Abstract
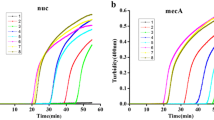
The aim of this study was to develop a rapid detection assay to identify methicillin-resistant Staphylococcus aureus by simultaneous testing for the mecA, nuc, and femB genes using the loop-mediated isothermal amplification (LAMP) method. LAMP primers were designed using online bio-software (http://primerexplorer.jp/e/), and amplification reactions were performed in an isothermal temperature bath. The products were then examined using 2% agarose gel electrophoresis. MecA, nuc, and femB were confirmed by triplex TaqMan real-time PCR. For better naked-eye inspection of the reaction result, hydroxy naphthol blue (HNB) was added to the amplification system. Within 60 min, LAMP successfully amplified the genes of interest under isothermal conditions at 63 °C. The results of 2% gel electrophoresis indicated that when the Mg2+ concentration in the reaction system was 6 μmol, the amplification of the mecA gene was relatively good, while the amplification of the nuc and femB genes was better at an Mg2+ concentration of 8 μmol. Obvious color differences were observed by adding 1 μL (3.75 mM) of HNB into 25 μL reaction system. The LAMP assay was applied to 128 isolates cases of methicillin-resistant Staphylococcus aureus, which were separated from the daily specimens and identified by Vitek microbial identification instruments. The results were identical for both LAMP and PCR. LAMP offers an alternative detection assay for mecA, nuc, and femB and is faster than other methods.


Similar content being viewed by others
References
Addicks JP, Gotting M, Jensen AM et al (2010) MRSA—current aspects of resistance, pathology, epidemiology and therapy. Versicherungsmedizin 62:183–188
Chambers HF, Deleo FR (2009) Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol 7:629–641. doi:10.1038/nrmicro2200
Davis MF, Peterson AE, Julian KG et al (2013) Household risk factors for colonization with multidrug-resistant Staphylococcus aureus isolates. PLoS ONE 8:e54733. doi:10.1371/journal.pone.0054733
Fishovitz J, Hermoso JA, Chang M et al (2014) Penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus. IUBMB Life 66:572–577. doi:10.1002/iub.1289
French GL (2009) Methods for screening for methicillin-resistant Staphylococcus aureus carriage. Clin Microbiol Infect 15(Suppl 7):10–16. doi:10.1111/j.1469-0691.2009.03092.x
Geiger K, Brown J (2013) Rapid testing for methicillin-resistant Staphylococcus aureus: implications for antimicrobial stewardship. Am J Health Syst Pharm 70:335–342. doi:10.2146/ajhp110724
Goto M, Honda E, Ogura A et al (2009) Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. Biotechniques 46:167–172. doi:10.2144/000113072
Hirvonen JJ (2014) The use of molecular methods for the detection and identification of methicillin-resistant Staphylococcus aureus. Biomark Med 8:1115–1125. doi:10.2217/bmm.14.60
Kim HJ, Kim HS, Lee JM et al (2016) Rapid detection of Pseudomonas aeruginosa and Acinetobacter baumannii Harboring bla(VIM-2), bla(IMP-1) and bla(OXA-23) genes by using loop-mediated isothermal amplification methods. Ann Lab Med 36:15–22. doi:10.3343/alm.2016.36.1.15
Kobayashi N, Wu H, Kojima K et al (1994) Detection of mecA, femA, and femB genes in clinical strains of staphylococci using polymerase chain reaction. Epidemiol Infect 113:259–266
Liu ZZ, Xiong YL, Fan XJ et al (2011) The correlation between expression level of femB and resistance phenotype of methicillin-resistant Staphylococcus aureus (MRSA). Sichuan Da Xue Xue Bao Yi Xue Ban 42:661–665
Montazeri EA, Khosravi AD, Jolodar A et al (2015) Identification of methicillin-resistant Staphylococcus aureus (MRSA) strains isolated from burn patients by multiplex PCR. Burns 41:590–594. doi:10.1016/j.burns.2014.08.018
Mori Y, Kanda H, Notomi T (2013) Loop-mediated isothermal amplification (LAMP): recent progress in research and development. J Infect Chemother 19:404–411. doi:10.1007/s10156-013-0590-0
Napolitano LM (2008) Early appropriate parenteral antimicrobial treatment of complicated skin and soft tissue infections caused by methicillin-resistant Staphylococcus aureus. Surg Infect 9(Suppl 1):s17–s27. doi:10.1089/sur.2008.063.supp (Larchmt)
Navarro MB, Huttner B, Harbarth S (2008) Methicillin-resistant Staphylococcus aureus control in the 21st century: beyond the acute care hospital. Curr Opin Infect Dis 21:372–379. doi:10.1097/QCO.0b013e3283013add
Notomi T, Okayama H, Masubuchi H et al (2000) Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 28:E63
Notomi T, Mori Y, Tomita N et al (2015) Loop-mediated isothermal amplification (LAMP): principle, features, and future prospects. J Microbiol 53:1–5. doi:10.1007/s12275-015-4656-9
Oteo J, Belen Aracil M (2015) Molecular characterization of resistance mechanisms: methicillin resistance Staphylococcus aureus, extended spectrum beta-lactamases and carbapenemases. Enferm Infecc Microbiol Clin 33(Suppl 2):27–33. doi:10.1016/S0213-005X(15)30012-4
Palavecino EL (2014) Rapid methods for detection of MRSA in clinical specimens. Methods Mol Biol 1085:71–83. doi:10.1007/978-1-62703-664-1_3
Pozzi C, Waters EM, Rudkin JK et al (2012) Methicillin resistance alters the biofilm phenotype and attenuates virulence in Staphylococcus aureus device-associated infections. PLoS Pathog 8:e1002626. doi:10.1371/journal.ppat.1002626
Sturenburg E (2009) Rapid detection of methicillin-resistant Staphylococcus aureus directly from clinical samples: methods, effectiveness and cost considerations. Ger Med Sci 7:Doc06. doi:10.3205/000065
Su J, Liu X, Cui H et al (2014) Rapid and simple detection of methicillin-resistance Staphylococcus aureus by orfX loop-mediated isothermal amplification assay. BMC Biotechnol 14:8. doi:10.1186/1472-6750-14-8
Sudhaharan S, Vanjari L, Mamidi N et al (2015) Evaluation of LAMP assay using phenotypic tests and conventional PCR for detection of nuc and mecA genes among clinical isolates of Staphylococcus spp. J Clin Diagn Res 9:DC06–09. doi:10.7860/JCDR/2015/13962.6315
Tacconelli E, De Angelis G, de Waure C et al (2009) Rapid screening tests for meticillin-resistant Staphylococcus aureus at hospital admission: systematic review and meta-analysis. Lancet Infect Dis 9:546–554. doi:10.1016/S1473-3099(09)70150-1
Towner KJ, Talbot DC, Curran R et al (1998) Development and evaluation of a PCR-based immunoassay for the rapid detection of methicillin-resistant Staphylococcus aureus. J Med Microbiol 47:607–613
Wang Y, Wang Y, Luo L et al (2015) Rapid and sensitive detection of Shigella spp. and Salmonella spp. by multiple endonuclease restriction real-time loop-mediated isothermal amplification technique. Front Microbiol 6:1400. doi:10.3389/fmicb.2015.01400
Wu R, Liu X, Guo B et al (2014) Development of double loop-mediated isothermal amplification to detect Listeria monocytogenes in food. Curr Microbiol 69:839–845. doi:10.1007/s00284-014-0661-1
Zervos M (2008) Treatment options for uncomplicated community-acquired skin and soft tissue infections caused by methicillin-resistant Staphylococcus aureus: oral antimicrobial agents. Surg Infect 9(Suppl 1):s29–s34. doi:10.1089/sur.2008.065.supp (Larchmt)
Zhang H, Feng J, Xue R et al (2014) Loop-mediated isothermal amplification assays for detecting Yersinia pseudotuberculosis in milk powders. J Food Sci 79:M967–M971. doi:10.1111/1750-3841.12436
Funding
This work was supported by the National Natural Science Foundation (No. 81401311) and the Capital Characteristic Clinical Application Research (WU JIEPING Foundation No. Z141107006614009). This work was also supported by the Navy General Hospital Innovation Cultivation Foundation (No. CXPY201412).
Author’s Contributions
CQY and ZQY conceived the study, collected and analyzed the data, and drafted the manuscript. GJW and LYJ conceived the project and provided technical support for data collection and analysis. CCG conceived the study and revised the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no competing interests.
Ethical Approval
The subjects of this work are bacteria that were separated and purified from clinical samples. This article does not contain any studies with human or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Chen, C., Zhao, Q., Guo, J. et al. Identification of Methicillin-Resistant Staphylococcus aureus (MRSA) Using Simultaneous Detection of mecA, nuc, and femB by Loop-Mediated Isothermal Amplification (LAMP). Curr Microbiol 74, 965–971 (2017). https://doi.org/10.1007/s00284-017-1274-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-017-1274-2