Abstract
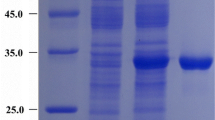
The arginine-degrading and ornithine-producing enzymes arginase has been used to treat arginine-dependent cancers. This study was carried out to obtain the microbial arginase from Bacillus subtilis, one of major microorganisms found in fermented foods such as Cheonggukjang. The gene encoding arginase was isolated from B. subtilis 168 and cloned into E. coli expression plasmid pET32a. The enzyme activity was detected in the supernatant of the transformed and IPTG induced cell-extract. Arginase was purified for homogeneity from the supernatant by affinity chromatography. The specific activity of the purified arginase was 150 U/mg protein. SDS-PAGE analysis revealed the molecular size to be 49 kDa (Trix·Tag, 6×His·Tag added size). The optimum pH and temperature of the purified enzyme with arginine as the substrate were pH 8.4 and 45°C, respectively. The Km and Vmax values of arginine for the enzyme were 4.6 mM and 133.0 mM/min/mg protein respectively. These findings can contribute in the development of functional fermented foods such as Cheonggukjang with an enhanced level of ornithine and pharmaceutical products by providing the key enzyme in arginine-degradation and ornithine-production.
Similar content being viewed by others
References
Arena, M.E., Saguir, F.M., Manca, M.C., and Nadra, M. 1999. Arginine, citrulline and ornithine metabolism by lactic acid bacteria from wine. Int. J. Food Microbiol. 52, 155–161.
Bewley, M.C., Lott, J.S., Baker, E.N., and Patchett, M.L. 1996. The cloning, expression and crystallization of a thermostable arginase. FEBS Lett. 386, 215–218.
Borkovich, K.A. and Weiss, R.L. 1987. Purification and characterization of arginase from Nerurospora crassa. J. Biol. Chem. 262, 7081–8086.
Boutin, J.P. 1982. Purification, properties and subunit structure of arginase from iris bulbs. Eur. J. Biochem. 127, 237–243.
Bradford, M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254.
Carvajal, N., Torres, C., Uribe, E., and Salas, M. 1995. Interaction of arginase with metal ions: Studies of the enzyme from human liver and comparison with other arginases. Comp. Biochem. Physiol. 112B, 153–159.
Cheng, P.N., Lam, T.L., Lam, W.M., Tsui, S.M., Cheng, A.W., Lo, W.H., and Leung, Y.C. 2007. Pegylated recombinant human arginase (rhArg-peg5,000mw) inhibits the in vitro and in vivo proliferation of human hepatocellular carcinoma through arginine depletion. Cancer Res. 67, 309–317.
Earl, A.M., Eppinger, M., Fricke, W.F., Rosovitz, M.J., Rasko, D.A., Daugherty, S., Losick, R., Kolter, R., and Ravel, J. 2012. Whole-genome sequences of Bacillus subtilis and close relatives. J. Bacteriol. 194, 2378–2379.
Green, S.M., Eisenstein, E., McPhie, P., and Hensley, P. 1990. The purification and characterization of arginase from Saccharomyces cerevisiae. J. Biol. Chem. 265, 1601–1607.
Hsueh, E.C., Knebel, S.M., Lo, W.H., Leung, Y.C., Cheng, P.N.M., and Hsueh, C.T. 2012. Deprivaion of arginine by recombinant human arginase in prostate cancer cells. J. Hematol. Oncol. 5, 17. Doi: 10.1186/1756-8722-5-17.
Hwang, J.H., Kim, H.E., and Cho, D.Y. 2001. Isolation and properties of arginase from a shade plant, ginseng (Panax ginseng C.A. Meyer) roots. Phytochem. 58, 1015–1024.
Kanda, M., Ohgishi, K., Hanawa, T., and Saito, Y. 1997. Arginase of Bacillus brevis Nagano: Purification, properties, and implication in gramicidin S biosynthesis. Arch. Biochem. Biophys. 344, 37–42.
Kim, J.E., Kim, S.Y., Lee, K.W., and Lee, H.J. 2009. Arginine deiminase originating from Lactococcus lactis spp. lactis American Type Culture Collection (ATCC) 7962 induces G1-phase cell-cycle arrest and apoptosis in SNU-1 stomach adenocarcinoma cells. Bri. J. Nutri. 102, 1469–1476.
Konst, P.M., Turras, P.M.C.C.D., Franssen, M.C.R., Scott, E.L., and Sanders, J.P.M. 2010. Stabilized and immobilized Bacillus subtilis arginase for the biobased production of nitrogen-containing chemicals. Adv. Synyh. Catal. 352, 1493–1502.
Kuensch, U., Temperli, A., and Mayer, K. 1974. Conversion of arginine to ornithine during malolactic fermentation in red Swiss wine. Am. J. Enol. Vitic. 25, 191–193.
Liu, S.Q., Holland, R., and Crow, V.L. 2003. The potential of dairy lactic acid bacteria to metabolise amino acids via non-transaminating reactions and endogenous transamination. Int. J. Food Microbiol. 86, 257–269.
McGee, D.J., Zabaleta, J., Viator, R.J., Testerman, T.L., Ochoa, A.C., and Mendz, G.L. 2004. Purification and characterization of Helicobacter pylori arginase, RocF: unique features among the arginase superfamily. Eur. J. Biochem. 271, 1952–1962.
Mendz, G.L., Holmes, E.M., and Ferrero, R.L. 1998. In situ characterization of Helicobacter pylori arginase. Biochim. Biophys. Acta. 1388, 465–477.
Nakamura, N., Fujita, M., and Kimura, K. 1973. Purification and properties of L-arginase from Bacillus subtilis. Agr. Biol. Chem. 37, 2827–2833.
Narimatsu S., Kazamori, D., Masuda, K., Katsu, T., Funae, Y., Naito, S., Nakura, H., Yamano, S., and Hanioka, N. 2009. The mechanism causing the difference in kinetic properties between rat CYP2D4 and human CYP2D6 in the oxidation of dextromethorphan and bufuralol. Biochem. Pharmacol. 77, 920–931.
Pahchett, M.L., Daniel, R.M., and Moran, H.W. 1991. Characterisation of arginase from the extreme thermophile Bacillus caldovelox. Biochim. Biophys. Acta 1077, 291–298.
Park, K.B., Yu, J.J., and Oh, S.H. 2010. Expression of arginase gene in Bacillus sp. enhances ornithine production. Program Abstr. 2010 Int’l Sympo. Annual Meeting., Abstr. P.5–05.
Peng, Y., Yang, X., and Zhang, Y. 2005. Microbial fibrinolytic enzymes: an overview of source, production, properties, and thrombolytic activity in vivo. Appl. Microbiol. Biotechnol. 69, 126–132.
Rijin, J.V., Berg, J.V.D., Teerlink, T., Kruyt, F.A.E., Schor, D.S.M., Lavalette, A.C.R.D., Berg, T.K.V.D., Jakobs, C., and Slotman, B.J. 2003. Changes in the ornithine cycle following ionising radiation cause a cytotoxic conditioning of the culture medium of H35 hepatoma cells. British J. Cancer 88, 447–454.
Shimotohno, K.W., Miwa, I., and Endo, T. 1997. Molecular cloning and nucleotide sequence of the arginase gene of Bacillus brevis TT02-8 and its expression in Escherichia coli. Biosci. Biotechnol. Biochem. 61, 1459–1464.
Sixt, S.U., Beiderlinden, M., Jennissen, H.P., and Peters, J. 2007. Extracellular proteasome in the human alveolar space: A new housekeeping enzyme? Am. J. Physiol. Lung Cell. Mol. Physiol. 292, L1280–L1288.
Spano, G., Chieppa, G., Benedue, L., and Massa, S. 2004. Expression analysis of putative arcA, arcB, arcC genes partially cloned from Lactobacillus plantarum isolated from wine. J. Appl. Microbiol. 96, 185–193.
Spolarics, Z. and Bond, J.S. 1989. Comparison of biochemical properties of liver arginase from streptozocin-induced diabetic and control mice. Arch. Biochem. Biophys. 274, 426–433.
Subrahmanyam, T.S. and Reddy, S.R. 1986. L-Ornithine and L-lysine need their α-carboxyl groups for effective inhibition of bovine liver arginase. Indian J. Biochem. Biophys. 23, 359–361.
Todd, C.D., Cooke, J.E.K., and Gifford, D.J. 2001. Purification and properties of Pinus taeda arginase from germinated seedlings. Plant Physiol. Biochem. 39, 1037–1045.
Tsui, S.M., Lam, W.M., Lam, T.L., Chong, H.C., So, P.K., Kwok, S.Y., Arnold, S., Cheng, P.N.M., Wheatley, D.N., Lo, W.H., and Leung, Y.C. 2009. Pegylated derivatives of recombinant human arginase (rhArgI) for sustained in vivo activity in cancer therapy: preparation, characterization and analysis of their pharmacodynamics in vivo and in vitro and action upon hepatocellular carcinoma cell (HCC). Cancer Cell International 9, 9. Doi:10. 1186/1475-2867-9-9.
Viator, R.J., Rest, R.F., Hildebrandt, E., and McGee, D.J. 2008. Characterization of Bacillus anthracis arginase: Effects of pH, temperature, and cell viability on metal preference. BMC Biochem. 9, 15. Doi:10.1186/1471-2091-9-15.
Wang, C., Du, M., Zheng, D., Kong, F., Zu, G., and Feng, Y. 2009. Purification and characterization of nattokinase from Bacillus subtilis natto B-12. J. Agric. Food Chem. 57, 9722–9729.
Yoshinori, M., Wong, A.H.K., and Jiang, B. 2005. Fibrinolytic enzymes in Asian traditional fermented foods. Food Res. Int. 38, 243–250.
Yu, J.J. and Oh, S.H. 2010. Isolation and characterization of lactic acid bacteria strains with ornithine producing capacity from natural sea salt. J. Microbiol. 48, 467–472.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, JJ., Park, KB., Kim, SG. et al. Expression, purification, and biochemical properties of arginase from Bacillus subtilis 168. J Microbiol. 51, 222–228 (2013). https://doi.org/10.1007/s12275-013-2669-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-013-2669-9