Abstract
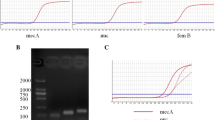
Staphylococcus aureus, including methicillin-resistant S. aureus (MRSA), is a major bacterial pathogen associated with nosocomial and community-acquired S. aureus infections all over the world. A rapid detection assay for staphylococcal gene of nuc and mecA is needed. In this study, a rapid identification assay based on the loop-mediated isothermal amplification (LAMP) method was established. PCR and LAMP assays were used to detect Staphylococcus aureus and other related species for nuc and mecA. With optimization of the primers and reaction temperature, the LAMP successfully amplified the genes under isothermal conditions at 62 °C within 60 min, of which the results were identical with those of the conventional PCR methods. The detection limits of the LAMP for nuc and mecA were 1.47 and 14.7 pg/μl DNA per tube, respectively, by naked eye inspections, while the detection limits of the PCR for nuc and mecA were 14.7 pg/μl and 147 pg/μl DNA, respectively. Finally, The LAMP method was then applied to clinical blood plaque samples. The LAMP and PCR demonstrated identical results for the plaque samples with the culture assay. Together, the LAMP offers an alternative detection assay for nuc and mecA with a great advantage of the sensitivity and rapidity.



Similar content being viewed by others
References
Tsuchiya, H., Sato, M., Miyazaki, T., Fujiwara, S., Tanigaki, S., Ohyama, M., Tanaka, T., & Iinuma, M. (1996). Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. Journal of Ethnopharmacology, 50, 27–34.
Khan, S., Rasheed, F., & Zahra, R. (2014). Genetic polymorphism of agr locus and antibiotic resistance of Staphylococcus aureus at two hospitals in Pakistan. Pakistan Journal of Medical Sciences, 30, 172–176.
Morell, E. A., & Balkin, D. M. (2010). Methicillin-resistant Staphylococcus aureus: a pervasive pathogen highlights the need for new antimicrobial development. The Yale Journal of Biology and Medicine, 83, 223–233.
Dulon, M., Haamann, F., Peters, C., Schablon, A., & Nienhaus, A. (2011). MRSA prevalence in European healthcare settings: a review. BMC Infectious Diseases, 11, 138.
Coughenour, C., Stevens, V., & Stetzenbach, L. D. (2011). An evaluation of methicillin-resistant Staphylococcus aureus survival on five environmental surfaces. Microbial Drug Resistance, 17, 457–461.
Fuda, C. C., Fisher, J. F., & Mobashery, S. (2005). Beta-lactam resistance in Staphylococcus aureus: the adaptive resistance of a plastic genome. Cellular and Molecular Life Sciences, 62, 2617–2633.
Shylaja, R., Thakasi, D. K., Murali, H. S., Reddy, K. P., & Batra, H. V. (2012). Application of a chimeric protein construct having enterotoxin B and toxic shock syndrome toxin domains of S. Aureus in immunodiagnostics. Indian Journal of Microbiology, 52, 449–455.
Li, S. J., Hu, D. L., Maina, E. K., Shinagawa, K., Omoe, K., & Nakane, A. (2011). Superantigenic activity of toxic shock syndrome toxin-1 is resistant to heating and digestive enzymes. Journal of Applied Microbiology, 110, 729–736.
Stoakes, L., Reyes, R., Daniel, J., Lennox, G., John, M. A., Lannigan, R., & Hussain, Z. (2006). Prospective comparison of a new chromogenic medium, MRSA select, to CHROMagar MRSA and mannitol-salt medium supplemented with oxacillin or cefoxitin for detection of methicillin-resistant Staphylococcus aureus. Journal of Clinical Microbiology, 44, 637–639.
Hirvonen, J. J., & Kaukoranta, S. S. (2013). GenomEra MRSA/SA, a fully automated homogeneous PCR assay for rapid detection of Staphylococcus aureus and the marker of methicillin resistance in various sample matrixes. Expert Review of Molecular Diagnostics, 13, 655–665.
Hoorfar, J. (2011). Rapid detection, characterization, and enumeration of foodborne pathogens. APMIS Supplementum, 133, 1–24.
Huletsky, A., Giroux, R., Rossbach, V., Gagnon, M., Vaillancourt, M., Bernier, M., Gagnon, F., Truchon, K., Bastien, M., Picard, F. J., Belkum, A., Ouellette, M., Roy, P. H., & Bergeron, M. G. (2004). New real-time PCR assay for rapid detection of methicillin-resistant Staphylococcus aureus directly from specimens containing a mixture of staphylococci. Journal of Clinical Microbiology, 42, 1875–1884.
Jonas, D., Speck, M., Daschner, F. D., & Grundmann, H. (2002). Rapid PCR-based identification of methicillin-resistant Staphylococcus aureus from screening swabs. Journal of Clinical Microbiology, 40, 1821–1823.
Notomi, T., Okayama, H., Masubuchi, H., Yonekawa, T., Watanabe, K., Amino, N., & Hase, T. (2000). Loop-mediated isothermal amplification of DNA. Nucleic Acids Research, 28, E63.
Koide, Y., Maeda, H., Yamabe, K., Naruishi, K., Yamamoto, T., Kokeguchi, S., & Takashiba, S. (2010). Rapid detection of mecA and spa by the loop-mediated isothermal amplification (LAMP) method. Letters in Applied Microbiology, 50, 386–392.
Lim, K. T., Teh, C. S., & Thong, K. L. (2013). Loop-mediated isothermal amplification assay for the rapid detection of Staphylococcus aureus. BioMed Research International, 2013, 895816.
Safavieh, M., Ahmed, M. U., Ng, A., & Zourob, M. (2014). High-throughput real-time electrochemical monitoring of LAMP for pathogenic bacteria detection. Biosensors and Bioelectronics, 58, 101–106.
Balbin, M. M., Belotindos, L. P., Abes, N. S., & Mingala, C. N. (2014). Caprine arthritis encephalitis virus detection in blood by loop-mediated isothermal amplification (LAMP) assay targeting the proviral gag region. Diagnostic Microbiology and Infectious Disease, 79, 39–42.
Yamazaki, M., Aoki, H., Iwade, Y., Matsumoto, M., Yamada, K., Yamamoto, H., Suzuki, M., Hiramatsu, R., & Minagawa, H. (2012). An enrichment medium for increasing a very small number of vibrio parahaemolyticus cells to the detection limit of the loop-mediated isothermal amplification (LAMP) assay. Japanese Journal of Infectious Diseases, 65, 111–116.
Ding, X., Nie, K., Zeng, Y. L., Wang, J., Shi, L., & Ma, X. J. (2013). Establishment of method and modification of colorimetric judgment on HIV-1 virus detection by reverse transcription loop-mediated isothermal amplification. Zhonghua Yu Fang Yi Xue Za Zhi, 47, 1045–1049.
Zhang, J., Feng, Y., Hu, D., Lv, H., Zhu, J., Cao, M., Zheng, F., Zhu, J., Gong, X., Hao, L., Srinivas, S., Ren, H., Qi, Z., Li, B., & Wang, C. (2013). Rapid and sensitive detection of H7N9 avian influenza virus by use of reverse transcription-loop-mediated isothermal amplification. Journal of Clinical Microbiology, 51, 3760–3764.
Hasani, A., Sheikhalizadeh, V., Hasani, A., Naghili, B., Valizadeh, V., & Nikoonijad, A. R. (2013). Methicillin resistant and susceptible Staphylococcus aureus: appraising therapeutic approaches in the Northwest of Iran. Iranian Journal of Microbiology, 5, 56–62.
Brukner, I., Oughton, M., Giannakakis, A., Kerzner, R., & Dascal, A. (2013). Significantly improved performance of a multitarget assay over a commercial SCCmec-based assay for methicillin-resistant Staphylococcus aureus screening: applicability for clinical laboratories. Journal of Molecular Diagnostics, 15, 577–580.
Yong, D., Toleman, M. A., Giske, C. G., Cho, H. S., Sundman, K., Lee, K., & Walsh, T. R. (2009). Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrobial Agents and Chemotherapy, 53, 5046–5054.
Mori, Y., Kitao, M., Tomita, N., & Notomi, T. (2004). Real-time turbidimetry of LAMP reaction for quantifying template DNA. Journal of Biochemical and Biophysical Methods, 59, 145–157.
Liu, W., Zou, D., Li, Y., Wang, X., He, X., Wei, X., Shao, C., Li, X., Shang, W., Yu, K., Liu, D., Li, Y., Guo, J., Yin, Z., & Yuan, J. (2012). Sensitive and rapid detection of the New Delhi metallo-beta-lactamase gene by loop-mediated isothermal amplification. Journal of Clinical Microbiology, 50, 1580–1585.
Acknowledgments
This work was supported by the two grants from the General Program of China during the 12th Five-Year Plan Period (No. CWS11J205) and the Military Major Special Project during the 12th Five-year Plan Period (No. 424135S).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xin-Ru Wang and Li-Fen Wu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, XR., Wu, LF., Wang, Y. et al. Rapid Detection of Staphylococcus Aureus by Loop-Mediated Isothermal Amplification. Appl Biochem Biotechnol 175, 882–891 (2015). https://doi.org/10.1007/s12010-014-1328-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1328-x