 (2022). https://doi.org/10.1007/s00284-022-02970-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-02970-x