Abstract
Background
Chemotherapy-induced nausea and vomiting (CINV) is a troublesome issue in chemotherapy for cancer patients. A second-generation 5HT3 receptor antagonist (5HT3RA), palonosetron, is effective and safe for the prevention of CINV in breast cancer patients treated with cyclophosphamide and anthracycline, but there is little data for malignant lymphoma. We conducted a prospective phase 2 study at a single institution to clarify the efficacy and safety of palonosetron in lymphoma patients.
Methods
Chemotherapy-naïve lymphoma patients who were treated with highly emetogenic chemotherapy (HEC) received a single intravenous bolus of palonosetron, 0.75 mg/body, before chemotherapy on day 1 during the first course of chemotherapy. The occurrence of CINV was assessed using the Multinational Association for Supportive Care in Cancer (MASCC) antiemesis tool, which was recorded by patients during the first course of chemotherapy.
Results
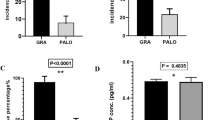
A total of 59 patients were enrolled, and 49 patients were eligible and evaluated. The complete response (CR) rate was 93.9% (95% confidence interval 83.1–98.7%) at 0–120 h post-chemotherapy. The proportion of patients who developed nausea of any grade and vomiting at 0–120 h post-chemotherapy was 34.7 and 6.1%, respectively. Although treatment-related adverse events were observed in 36 (73.5%) patients, these were mild and they recovered by the next cycle of chemotherapy.
Conclusion
The present study demonstrated that a single dose of palonosetron was highly effective and safe for the prevention of CINV in lymphoma patients.



Similar content being viewed by others
References
Japan Society of Clinical Oncology (2015) Guideline: appropriate use of antiemetics version 2.0 (in Japanese). Kanehara & Co., Ltd., Tokyo
Basch E, Prestrud AA, Hesketh PJ et al (2011) American Society of Clinical Oncology. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 29:4189–4198
National Comprehensive Cancer Network (2016) NCCN Clinical practice guidelines in oncology: antiemesis version 2. [http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf]. Accessed Feb 2017
Roila F, Molassiotis A, Herrstedt J et al (2016) 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 27(suppl 5):v119–v133
Tamura K, Aiba K, Saeki T et al (2015) Testing the effectiveness of antiemetic guidelines: results of a prospective registry by the CINV Study Group of Japan. Int J Clin Oncol 20:855–865
Takahashi T, Kumanomidou S, Takami S et al (2016) A retrospective study of R-CHOP/CHOP therapy-induced nausea and vomiting in non-Hodgkin’s lymphoma patients: a comparison of intravenous and oral 5-HT3 receptor antagonists. Int J Hematol 104:378–383
Wong EH, Clark R, Leung E et al (1995) The interaction of RS 25259-197, a potent and selective antagonist, with 5-HT3 receptors, in vitro. Br J Pharmacol 114:851–859
Rojas C, Stathis M, Thomas AG et al (2008) Palonosetron exhibits unique molecular interactions with the 5-HT3 receptor. Anesth Analg 107:469–478
Saito M, Aogi K, Sekine I et al (2009) Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol 10:115–124
Suzuki K, Yamanaka T, Hashimoto H et al (2016) Randomized, double-blind, phase III trial of palonosetron versus granisetron in the triplet regimen for preventing chemotherapy-induced nausea and vomiting after highly emetogenic chemotherapy: TRIPLE study. Ann Oncol 27:1601–1606
Di Renzo N, Montanini A, Mannina D et al (2011) Single-dose palonosetron for prevention of chemotherapy-induced nausea and vomiting in patients with aggressive non-Hodgkin’s lymphoma receiving moderately emetogenic chemotherapy containing steroids: results of a phase II study from the Gruppo Italiano per lo Studio dei Linfomi (GISL). Support Care Cancer 19:1505–1510
Choi BS, Borsaru GP, Ballinari G et al (2014) Multicenter phase IV study of palonosetron in the prevention of chemotherapy-induced nausea and vomiting (CINV) in patients with non-Hodgkin lymphomas undergoing repeated cycles of moderately emetogenic chemotherapy. Leuk Lymphoma 55:544–550
Miyata Y, Yakushijin K, Inui Y et al (2016) A prospective study of the antiemetic effect of palonosetron in malignant lymphoma patients treated with the CHOP regimen. Int J Hematol 104:682–691
Molassiotis A, Coventry PA, Stricker CT et al (2007) Validation and psychometric assessment of a short clinical scale to measure chemotherapy-induced nausea and vomiting: the MASCC antiemesis tool. J Pain Symptom Manage 34:148–159
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458
Sekine I, Segawa Y, Kubota K et al (2013) Risk factors of chemotherapy-induced nausea and vomiting: index for personalized antiemetic prophylaxis. Cancer Sci 104:711–717
Talley NJ, Phillips SF, Haddad A et al (1990) GR 38032F (ondansetron), a selective 5HT3 receptor antagonist, slows colonic transit in healthy man. Dig Dis Sci 35:477–480
Popovic M, Warr DG, Deangelis C et al (2014) Efficacy and safety of palonosetron for the prophylaxis of chemotherapy-induced nausea and vomiting (CINV): a systematic review and meta-analysis of randomized controlled trials. Support Care Cancer 22:1685–1697
Aapro M, Rugo H, Rossi G et al (2014) A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol 25:1328–1333
Navari RM, Qin R, Ruddy KJ et al (2016) Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med 375:134–142
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Tsutomu Takahashi received honoraria from Kyowa Hakko Kirin Co., Ltd., and Chugai Pharmaceutical Co., Ltd. Takaaki Miyake received honoraria from Kyowa Hakko Kirin Co., Ltd., and Chugai Pharmaceutical Co., Ltd. Ritsuro Suzuki received honoraria from Kyowa Hakko Kirin Co., Ltd., and Chugai Pharmaceutical Co., Ltd. Junji Suzumiya received honoraria from Chugai Pharmaceutical Co., Ltd., and received research funding from Kyowa Hakko Kirin Co., Ltd., Chugai Pharmaceutical Co., Ltd., and Astellas Pharma Inc. The other authors have no conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Takahashi, T., Okada, T., Ikejiri, F. et al. A prospective study of palonosetron for prevention of chemotherapy-induced nausea and vomiting in malignant lymphoma patients following highly emetogenic chemotherapy. Int J Clin Oncol 23, 189–194 (2018). https://doi.org/10.1007/s10147-017-1173-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-017-1173-3