Abstract
Background
Chemotherapy-induced nausea and vomiting (CINV) is considered one of the most serious adverse events affecting chemotherapy-receiving cancer patients. It dramatically affects their food intake, nutritional status and more importantly their quality of life. We can observe CINV in highly emetogenic chemotherapy (HEC) such as adriamycin–cyclophosphamide combination (AC) in breast cancer patients and cisplatin-based regimens in other cancer types. This study aimed to evaluate the antiemetic efficacy of palonosetron (PALO) over granisetron (GRA) in combination with dexamethasone for multiple highly emetogenic chemotherapy drugs (HEC), especially in chemotherapy regimens in Egyptian breast cancer patients and cisplatin-based regimens in other diseases.
Patients and methods
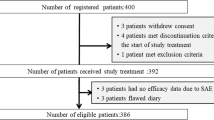
An open-label randomized trial was carried out, including 115 patients receiving at least four cycles of highly emetogenic chemotherapy regimens. All patients received dexamethasone in combination with the 5-HT3 receptor antagonist. We recorded patients' clinical and biochemical characteristics and withdraw blood samples to monitor serum substance P and serotonin in correlation with chemotherapy-induced nausea and vomiting (CINV). We use the MASCC antiemetic tool in the acute phase (0–24 hr) and delayed phase (24–120 h) to evaluate patient outcomes in both stages after each chemotherapy cycle.
Results
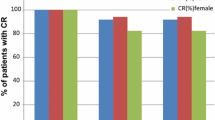
In (PALO) group, only 7.84% of patients showed acute vomiting, and 11.76% showed acute nausea, whereas 43.75% of patients showed acute vomiting and 89.06% showed acute nausea in (GRA) group (P < 0.0001). For delayed CINV, 23.53% of patients showed delayed vomiting, and 47.06% showed delayed nausea in the (PALO) group, while 82.81% of patients showed delayed emesis, and 92.19% showed delayed nausea in (GRA) group (P < 0.0001). The study showed that PALO is a cost-effective choice when compared to GRA in CINV prevention as 45.10% of patients in (PALO) required additional rescue medications (Domperidone 10 mg orally three times per day plus Trimebutine 200 mg orally three times per week both for 5 days), while 95.24% in the (GRA) group used the same medications. Adverse events of both antiemetic drugs (PALO and GRA) include headaches and constipation and QTc prolongation reports, mostly mild to moderate, with relatively low rates among the two groups.
Conclusion
Palonosetron, combined with dexamethasone, is more effective than granisetron and dexamethasone combination against both acute and delayed emesis induced by highly emetogenic chemotherapy (HEC) cisplatin-based protocols and the combination of cyclophosphamide and anthracyclines (AC). Medical team members should make more efforts, especially clinical pharmacy personnel, to monitor medications' effectiveness and help the medical team achieve a suitable and reliable care plan.




Similar content being viewed by others
Data availability
The authors confirm that the data supporting this study's findings are available within the article [and] its supplementary material.
References
Jordan K et al (2007) A meta-analysis comparing the efficacy of four 5-HT3-receptor antagonists for acute chemotherapy-induced emesis. Support Care Cancer 15(9):1023–1033
Roscoe JA et al (2012) Prevention of delayed nausea: a University of Rochester Cancer Center Community Clinical Oncology Program study of patients receiving chemotherapy. J Clin Oncol 30(27):3389–3395
Saito M et al (2009) Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol 10(2):115–124
Kubota K et al (2016) Control of nausea with palonosetron versus granisetron, both combined with dexamethasone, in patients receiving cisplatin- or anthracycline plus cyclophosphamide-based regimens. Support Care Cancer 24(9):4025–4033
Aapro M et al (2010) Double-blind, randomised, controlled study of the efficacy and tolerability of palonosetron plus dexamethasone for 1 day with or without dexamethasone on days 2 and 3 in the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy. Ann Oncol 21(5):1083–1088
Hesketh PJ (2008) Chemotherapy-induced nausea and vomiting. N Engl J Med 358(23):2482–2494
Gralla RJ et al (1999) Recommendations for the use of antiemetics: evidence-based, clinical practice guidelines. American Society of Clinical Oncology. J Clin Oncol 17(9):2971–2994
Schwartzberg L et al (2014) Pooled analysis of phase III clinical studies of palonosetron versus ondansetron, dolasetron, and granisetron in the prevention of chemotherapy-induced nausea and vomiting (CINV). Support Care Cancer 22(2):469–477
Botrel TE et al (2011) Efficacy of palonosetron (PAL) compared to other serotonin inhibitors (5-HT3R) in preventing chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderately or highly emetogenic (MoHE) treatment: systematic review and meta-analysis. Support Care Cancer 19(6):823–832
Geling O, Eichler H-G (2005) Should 5-hydroxytryptamine-3 receptor antagonists be administered beyond 24 hours after chemotherapy to prevent delayed emesis? Syst Re-Eval Clin Evid Drug Cost Implic 23(6):1289–1294
Maemondo M et al (2009) A phase II study of palonosetron combined with dexamethasone to prevent nausea and vomiting induced by highly emetogenic chemotherapy. Ann Oncol 20(11):1860–1866
Aapro MS et al (2006) A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol 17(9):1441–1449
Berger MJ et al (2017) NCCN guidelines insights: antiemesis, version 2.2017. J Natl Compr Canc Netw 15(7):883–893
Jordan K et al (2011) Antiemetics in children receiving chemotherapy. MASCC/ESMO guideline update 2009. Support Care Cancer. 19 Suppl 1:S37-42
Hesketh PJ, Palmas M, Nicolas P (2018) Preventing chemotherapy-induced nausea and vomiting in patients with lung cancer: efficacy of NEPA (netupitant-palonosetron), the first combination antiemetic. Support Care Cancer 26(4):1151–1159
Roila F et al (2016) 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 27(suppl 5):v119–v133
Hesketh PJ, Bohlke K, Kris MG (2017) Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update Summary. J Oncol Pract 13(12):825–830
Okada Y et al (2019) One-day versus three-day dexamethasone in combination with palonosetron for the prevention of chemotherapy-induced nausea and vomiting: a systematic review and individual patient data-based meta-analysis. Oncologist 24(12):1593–1600
Celio L et al (2019) Impact of dexamethasone-sparing regimens on delayed nausea caused by moderately or highly emetogenic chemotherapy: a meta-analysis of randomised evidence. BMC Cancer 19(1):1268
Simino GP et al (2016) Efficacy, safety and effectiveness of ondansetron compared to other serotonin-3 receptor antagonists (5-HT3RAs) used to control chemotherapy-induced nausea and vomiting: systematic review and meta-analysis. Expert Rev Clin Pharmacol 9(9):1183–1194
Rojas C et al (2008) Palonosetron exhibits unique molecular interactions with the 5-HT3 receptor. Anesth Analg 107(2):469–478
Kitamura H et al (2015) Palonosetron with aprepitant plus dexamethasone to prevent chemotherapy-induced nausea and vomiting during gemcitabine/cisplatin in urothelial cancer patients. Int J Urol 22(10):911–914
Price KL et al (2016) Palonosetron-5-HT3 receptor interactions as shown by a binding protein cocrystal structure. ACS Chem Neurosci 7(12):1641–1646
Rojas C et al (2010) The antiemetic 5-HT3 receptor antagonist palonosetron inhibits substance P-mediated responses in vitro and in vivo. J Pharmacol Exp Ther 335(2):362–368
Zabirowicz ES, Gan TJ (2019) 34 - Pharmacology of postoperative nausea and vomiting. In: Hemmings HC, Egan TD (eds) Pharmacology and physiology for anesthesia (Second Edition). Elsevier, Philadelphia, pp 671–692
Thomas AG et al (2014) Netupitant and palonosetron trigger NK1 receptor internalization in NG108-15 cells. Exp Brain Res 232(8):2637–2644
Kimura H et al (2015) Efficacy of triplet regimen antiemetic therapy for chemotherapy-induced nausea and vomiting (CINV) in bone and soft tissue sarcoma patients receiving highly emetogenic chemotherapy, and an efficacy comparison of single-shot palonosetron and consecutive-day granisetron for CINV in a randomized, single-blinded crossover study. Cancer Med 4(3):333–341
Darmani NA et al (2011) Synergistic antiemetic interactions between serotonergic 5-HT3 and tachykininergic NK1-receptor antagonists in the least shrew (Cryptotis parva). Pharmacol Biochem Behav 99(4):573–579
Minami M et al (2001) Effects of CP-99, 994, a tachykinin NK(1) receptor antagonist, on abdominal afferent vagal activity in ferrets: evidence for involvement of NK(1) and 5-HT(3) receptors. Eur J Pharmacol 428(2):215–220
Rojas C et al (2010) Palonosetron triggers 5-HT(3) receptor internalization and causes prolonged inhibition of receptor function. Eur J Pharmacol 626(2–3):193–199
Genazzani AA, Billington RA (2008) Pharmacokinetic and pharmacodynamic features of palonosetron. Tumori 94(2):suppl 6-13
Osawa H, Goto H, Myojo T (2013) Comparison of antiemesis effects of granisetron, aprepitant and dexamethasone to palonosetron, aprepitant and dexamethasone in treatment of high-emetic risk chemotherapy-induced nausea and vomiting - a retrospective study for efficacy and safety in a single institute. Gan To Kagaku Ryoho 40(5):617–621
Omar NE et al (2020) Perceptions and expectations of health care providers towards clinical pharmacy services at a tertiary cancer centre in Qatar. J Oncol Pharm Pract 26(5):1086–1096
Suzuki K et al (2016) Randomized, double-blind, phase III trial of palonosetron versus granisetron in the triplet regimen for preventing chemotherapy-induced nausea and vomiting after highly emetogenic chemotherapy: TRIPLE study. Ann Oncol 27(8):1601–1606
Toda H et al (2017) Antiemetic effectiveness and cost-saving of aprepitant plus granisetron is superior to palonosetron in gastrointestinal cancer patients who received moderately emetogenic chemotherapy. J Cancer 8(8):1371–1377
Du Q et al (2017) Economic evaluation of 5-HT3 receptor antagonists in combination with dexamethasone for the prevention of “overall” nausea and vomiting following highly emetogenic chemotherapy in Chinese adult patients. J Oncol Pharm Pract 23(6):403–412
Acknowledgments
The authors declare that the abstract and preliminary subjective data has been presented in the ESMO breast cancer virtual meeting in MAY 2020 citation: Annals of Oncology (2020) 31 (suppl_2): S83-S87. 10.1016/annonc/annonc123.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
MAM: checking and optimizing of the supportive care plan for all patients, also the assurance of proper patient recruitment and teaching them about study care plan according to the declaration of Helsinki and finally access different clinical response. GAAEE: supervision all the clinical pharmacy interventions and clinical outcome measures as a professor of clinical pharmacy in the faculty of pharmacy. HAT: as a professor and chairman of the medical oncology department at the Faculty of Medicine Tanta University, he played an essential role in designing study protocol and a significant percentage of the study population recruited from this Department.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that they are relevant to this article's content.
Ethics approval
Approval was obtained from the ethics committee of Tanta university. The procedures used in this study adhere to the tenets of the Declaration of Helsinki 1964.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Additional informed consent was obtained from all individual participants for identifying information in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
A. Mahrous, M., A. El-Azab, G. & A. Tawfik, H. Evaluation of clinical outcomes and efficacy of palonosetron and granisetron in combination with dexamethasone in Egyptian patients receiving highly emetogenic chemotherapy. Cancer Chemother Pharmacol 88, 121–129 (2021). https://doi.org/10.1007/s00280-021-04257-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-021-04257-7