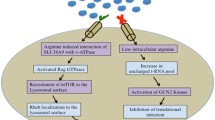
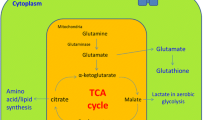
Abstract
Cancer is the second leading cause of death globally. Chemotherapy and radiation therapy and other medications are employed to treat various types of cancer. However, each treatment has its own set of side effects, owing to its low specificity. As a result, there is an urgent need for newer therapeutics that do not disrupt healthy cells’ normal functioning. Depriving nutrient or non/semi-essential amino acids to which cancerous cells are auxotrophic remains one such promising anticancer strategy. l-Arginine (Arg) is a semi-essential vital amino acid involved in versatile metabolic processes, signaling pathways, and cancer cell proliferation. Hence, the administration of Arg depriving enzymes (ADE) such as arginase, arginine decarboxylase (ADC), and arginine deiminase (ADI) could be effective in cancer therapy. The Arg auxotrophic cancerous cells like hepatocellular carcinoma, human colon cancer, leukemia, and breast cancer cells are sensitive to ADE treatment due to low expression of crucial enzymes argininosuccinate synthetase (ASS), argininosuccinate lyase (ASL), and ornithine transcarbamylase (OCT). These therapeutic enzyme treatments induce cell death through inducing autophagy, apoptosis, generation of oxidative species, i.e., oxidative stress, and arresting the progression and expansion of cancerous cells at certain cell cycle checkpoints. The enzymes are undergoing clinical trials and could be successfully exploited as potential anticancer agents in the future.








Similar content being viewed by others
Abbreviations
- Arg:
-
L-Arginine
- ADE:
-
Arginine depriving enzymes
- ADC:
-
Arginine decarboxylase
- ADI:
-
Arginine deiminase
- ASS:
-
Argininosuccinate synthetase
- OCT:
-
Ornithine transcarbamylase
- OAT:
-
Ornithine aminotransferase
- ODC:
-
Ornithine decarboxylase
- PEG:
-
Polyethylene glycol
- hArg:
-
Human arginase
- rhArg:
-
Recombinant human arginase
- NO:
-
Nitric oxide
References
Chandra S (2012) Endophytic fungi: novel sources of anticancer lead molecules. Appl Microbiol Biotechnol 95:47–59. https://doi.org/10.1007/s00253-012-4128-7
Wu G (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37:1–17. https://doi.org/10.1007/s00726-009-0269-0
Oberkersch RE, Santoro MM (2019) Role of amino acid metabolism in angiogenesis. Vascul Pharmacol 112:17–23. https://doi.org/10.1016/j.vph.2018.11.001
Icard P, Lincet H (2012) A global view of the biochemical pathways involved in the regulation of the metabolism of cancer cells. Biochim Biophys Acta Rev Cancer 1826:423–433. https://doi.org/10.1016/j.bbcan.2012.07.001
Lukey MJ, Katt WP, Cerione RA (2017) Targeting amino acid metabolism for cancer therapy. Drug Discov 22:796–804. https://doi.org/10.1016/j.drudis.2016.12.003
Scott L, Lamb J, Smith S, Wheatley DN (2000) Single amino acid (arginine) deprivation: rapid and selective death of cultured transformed and malignant cells. Br J Cancer 83:800–810. https://doi.org/10.1054/bjoc.2000.1353
Caldwell RB, Toque HA, Narayanan SP, Caldwell RW (2015) Arginase: an old enzyme with new tricks. Trends Pharmacol Sci 36:395–405. https://doi.org/10.1016/j.tips.2015.03.006
Jahani M, Noroznezhad F, Mansouri K (2018) Arginine: challenges and opportunities of this two-faced molecule in cancer therapy. Biomed Pharmacother 102:594–601. https://doi.org/10.1016/j.biopha.2018.02.109
Huang CC, Tsai ST, Kuo CC et al (2012) Arginine deprivation as a new treatment strategy for head and neck cancer. Oral Oncol 48:1227–1235. https://doi.org/10.1016/j.oraloncology.2012.06.004
Gilroy E (1930) The influence of arginine upon the growth rate of a transplantable tumour in the mouse. Biochem J 24:589–595
Patil MD, Bhaumik J, Babykutty S et al (2016) Arginine dependence of tumor cells: targeting a chink in cancer’s armor. Oncogene 35:4957–9724. https://doi.org/10.1038/onc.2016.37
dos Reis KC, Coimbra JM, Duarte WF et al (2019) Biological treatment of vinasse with yeast and simultaneous production of single-cell protein for feed supplementation. Int J Environ Sci Technol 16:763–774. https://doi.org/10.1007/s13762-018-1709-8
Miyazaki K, Takaku H, Umeda M et al (1990) Potent growth inhibition of human tumor cells in culture by arginine deiminase purified from a culture medium of a Mycoplasma-infected cell line. Cancer Res 50:4522–4527
Delman KA, Brown TD, Thomas M et al (2005) Phase I/II trial of pegylated arginine deiminase (ADI-PEG20) in unresectable hepatocellular carcinoma. J Clin Oncol 23:4139–4139. https://doi.org/10.1200/jco.2005.23.16_suppl.4139
Feun LG, Savaraj N, Marini A et al (2006) Phase II study of pegylated arginine deiminase (ADI-PEG20), a novel targeted therapy for melanoma. J Clin Oncol 24:8045–8045. https://doi.org/10.1200/jco.2006.24.18_suppl.8045
Ott PA, Carvajal RD, Pandit-Taskar N et al (2013) Phase I/II study of pegylated arginine deiminase (ADI-PEG 20) in patients with advanced melanoma. Invest New Drugs 31:425–434. https://doi.org/10.1007/s10637-012-9862-2
Regunathan S, Piletz JE (2003) Regulation of inducible nitric oxide synthase and agmatine synthesis in macrophages and astrocytes. Ann N Y Acad Sci 1009:20–29. https://doi.org/10.1196/annals.1304.002
Cheng PN-M, Lam T-L, Lam W-M et al (2007) Pegylated recombinant human arginase (rhArg-peg5,000mw) inhibits the in vitro and in vivo proliferation of human hepatocellular carcinoma through arginine depletion. Cancer Res 67:309–317. https://doi.org/10.1158/0008-5472.CAN-06-1945
Hsueh EC, Knebel SM, Lo W-H et al (2012) Deprivation of arginine by recombinant human arginase in prostate cancer cells. J Hematol Oncol 5:17. https://doi.org/10.1186/1756-8722-5-17
Glazer ES, Stone EM, Zhu C et al (2011) Bioengineered human arginase I with enhanced activity and stability controls hepatocellular and pancreatic carcinoma xenografts. Transl Oncol 4:138–146. https://doi.org/10.1593/tlo.10265
Yang JS, Wang CC, Qiu JD et al (2021) Arginine metabolism: a potential target in pancreatic cancer therapy. Chin Med J 134:28–37. https://doi.org/10.1097/CM9.0000000000001216
Hernandez CP, Morrow K, Lopez-Barcons LA et al (2010) Pegylated arginase I: a potential therapeutic approach in T-ALL. Blood 115:5214–5221. https://doi.org/10.1182/blood-2009-12-258822
Khoury O, Ghazale N, Stone E et al (2015) Human recombinant arginase I (Co)-PEG5000 [HuArgI (Co)-PEG5000]-induced arginine depletion is selectively cytotoxic to human glioblastoma cells. J Neuro-Oncol 122:75–85. https://doi.org/10.1007/s11060-014-1698-5
Singh R, Pervin S, Karimi A et al (2000) Arginase activity in human breast cancer cell lines: Nω-Hydroxy-l-arginine selectively inhibits cell proliferation and induces apoptosis in MDA-MB-468 Cells. Cancer Res 60:3305–3312
Zeng X, Li Y, Fan J et al (2013) Recombinant human arginase induced caspase-dependent apoptosis and autophagy in non-Hodgkin’s lymphoma cells. Cell Death Dis 4:e840. https://doi.org/10.1038/cddis.2013.359
Tomlinson C, Rafii M, Sgro M et al (2011) Arginine is synthesized from proline, not glutamate, in enterally fed human preterm neonates. Pediatr Res 69:46–50. https://doi.org/10.1203/PDR.0b013e3181fc6ab7
Riess C, Shokraie F, Classen CF et al (2018) Arginine-depleting enzymes—an increasingly recognized treatment strategy for therapy-refractory malignancies. Cell Physiol Biochem 51:854–870. https://doi.org/10.1159/000495382
Xiong L, Teng JLL, Botelho MG et al (2016) Arginine metabolism in bacterial pathogenesis and cancer therapy. Int J Mol Sci 17:363. https://doi.org/10.3390/ijms17030363
Pasut G, Sergi M, Veronese FM (2008) Anti-cancer PEG-enzymes: 30 years old, but still a current approach. Adv Drug Deliv Rev 60:69–78. https://doi.org/10.1016/j.addr.2007.04.018
Vellard M (2003) The enzyme as drug: application of enzymes as pharmaceuticals. Curr Opin Biotechnol 14:444–450. https://doi.org/10.1016/S0958-1669(03)00092-2
Lukey MJ, Wilson KF, Cerione RA (2013) Therapeutic strategies impacting cancer cell glutamine metabolism. Future Med Chem 5:1685–1700. https://doi.org/10.4155/fmc.13.130
Katt WP, Lukey MJ, Cerione RA (2017) A tale of two glutaminases: homologous enzymes with distinct roles in tumorigenesis. Future Med Chem 9:223–243. https://doi.org/10.4155/fmc-2016-0190
Bansal S, Srivastava A, Mukherjee G et al (2012) Hyperthermophilic asparaginase mutants with enhanced substrate affinity and antineoplastic activity: structural insights on their mechanism of action. FASEB J 26:1161–1171. https://doi.org/10.1096/fj.11-191254
Kundu B, Bansal S, Mishra P (2016) Mutants of L-asparaginase. Patent, US9322008B2
Pardee AB (1974) A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci 71:1286–1290. https://doi.org/10.1073/pnas.71.4.1286
Müller HJ, Boos J (1998) Use of L-asparaginase in childhood ALL. Crit Rev Oncol Hematol 28:97–113. https://doi.org/10.1016/S1040-8428(98)00015-8
Icard P, Fournel L, Wu Z et al (2019) Interconnection between metabolism and cell cycle in cancer. Trends Biochem Sci 44:490–521. https://doi.org/10.1016/j.tibs.2018.12.007
Wheatley DN (2005) Arginine deprivation and metabolomics: Important aspects of intermediary metabolism in relation to the differential sensitivity of normal and tumour cells. Semin Cancer Biol 15:247–253. https://doi.org/10.1016/j.semcancer.2005.04.002
Ascierto PA, Scala S, Castello G et al (2005) Pegylated arginine deiminase treatment of patients with metastatic melanoma: results from phase I and II studies. J Clin Oncol 23:7660–7668. https://doi.org/10.1200/JCO.2005.02.0933
Delage B, Luong P, Maharaj L et al (2012) Promoter methylation of argininosuccinate synthetase-1 sensitises lymphomas to arginine deiminase treatment, autophagy and caspase-dependent apoptosis. Cell Death Dis 3:e342. https://doi.org/10.1038/cddis.2012.83
Qiu F, Huang J, Sui M (2015) Targeting arginine metabolism pathway to treat arginine-dependent cancers. Cancer Lett 364:1–7. https://doi.org/10.1016/j.canlet.2015.04.020
Huang H-L, Chen W-C, Hsu H-P et al (2015) Argininosuccinate lyase is a potential therapeutic target in breast cancer. Oncol Rep 34:3131–3139. https://doi.org/10.3892/or.2015.4280
Hung Y-H, Huang H-L, Chen W-C et al (2017) Argininosuccinate lyase interacts with cyclin A2 in cytoplasm and modulates growth of liver tumor cells. Oncol Rep 37:969–978. https://doi.org/10.3892/or.2016.5334
Vardon A, Dandapani M, Cheng D et al (2017) Arginine auxotrophic gene signature in paediatric sarcomas and brain tumours provides a viable target for arginine depletion therapies. Oncotarget 8:63506–63517. https://doi.org/10.18632/oncotarget.18843
Feun L, You M, Wu CJ et al (2008) Arginine deprivation as a targeted therapy for cancer. Curr Pharm Des 14:1049–1057. https://doi.org/10.2174/138161208784246199
Yeatman TJ, Risley GL, Brunson ME (1991) Depletion of dietary arginine inhibits growth of metastatic tumor. Arch Surg 126:1376–1381. https://doi.org/10.1001/archsurg.1991.01410350066010 (Discussion 1381-1382)
Gonzalez GG, Byus CV (1991) Effect of dietary arginine restriction upon ornithine and polyamine metabolism during two-stage epidermal carcinogenesis in the mouse. Cancer Res 51:2932–2939
Abdelmagid SA, Rickard JA, McDonald WJ et al (2011) CAT-1-mediated arginine uptake and regulation of nitric oxide synthases for the survival of human breast cancer cell lines. J Cell Biochem 112:1084–1092. https://doi.org/10.1002/jcb.23022
Sosa V, Moliné T, Somoza R et al (2013) Oxidative stress and cancer: an overview. Ageing Res Rev 12:376–390. https://doi.org/10.1016/j.arr.2012.10.004
Changou CA, Chen Y-R, Xing L et al (2014) Arginine starvation-associated atypical cellular death involves mitochondrial dysfunction, nuclear DNA leakage, and chromatin autophagy. Proc Natl Acad Sci 111:14147–14152. https://doi.org/10.1073/pnas.1404171111
Rybstein MD, Pedro JMBS, Kroemer G, Galluzzi L (2018) The autophagic network and cancer. Nat Cell Biol 20:243–251. https://doi.org/10.1038/s41556-018-0042-2
Delage B, Fennell DA, Nicholson L et al (2010) Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int J Cancer 126:2762–2772. https://doi.org/10.1002/ijc.25202
Rabinovich S, Adler L, Yizhak K et al (2015) Diversion of aspartate in ASS1-deficient tumors fosters de novo pyrimidine synthesis. Nature 527:379–383. https://doi.org/10.1038/nature15529
Kim RH, Coates JM, Bowles TL et al (2009) Arginine deiminase as a novel therapy for prostate cancer induces autophagy and caspase-independent apoptosis. Cancer Res 69:700–708. https://doi.org/10.1158/0008-5472.CAN-08-3157
O’Sullivan D, Sanin DE, Pearce EJ, Pearce EL (2019) Metabolic interventions in the immune response to cancer. Nat Rev Immunol 19:324–335. https://doi.org/10.1038/s41577-019-0140-9
Molderings GJ, Kribben B, Heinen A et al (2004) Intestinal tumor and agmatine (decarboxylated arginine): low content in colon carcinoma tissue specimens and inhibitory effect on tumor cell proliferation in vitro. Cancer 101:858–868. https://doi.org/10.1002/cncr.20407
Mayeur C, Veuillet G, Michaud M et al (2005) Effects of agmatine accumulation in human colon carcinoma cells on polyamine metabolism, DNA synthesis and the cell cycle. Biochim Biophys Acta Mol Cell Res 1745:111–123. https://doi.org/10.1016/j.bbamcr.2004.12.004
Wolf C, Brüss M, Hanisch B et al (2007) Molecular basis for the antiproliferative effect of agmatine in tumor cells of colonic, hepatic, and neuronal origin. Mol Pharmacol 71:276–283. https://doi.org/10.1124/mol.106.028449
Haenisch B, Bönisch H, Cichon S et al (2011) Effects of exogenous agmatine in human leukemia HMC-1 and HL-60 cells on proliferation, polyamine metabolism and cell cycle. Leuk Res 35:1248–1253. https://doi.org/10.1016/j.leukres.2010.12.023
Weickmann JL, Fahrney DE (1977) Arginine deiminase from Mycoplasma arthritidis. Evidence for multiple forms. J Biol Chem 252:2615–2620
Cunin R, Glansdorff N, Piérard A, Stalon V (1986) Biosynthesis and metabolism of arginine in bacteria. Microbiol Rev 50:314–352
Ryan S, Begley M, Gahan CGM, Hill C (2009) Molecular characterization of the arginine deiminase system in Listeria monocytogenes: regulation and role in acid tolerance. Environ Microbiol 11:432–445. https://doi.org/10.1111/j.1462-2920.2008.01782.x
Ba D, Mc F, Ah D et al (2000) Characterization of an isogenic mutant of Streptococcus pyogenes Manfredo lacking the ability to make streptococcal acid glycoprotein. Infect Immun 68:2441–2448. https://doi.org/10.1128/iai.68.5.2441-2448.2000
Xiong L, Teng JL, Watt RM et al (2014) Arginine deiminase pathway is far more important than urease for acid resistance and intracellular survival in Laribacter hongkongensis: a possible result of arc gene cassette duplication. BMC Microbiol 14:1–12. https://doi.org/10.1186/1471-2180-14-42
Choi Y, Choi J, Groisman EA et al (2012) Expression of STM4467-encoded arginine deiminase controlled by the STM4463 regulator contributes to Salmonella enterica Serovar typhimurium virulence. Infect Immun 80:4291–4297. https://doi.org/10.1128/IAI.00880-12
Takaku H, Takase M, Abe S et al (1992) In vivo anti-tumor activity of arginine deiminase purified from Mycoplasma arginini. Int J Cancer 51:244–249. https://doi.org/10.1002/ijc.2910510213
Maghnouj A, Abu-Bakr AAW, Baumberg S et al (2000) Regulation of anaerobic arginine catabolism in Bacillus licheniformis by a protein of the Crp/Fnr family. FEMS Microbiol Lett 191:227–234. https://doi.org/10.1111/j.1574-6968.2000.tb09344.x
Zúñiga M, Champomier-Verges M, Zagorec M, Pérez-Martínez G (1998) Structural and functional analysis of the gene cluster encoding the enzymes of the arginine deiminase pathway of Lactobacillus sake. J Bacteriol 180:4154–4159
Griswold A, Chen Y-YM, Snyder JA, Burne RA (2004) Characterization of the arginine deiminase operon of Streptococcus rattus FA-1. Appl Environ Microbiol 70:1321–1327. https://doi.org/10.1128/AEM.70.3.1321-1327.2004
Gamper M, Zimmermann A, Haas D (1991) Anaerobic regulation of transcription initiation in the arcDABC operon of Pseudomonas aeruginosa. J Bacteriol 173:4742–4750
Takaku H, Misawa S, Hayashi H, Miyazaki K (1993) Chemical modification by polyethylene glycol of the anti-tumor enzyme arginine deiminase from Mycoplasma arginini. Japn J Cancer Res 84:1195–1200. https://doi.org/10.1111/j.1349-7006.1993.tb02821.x
Takaku H, Matsumoto M, Misawa S, Miyazaki K (1995) Anti-tumor activity of arginine deiminase from mycoplasma arginini and its growth-inhibitory mechanism. Japn J Cancer Res 86:840–846. https://doi.org/10.1111/j.1349-7006.1995.tb03094.x
Sugimura K, Ohno T, Kusuyama T, Azuma I (1992) High sensitivity of human melanoma cell lines to the growth inhibitory activity of mycoplasmal arginine deiminase in vitro. Melanoma Res 2:191–196. https://doi.org/10.1097/00008390-199209000-00007
Gong H, Zölzer F, von Recklinghausen G et al (2000) Arginine deiminase inhibits proliferation of human leukemia cells more potently than asparaginase by inducing cell cycle arrest and apoptosis. Leukemia 14:826–829. https://doi.org/10.1038/sj.leu.2401763
Kelly MP, Jungbluth AA, Wu B-W et al (2012) Arginine deiminase PEG20 inhibits growth of small cell lung cancers lacking expression of argininosuccinate synthetase. Br J Cancer 106:324–332. https://doi.org/10.1038/bjc.2011.524
Ensor CM, Holtsberg FW, Bomalaski JS, Clark MA (2002) Pegylated arginine deiminase (ADI-SS PEG20,000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer Res 62:5443–5450
Holtsberg FW, Ensor CM, Steiner MR et al (2002) Poly(ethylene glycol) (PEG) conjugated arginine deiminase: effects of PEG formulations on its pharmacological properties. J Control Release 80:259–271. https://doi.org/10.1016/S0168-3659(02)00042-1
Thongkum A, Wu C, Li Y-Y et al (2017) The combination of arginine deprivation and 5-fluorouracil improves therapeutic efficacy in argininosuccinate synthetase negative hepatocellular carcinoma. Int J Mol Sci 18:1175. https://doi.org/10.3390/ijms18061175
Liu J, Ma J, Wu Z et al (2014) Arginine deiminase augments the chemosensitivity of argininosuccinate synthetase-deficient pancreatic cancer cells to gemcitabine via inhibition of NF-κB signaling. BMC Cancer 14:1–17. https://doi.org/10.1186/1471-2407-14-686
Savaraj N, Wu C, Li Y-Y et al (2015) Targeting argininosuccinate synthetase negative melanomas using combination of arginine degrading enzyme and cisplatin. Oncotarget 6:6295–6309. https://doi.org/10.18632/oncotarget.3370
Jones JB (1981) The effect of arginine deiminase on murine leukemic lymphoblasts. PhD Thesis, University of Oklahoma Health Sciences Center
Misawa S, Aoshima M, Takaku H et al (1994) High-level expression of mycoplasma arginine deiminase in Escherichia coli and its efficient renaturation as an anti-tumor enzyme. J Biotechnol 36:145–155. https://doi.org/10.1016/0168-1656(94)90050-7
Gong H, Zölzer F, von Recklinghausen G et al (1999) Arginine deiminase inhibits cell proliferation by arresting cell cycle and inducing apoptosis. Biochem Biophys Res Commun 261:10–14. https://doi.org/10.1006/bbrc.1999.1004
Beloussow K, Wang L, Wu J et al (2002) Recombinant arginine deiminase as a potential anti-angiogenic agent. Cancer Lett 183:155–162. https://doi.org/10.1016/S0304-3835(01)00793-5
Shen L-J, Lin W-C, Beloussow K, Shen W-C (2003) Resistance to the anti-proliferative activity of recombinant arginine deiminase in cell culture correlates with the endogenous enzyme, argininosuccinate synthetase. Cancer Lett 191:165–170. https://doi.org/10.1016/S030-43835(02)00693-6
Noh E-J, Kang S-W, Shin Y-J et al (2004) Arginine deiminase enhances dexamethasone-induced cytotoxicity in human T-lymphoblastic leukemia CCRF-CEM cells. Int J Cancer 112:502–508. https://doi.org/10.1002/ijc.20435
Yoon C-Y, Shim Y-J, Kim E-H et al (2007) Renal cell carcinoma does not express argininosuccinate synthetase and is highly sensitive to arginine deprivation via arginine deiminase. Int J Cancer 120:897–905. https://doi.org/10.1002/ijc.22322
Bowles TL, Kim R, Galante J et al (2008) Pancreatic cancer cell lines deficient in argininosuccinate synthetase are sensitive to arginine deprivation by arginine deiminase. Int J Cancer 123:1950–1955. https://doi.org/10.1002/ijc.23723
You M, Savaraj N, Wu C et al (2010) Abstract 61: enhancing arginine deprivation therapy in melanoma by combining with cisplatin. Cancer Res 70:61–61. https://doi.org/10.1158/1538-7445.AM10-61
Izzo F, Marra P, Beneduce G et al (2004) Pegylated arginine deiminase treatment of patients with unresectable hepatocellular carcinoma: results from phase I/II studies. J Clin Oncol 22:1815–1822. https://doi.org/10.1200/JCO.2004.11.120
Yang TS, Lu SN, Chao Y et al (2010) A randomised phase II study of pegylated arginine deiminase (ADI-PEG 20) in Asian advanced hepatocellular carcinoma patients. Br J Cancer 103:954–960. https://doi.org/10.1038/sj.bjc.6605856
Lowery MA, Yu KH, Kelsen DP et al (2017) A phase 1/1B trial of ADI-PEG 20 plus nab-paclitaxel and gemcitabine in patients with advanced pancreatic adenocarcinoma. Cancer 123:4556–4565. https://doi.org/10.1002/cncr.30897
Beddowes E, Spicer J, Chan PY et al (2017) Phase 1 Dose-escalation study of pegylated arginine deiminase, cisplatin, and pemetrexed in patients with argininosuccinate synthetase 1–deficient thoracic cancers. J Clin Oncol 35:1778–1785. https://doi.org/10.1200/JCO.2016.71.3230
Szlosarek PW, Steele JP, Nolan L et al (2017) Arginine deprivation with pegylated arginine deiminase in patients with argininosuccinate synthetase 1-deficient malignant pleural mesothelioma: a randomized clinical trial. JAMA Oncol 3:58–66. https://doi.org/10.1001/jamaoncol.2016.3049
Harding JJ, Do RK, Dika IE et al (2018) A phase 1 study of ADI-PEG 20 and modified FOLFOX6 in patients with advanced hepatocellular carcinoma and other gastrointestinal malignancies. Cancer Chemother Pharmacol 82:429–440. https://doi.org/10.1007/s00280-018-3635-3
Hall PE, Lewis R, Syed N et al (2019) A Phase I study of pegylated arginine deiminase (pegargiminase), cisplatin, and pemetrexed in argininosuccinate synthetase 1-deficient recurrent high-grade glioma. Clin Cancer Res 25:2708–2716. https://doi.org/10.1158/1078-0432
Tsai HJ, Hsiao HH, Hsu YT et al (2021) Phase I study of ADI-PEG20 plus low-dose cytarabine for the treatment of acute myeloid leukemia. Cancer Med 10:2946–2955. https://doi.org/10.1002/cam4.3871
Yao S, Janku F, Subbiah V et al (2021) Phase 1 trial of ADI-PEG20 plus cisplatin in patients with pretreated metastatic melanoma or other advanced solid malignancies. Br J Cancer 124:1533–1539. https://doi.org/10.1038/s41416-020-01230-8
Bewley MC, Lott JS, Baker EN, Patchett ML (1996) The cloning, expression and crystallisation of a thermostable arginase. FEBS Lett 386:215–218. https://doi.org/10.1016/0014-5793(96)00459-0
Muszynska G, Severina LO, Lobyreva LW (1972) Characteristics of arginases from plant, ureotelic and uricotelic organisms. Acta Biochim Pol 19:109–116
Dizikes GJ, Grody WW, Kern RM, Cederbaum SD (1986) Isolation of human liver arginase cDNA and demonstration of nonhomology between the two human arginase genes. Biochem Biophys Res Commun 141:53–59. https://doi.org/10.1016/S0006-291X(86)80333-3
Gotoh T, Sonoki T, Nagasaki A et al (1996) Molecular cloning of cDNA for nonhepatic mitochondrial arginase (arginase II) and comparison of its induction with nitric oxide synthase in a murine macrophage-like cell line. FEBS Lett 395:119–122. https://doi.org/10.1016/0014-5793(96)01015-0
Vockley JG, Jenkinson CP, Shukla H et al (1996) Cloning and characterization of the human type II arginase gene. Genomics 38:118–123. https://doi.org/10.1006/geno.1996.0606
Cama E, Pethe S, Boucher J-L et al (2004) Inhibitor coordination interactions in the binuclear manganese cluster of arginase I. Biochemistry 43:8987–8999. https://doi.org/10.1021/bi0491705
Morris SM (2009) Recent advances in arginine metabolism: roles and regulation of the arginases. Br J Pharmacol 157:922–930. https://doi.org/10.1111/j.1476-5381.2009.00278.x
Dzik JM (2014) Evolutionary roots of arginase expression and regulation. Front Immunol 5:544. https://doi.org/10.3389/fimmu.2014.00544
Legaz ME, Fontaniella B, Millanes AM, Vicente C (2004) Secreted arginases from phylogenetically far-related lichen species act as cross-recognition factors for two different algal cells. Eur J Cell Biol 83:435–446. https://doi.org/10.1078/0171-9335-00384
Labudda M, Różańska E, Cieśla J et al (2016) Arginase activity in Arabidopsis thaliana infected with Heterodera schachtii. Plant Pathol 65:1529–1538. https://doi.org/10.1111/ppa.12537
Shi H, Ye T, Chen F et al (2013) Manipulation of arginase expression modulates abiotic stress tolerance in Arabidopsis: effect on arginine metabolism and ROS accumulation. J Exp Bot 64:1367–1379. https://doi.org/10.1093/jxb/ers400
Todd CD, Gifford DJ (2002) The role of the megagametophyte in maintaining loblolly pine (Pinus taeda L.) seedling arginase gene expression in vitro. Planta 215:110–118. https://doi.org/10.1007/s00425-001-0720-2
Spano G, Chieppa G, Beneduce L, Massa S (2004) Expression analysis of putative arcA, arcB and arcC genes partially cloned from Lactobacillus plantarum isolated from wine. J Appl Microbiol 96:185–193. https://doi.org/10.1046/j.1365-2672.2003.02132.x
Arena ME, Saguir FM, Manca de Nadra MC (1999) Arginine, citrulline and ornithine metabolism by lactic acid bacteria from wine. Int J Food Microbiol 52:155–161. https://doi.org/10.1016/S0168-1605(99)00133-6
Yu JJ, Oh SH (2010) Isolation and characterization of lactic acid bacteria strains with ornithine producing capacity from natural sea salt. J Microbiol 48:467–472. https://doi.org/10.1007/s12275-010-0204-9
Kim JE, Kim SY, Lee KW, Lee HJ (2009) Arginine deiminase originating from Lactococcus lactis ssp. lactis American Type Culture Collection (ATCC) 7962 induces G1-phase cell-cycle arrest and apoptosis in SNU-1 stomach adenocarcinoma cells. Br J Nutr 102:1469–1476. https://doi.org/10.1017/S0007114509990432
McGee DJ, Radcliff FJ, Mendz GL et al (1999) Helicobacter pylori rocF is required for arginase activity and acid protection in vitro but is not essential for colonization of mice or for urease activity. J Bacteriol 181:7314–7322. https://doi.org/10.1128/JB.181.23.7314-7322.1999
Patchett ML, Daniel RM, Morgan HW (1991) Characterisation of arginase from the extreme thermophile ‘Bacillus caldovelox’. Biochim Biophys Acta Protein Struct Mol Enzymol 1077:291–298. https://doi.org/10.1016/0167-4838(91)90543-9
Könst PM, Turras PMCCD, Franssen MCR et al (2010) Stabilized and immobilized Bacillus subtilis arginase for the biobased production of nitrogen-containing chemicals. Adv Synth Catal 352:1493–1502. https://doi.org/10.1002/adsc.201000034
Nakamura N, Fujita M, Kimura K (1973) Purification and properties of L-arginase from Bacillus subtilis. Agric Biol Chem 37:2827–2833. https://doi.org/10.1080/00021369.1973.10861080
Shimotohno KW, Iida J, Takizawa N, Endo T (1994) Purification and characterization of arginine amidinohydrolase from Bacillus brevis TT02-8. Biosci Biotechnol Biochem 58:1045–1049. https://doi.org/10.1271/bbb.58.1045
Kanda M, Ohgishi K, Hanawa T, Saito Y (1997) Arginase of Bacillus brevis Nagano: purification, properties, and implication in gramicidin s biosynthesis. Arch Biochem Biophys 344:37–42. https://doi.org/10.1006/abbi.1997.0174
Yu JJ, Park KB, Kim SG, Oh SH (2013) Expression, purification, and biochemical properties of arginase from Bacillus subtilis 168. J Microbiol 51:222–228. https://doi.org/10.1007/s12275-013-2669-9
Huang K, Zhang T, Jiang B et al (2016) Characterization of a thermostable arginase from Rummeliibacillus pycnus SK31.001. J Mol Catal B: Enzym 133:68–75. https://doi.org/10.1016/j.molcatb.2016.11.020
Husain I, Bala K, Wani A et al (2017) Arginase purified from endophytic Pseudomonas aeruginosa IH2: induce apoptosis through both cell cycle arrest and MMP loss in human leukemic HL-60 cells. Chem Biol Interact 274:35–49. https://doi.org/10.1016/j.cbi.2017.07.001
Borkovich KA, Weiss RL (1987) Purification and characterization of arginase from Neurospora crassa. J Biol Chem 262:7081–7086
El-Sayed AS, Shindia AA, Diab AA, Rady AM (2014) Purification and immobilization of l-arginase from thermotolerant Penicillium chrysogenum KJ185377.1; with unique kinetic properties as thermostable anticancer enzyme. Arch Pharm Res. https://doi.org/10.1007/s12272-014-0498-y
Dzikowska A, Kacprzak M, Tomecki R et al (2003) Specific induction and carbon/nitrogen repression of arginine catabolism gene of Aspergillus nidulans–functional in vivo analysis of the otaA promoter. Fungal Genet Biol 38:175–186. https://doi.org/10.1016/s1087-1845(02)00522-4
Green SM, Eisenstein E, McPhie P, Hensley P (1990) The purification and characterization of arginase from Saccharomyces cerevisiae. J Biol Chem 265:1601–1607
Benucci I, Fiorelli V, Lombardelli C et al (2017) Kinetic characterization of arginase from Saccharomyces cerevisiae during alcoholic fermentation at different temperatures. LWT-Food Sci Technol 82:268–273. https://doi.org/10.1016/j.lwt.2017.04.044
Romagnoli G, Verhoeven MD, Mans R et al (2014) An alternative, arginase-independent pathway for arginine metabolism in Kluyveromyces lactis involves guanidinobutyrase as a key enzyme. Mol Microbiol 93:369–389. https://doi.org/10.1111/mmi.12666
Molina MC, Stocker-Wörgötter E, Turk R et al (1998) Secreted, glycosylated arginase from Xanthoria parietina thallus induces loss of cytoplasmic material from Xanthoria photobionts. Cell Adhes Commun 6:481–490. https://doi.org/10.3109/15419069809010796
Díaz E-M, Sacristán M, Legaz M-E, Vicente C (2009) Isolation and characterization of a cyanobacterium-binding protein and its cell wall receptor in the lichen Peltigera canina. Plant Signal Behav 4:598–603. https://doi.org/10.4161/psb.4.7.9164
Boutin J-P (1982) Purification, Properties and subunit structure of arginase from iris bulbs. Eur J Biochem 127:237–243. https://doi.org/10.1111/j.1432-1033.1982.tb06861.x
Hwang HJ, Kim EH, Cho YD (2001) Isolation and properties of arginase from a shade plant, ginseng (Panax ginseng C.A. Meyer) roots. Phytochemistry 58:1015–1024. https://doi.org/10.1016/S0031-9422(01)00392-2
Splittstoesser WE, Fowden L (1973) Ornithine transaminase from Cucurbita maxima cotyledons. Phytochemistry 12:785–790. https://doi.org/10.1016/0031-9422(73)80678-8
Kollöffel C, van Dijke HD (1975) Mitochondrial arginase activity from cotyledons of developing and germinating seeds of Vicia faba L. Plant Physiol 55:507–510. https://doi.org/10.1104/pp.55.3.507
Zonia LE, Stebbins NE, Polacco JC (1995) Essential role of urease in germination of nitrogen-limited Arabidopsis thaliana seeds. Plant Physiol 107:1097–1103. https://doi.org/10.1104/pp.107.4.1097
Alabadi D, Aguero MS, Perez-Amador MA, Carbonell J (1996) Arginase, arginine decarboxylase, ornithine decarboxylase, and polyamines in tomato ovaries (changes in unpollinated ovaries and parthenocarpic fruits induced by auxin or gibberellin). Plant Physiol 112:1237–1244. https://doi.org/10.1104/pp.112.3.1237
Downum KR, Rosenthal GA, Cohen WS (1983) l-Arginine and l-canavanine metabolism in jack bean, Canavalia ensiformis (L.) DC. and SOYBEAN, Glycine max (L.) Merr. Plant Physiol 73:965–968. https://doi.org/10.1104/pp.73.4.965
Goldraij A, Polacco JC (1999) Arginase is inoperative in developing soybean embryos. Plant Physiol 119:297. https://doi.org/10.1104/pp.119.1.297
Desai HV (1983) Purification and properties of arginase from Arachis hypogea L. seedlings. Indian J Biochem Biophys 20(4):236–237
Siddappa S, Basrur V, Ravishankar Rai V, Marathe GK (2018) Biochemical and functional characterization of an atypical plant l-arginase from Cilantro (Coriandrum sativam L.). Int J Biol Macromol 118:844–856. https://doi.org/10.1016/j.ijbiomac.2018.06.096
Bach SJ, Hawkins RA, Swaine D (1963) A short method for the purification of arginase from ox liver. Biochem J 89:263–265
Bach SJ, Swaine D (1965) The effect of arginase on the retardation of tumour growth. Br J Cancer 19:379–386
Savoca KV, Abuchowski A, van Es T et al (1979) Preparation of a non-immunogenic arginase by the covalent attachment of polyethylene glycol. Biochim Biophys Acta 578:47–53. https://doi.org/10.1016/0005-2795(79)90111-9
Savoca KV, Davis FF, Van TE et al (1984) Cancer therapy with chemically modified enzymes. II. The therapeutic effectiveness of arginase, and arginase modified by the covalent attachment of polyethylene glycol, on the taper liver tumor and the L5178Y murine leukemia. Cancer Biochem Biophys 7:261–268
Stone EM, Glazer ES, Chantranupong L et al (2010) Replacing Mn2+ with Co2+ in human arginase I enhances cytotoxicity toward l-arginine auxotrophic cancer cell lines. ACS Chem Biol 5:333–342. https://doi.org/10.1021/cb900267j
Tanios R, Bekdash A, Kassab E et al (2013) Human recombinant arginase I(Co)-PEG5000 [HuArgI(Co)-PEG5000]-induced arginine depletion is selectively cytotoxic to human acute myeloid leukemia cells. Leuk Res 37:1565–1571. https://doi.org/10.1016/j.leukres.2013.08.007
Yoon J-K, Frankel AE, Feun LG et al (2012) Arginine deprivation therapy for malignant melanoma. Clin Pharmacol 5:11–19. https://doi.org/10.2147/CPAA.S37350
Luke JJ, Schwartz GK (2013) Chemotherapy in the management of advanced cutaneous malignant melanoma. Clin Dermatol 31:290–297. https://doi.org/10.1016/j.clindermatol.2012.08.016
Moradpour D, Blum HE (2005) Pathogenesis of hepatocellular carcinoma. Eur J Gastroenterol Hepatol 17:477. https://doi.org/10.1097/00042737-200505000-00002
Blum HE (2005) Hepatocellular carcinoma: therapy and prevention. World J Gastroenterol 11:7391–7400. https://doi.org/10.3748/wjg.v11.i47.7391
Chrzanowska A, Krawczyk M, Baranczyk-Kuzma A (2008) Changes in arginase isoenzymes pattern in human hepatocellular carcinoma. Biochem Biophys Res Commun 377:337–340. https://doi.org/10.1016/j.bbrc.2008.09.093
Cheng PN, Lo TWH, Leung TWH et al (2005) Pegylated recombinant human arginase (rhArg-peg 5000mw) has in vitro and in vivo anti-proliferative potential and apoptotic activities in human hepatocellular varcinoma (HCC). J Clin Oncol 23:3179–3179. https://doi.org/10.1200/jco.2005.23.16_suppl.3179
Tsui SM, Lam WM, Lam TL et al (2009) Pegylated derivatives of recombinant human arginase (rhArg1) for sustained in vivo activity in cancer therapy: preparation, characterization and analysis of their pharmacodynamics in vivo and in vitro and action upon hepatocellular carcinoma cell (HCC). Cancer Cell Int 9:1-13. https://doi.org/10.1186/1475-2867-9-9
Zou S, Wang X, Liu P et al (2019) Arginine metabolism and deprivation in cancer therapy. Biomed Pharmacother 118:109210. https://doi.org/10.1016/j.biopha.2019.109210
D’Antonio EL, Hai Y, Christianson DW (2012) Structure and function of non-native metal clusters in human arginase I. Biochemistry 51:8399–8409. https://doi.org/10.1021/bi301145n
Caldwell RW, Rodriguez PC, Toque HA et al (2018) Arginase: a multifaceted enzyme important in health and disease. Physiol Rev 98:641–665. https://doi.org/10.1152/physrev.00037.2016
Storr JM, Burton AF (1974) The effects of arginine deficiency on lymphoma cells. Br J Cancer 30:50. https://doi.org/10.1038/bjc.1974.112
Nelson JA, Carpenter JW, Morris HP (1975) Growth inhibitory and arginase activities in liver and hepatoma extracts. Proc Soc Expl Biol Med 149:900–902. https://doi.org/10.3181/00379727-149-38923
Cheng N, Leung Y, Lo W (2005) Pharmaceutical preparation and method of treatment of human malignancies with arginine deprivation. Patent, EP1908478B1
Lam TL, Wong GKY, Chong HC et al (2009) Recombinant human arginase inhibits proliferation of human hepatocellular carcinoma by inducing cell cycle arrest. Cancer Lett 277:91–100. https://doi.org/10.1016/j.canlet.2008.11.031
Ellyard JI, Quah BJC, Simson L, Parish CR (2010) Alternatively activated macrophage possess antitumor cytotoxicity that is induced by IL-4 and mediated by arginase-1. J Immunother 33:443–452. https://doi.org/10.1097/CJI.0b013e3181cd8746
Lam TL, Wong GKY, Chow HY et al (2011) Recombinant human arginase inhibits the in vitro and in vivo proliferation of human melanoma by inducing cell cycle arrest and apoptosis. Pigm Cell Melanoma Res 24:366–376. https://doi.org/10.1111/j.1755-148X.2010.00798.x
Wang Z, Shi X, Li Y et al (2014) Involvement of autophagy in recombinant human arginase-induced cell apoptosis and growth inhibition of malignant melanoma cells. Appl Microbiol Biotechnol 98:2485–2494. https://doi.org/10.1007/s00253-013-5118-0
Wells JW, Evans CH, Scott MC et al (2013) Arginase treatment prevents the recovery of canine lymphoma and osteosarcoma cells resistant to the toxic effects of prolonged arginine deprivation. PLoS ONE 8:e54464. https://doi.org/10.1371/journal.pone.0054464
Wang Z, Shi X, Li Y et al (2014) Blocking autophagy enhanced cytotoxicity induced by recombinant human arginase in triple-negative breast cancer cells. Cell Death Dis 5:e1563–e1563. https://doi.org/10.1038/cddis.2014.503
Lin C, Wang Z, Li L et al (2015) The role of autophagy in the cytotoxicity induced by recombinant human arginase in laryngeal squamous cell carcinoma. Appl Microbiol Biotechnol 99:8487–8494. https://doi.org/10.1007/s00253-015-6565-6
Yu KM, Pang TP, Cutler M et al (2021) Rational design, engineer, and characterization of a novel pegylated single isomer human arginase for arginine depriving anti-cancer treatment. Life Sci 264:118674. https://doi.org/10.1016/j.lfs.2020.118674
Zhao Z, Zhang P, Li W et al (2021) Pegylated recombinant human arginase 1 induces autophagy and apoptosis via the ROS-activated AKT/mTOR pathway in bladder cancer cells. Oxid Med Cell Longev 2021:5510663. https://doi.org/10.1155/2021/5510663
El-Mais N, Fakhoury I, Abdellatef S et al (2021) Human recombinant arginase I [HuArgI (Co)-PEG5000]-induced arginine depletion inhibits ovarian cancer cell adhesion and migration through autophagy-mediated inhibition of RhoA. J Ovarian Res 14:13. https://doi.org/10.1186/s13048-021-00767-3
Cheng PNM, Leung YC, Lo WH et al (2005) Remission of hepatocellular carcinoma with arginine depletion induced by systemic release of endogenous hepatic arginase due to transhepatic arterial embolisation, augmented by high-dose insulin: arginase as a potential drug candidate for hepatocellular carcinoma. Cancer Lett 224:67–80. https://doi.org/10.1016/j.canlet.2004.10.050
Yau T, Cheng PN, Chan P et al (2013) A phase 1 dose-escalating study of pegylated recombinant human arginase 1 (Peg-rhArg1) in patients with advanced hepatocellular carcinoma. Invest New Drugs 31:99–107. https://doi.org/10.1007/s10637-012-9807-9
Yau T, Chiu J, Ng Chi T et al (2017) A phase 1 study of recombinant human arginase 1 (PEG-BCT-100) in combination with capecitabine and oxaliplatin in advanced hepatocellular carcinoma patients. Ann Oncol 28:54. https://doi.org/10.1093/annonc/mdx261.141
De Santo C, Cheng P et al (2018) Metabolic therapy with PEG-arginase induces a sustained complete remission in immunotherapy-resistant melanoma. J hemato oncol 11:1–5. https://doi.org/10.1186/s13045-018-0612-6
Chan SL, Cheng PNM, Liu AM et al (2021) A phase II clinical study on the efficacy and predictive biomarker of pegylated recombinant arginase on hepatocellular carcinoma. Invest New Drugs. https://doi.org/10.1007/s10637-021-01111-8
Burrage LC, Sun Q, Elsea SH et al (2015) Human recombinant arginase enzyme reduces plasma arginine in mouse models of arginase deficiency. Hum Mol Genet 24:6417–6427. https://doi.org/10.1093/hmg/ddv352
Zhang Y, Chung S-F, Tam S-Y et al (2021) Arginine deprivation as a strategy for cancer therapy: an insight into drug design and drug combination. Cancer Lett 502:58–70. https://doi.org/10.1016/j.canlet.2020.12.041
Fultang L, Vardon A, De Santo C, Mussai F (2016) Molecular basis and current strategies of therapeutic arginine depletion for cancer. Int J Cancer 139:501–509. https://doi.org/10.1002/ijc.30051
Sadarangani V, Rahman S, Sau AK (2018) Metal ions-induced stability and function of bimetallic human arginase-I, a therapeutically important enzyme. Biochim Biophys Acta 1866:1153–1164. https://doi.org/10.1016/j.bbapap.2018.08.006
Li L, Wang Y, Chen J et al (2013) An engineered arginase FC protein inhibits tumor growth in vitro and in vivo. Evid Based Complement Alternat Med. https://doi.org/10.1155/2013/423129
Suman M, Rajnikant M (2021) Impact of suitable control on a uniform interpretation of units for arginase assay. Biochem Biophys Rep 25:100910. https://doi.org/10.1016/j.bbrep.2021.100910
McGee DJ, Zabaleta J, Viator RJ et al (2004) Purification and characterization of Helicobacter pylori arginase, RocF: unique features among the arginase superfamily. Eur J Biochem 271:1952–1962. https://doi.org/10.1111/j.1432-1033.2004.04105.x
Stasyk OV, Boretsky YR, Gonchar MV, Sibirny AA (2015) Recombinant arginine-degrading enzymes in metabolic anticancer therapy and bioanalytics. Cell Biol Int 39:246–252. https://doi.org/10.1002/cbin.10383
Stasyuk N, Gayda G, Zakalskiy A et al (2018) Highly selective apo-arginase based method for sensitive enzymatic assay of manganese (II) and cobalt (II) ions. Spectrochim Acta A Mol Biomol Spectrosc 193:349–356. https://doi.org/10.1016/j.saa.2017.12.031
Dillon BJ, Prieto VG, Curley SA et al (2004) Incidence and distribution of argininosuccinate synthetase deficiency in human cancers. Cancer 100:826–833. https://doi.org/10.1002/cncr.20057
Zarei M, Nezafat N, Rahbar MR et al (2019) Decreasing the immunogenicity of arginine deiminase enzyme via structure-based computational analysis. J Biomol Struct Dyn 37:523–536. https://doi.org/10.1080/07391102.2018.1431151
Phillips MM, Sheaff MT, Szlosarek PW (2013) Targeting arginine-dependent cancers with arginine-degrading enzymes: opportunities and challenges. Cancer Res Treat 45:251–262. https://doi.org/10.4143/crt.2013.45.4.251
Kuo MT, Savaraj N, Feun LG (2010) Targeted cellular metabolism for cancer chemotherapy with recombinant arginine-degrading enzymes. Oncotarget 1:246–251. https://doi.org/10.18632/oncotarget.135
Thomas JB, Holtsberg FW, Ensor CM et al (2002) Enzymic degradation of plasma arginine using arginine deiminase inhibits nitric oxide production and protects mice from the lethal effects of tumour necrosis factor α and endotoxin. Biochem J 363:581–587. https://doi.org/10.1042/bj3630581
Colasanti M, Suzuki H (2000) The dual personality of NO. Trends Pharmacol Sci 21:249–252. https://doi.org/10.1016/S0165-6147(00)01499-1
Dillon BJ, Holtsberg FW, Ensor CM et al (2002) Biochemical characterization of the arginine degrading enzymes arginase and arginine deiminase and their effect on nitric oxide production. Med Sci Monit 8:BR248-253
Bel’skaya LV, Sarf EA, Kosenok VK (2021) Indicators of L-arginine metabolism in saliva: a focus on breast cancer. J Oral Biosci 63:52–57. https://doi.org/10.1016/j.job.2020.12.002
Zhang T, Guo Y, Zhang H et al (2013) Arginase from Bacillus thuringiensis SK 20.001: purification, characteristics, and implications for L-ornithine biosynthesis. Process Biochem 48:663–668. https://doi.org/10.1016/j.procbio.2013.02.023
Song W, Niu P, Chen X, Liu L (2014) Enzymatic production of L-ornithine from L-arginine with recombinant thermophilic arginase. J Mol Catal B: Enzym 110:1–7. https://doi.org/10.1016/j.molcatb.2014.09.005
Zhang X, Liu J, Yu X et al (2015) High-level expression of human arginase I in Pichia pastoris and its immobilization on chitosan to produce L-ornithine. BMC Biotechnol 15:1–10. https://doi.org/10.1186/s12896-015-0184-2
Acknowledgements
The authors acknowledge Dr Ankit Shrivastava, LPVD, Rocky Mountain Laboratories, NIAID, NIH, Hamilton, MT, 59840, USA, for his valuable suggestions during the drafting of the current study.
Funding
The authors acknowledge the Jaypee University of Information Technology Waknaghat, Solan, Himachal Pradesh, India, for providing infrastructure and financial support for carrying out the present work.
Author information
Authors and Affiliations
Contributions
NK delineated, conducted the literature work, and wrote the manuscript. SB analyzes, reads, and approved the final submitted data.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumari, N., Bansal, S. Arginine depriving enzymes: applications as emerging therapeutics in cancer treatment. Cancer Chemother Pharmacol 88, 565–594 (2021). https://doi.org/10.1007/s00280-021-04335-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-021-04335-w