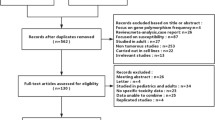
Abstract
Purpose
Methotrexate chemotherapy is associated with various toxicities which can result in the interruption or discontinuation of treatment and a subsequently raised risk of relapse. This umbrella systematic review was conducted to synthesize the results of all existing systematic reviews that investigate the pharmacogenetics of methotrexate-induced toxicity, with the aim of developing a comprehensive reference for personalized medicine.
Methods
Databases searched were PubMed, Embase, JBI Database of Systematic Reviews and Implementation Reports, DARE, and ProQuest. Papers were critically appraised by two reviewers, and data were extracted using a standardized tool.
Results
Three systematic reviews on methotrexate-induced toxicity were included in the review. Meta-analyses were reported across Asian, Caucasian, pediatric and adult patients for the MTHFR C677T and A1298C polymorphisms. Toxicity outcomes included different forms of hematologic, ectodermal and hepatic toxicities. Results varied considerably depending on the patient groups and subgroups investigated in the different systematic reviews, as well as the genetic models utilized. However, significant associations were found between the MTHFR C677T allele and; hepatic toxicity, myelosuppression, oral mucositis, gastrointestinal toxicity, and skin toxicity. Additionally, limited evidence suggests that the MTHFR A1298C polymorphism may be associated with decreased risk of skin toxicity and leukopenia.
Conclusion
This umbrella systematic review has synthesized the best available evidence on the pharmacogenetics of methotrexate toxicity. The next step in making personalized medicine for methotrexate therapy a clinical reality is research on the effectiveness and cost-effectiveness of MTHFR genotype testing to enable the close monitoring of at-risk patients for the timely initiation of rescue therapies.

Similar content being viewed by others
References
Krajinovic M, Moghrabi A (2004) Pharmacogenetics of methotrexate. Pharmacogenomics 5(7):819–834. doi:10.1517/14622416.5.7.819
Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA, van den Heuvel LP et al (1995) A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 10(1):111–113. doi:10.1038/ng0595-111
Weisberg I, Tran P, Christensen B, Sibani S, Rozen R (1998) A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab 64(3):169–172. doi:10.1006/mgme.1998.2714
Weisberg IS, Jacques PF, Selhub J, Bostom AG, Chen Z, Curtis Ellison R, Eckfeldt JH, Rozen R (2001) The 1298A→C polymorphism in methylenetetrahydrofolate reductase (MTHFR): in vitro expression and association with homocysteine. Atherosclerosis 156(2):409–415
Studies N-NWGoRiA, Chanock SJ, Manolio T, Boehnke M, Boerwinkle E, Hunter DJ, Thomas G, Hirschhorn JN, Abecasis G, Altshuler D, Bailey-Wilson JE, Brooks LD, Cardon LR, Daly M, Donnelly P, Fraumeni JF Jr, Freimer NB, Gerhard DS, Gunter C, Guttmacher AE, Guyer MS, Harris EL, Hoh J, Hoover R, Kong CA, Merikangas KR, Morton CC, Palmer LJ, Phimister EG, Rice JP, Roberts J, Rotimi C, Tucker MA, Vogan KJ, Wacholder S, Wijsman EM, Winn DM, Collins FS (2007) Replicating genotype-phenotype associations. Nature 447(7145):655–660. doi:10.1038/447655a
Moola S, Munn Z, Sears K, Sfetcu R, Currie M, Lisy K, Tufanaru C, Qureshi R, Mattis P, Mu P (2015) Conducting systematic reviews of association (etiology): the Joanna Briggs Institute’s approach. Int J Evid Based Healthc 13(3):163–169. doi:10.1097/XEB.0000000000000064
Munn Z, Tufanaru C, Aromataris E (2014) JBI’s systematic reviews: data extraction and synthesis. Am J Nurs 114(7):49–54. doi:10.1097/01.NAJ.0000451683.66447.89
Tufanaru C, Munn Z, Stephenson M, Aromataris E (2015) Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc 13(3):196–207. doi:10.1097/XEB.0000000000000065
Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P (2015) Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc 13(3):132–140. doi:10.1097/XEB.0000000000000055
Aplenc R, Thompson J, Han P, La M, Zhao H, Lange B, Rebbeck T (2005) Methylenetetrahydrofolate reductase polymorphisms and therapy response in pediatric acute lymphoblastic leukemia. Cancer Res 65(6):2482–2487. doi:10.1158/0008-5472.CAN-04-2606
Imanishi H, Okamura N, Yagi M, Noro Y, Moriya Y, Nakamura T, Hayakawa A, Takeshima Y, Sakaeda T, Matsuo M, Okumura K (2007) Genetic polymorphisms associated with adverse events and elimination of methotrexate in childhood acute lymphoblastic leukemia and malignant lymphoma. J Hum Genet 52(2):166–171. doi:10.1007/s10038-006-0096-z
Seidemann K, Book M, Zimmermann M, Meyer U, Welte K, Stanulla M, Reiter A (2006) MTHFR 677 (C→T) polymorphism is not relevant for prognosis or therapy-associated toxicity in pediatric NHL: results from 484 patients of multicenter trial NHL-BFM 95. Ann Hematol 85(5):291–300. doi:10.1007/s00277-005-0072-2
Ruiz-Arguelles GJ, Coconi-Linares LN, Garces-Eisele J, Reyes-Nunez V (2007) Methotrexate-induced mucositis in acute leukemia patients is not associated with the MTHFR 677T allele in Mexico. Hematology 12(5):387–391. doi:10.1080/10245330701448479
van Kooten Niekerk PB, Schmiegelow K, Schroeder H (2008) Influence of methylene tetrahydrofolate reductase polymorphisms and coadministration of antimetabolites on toxicity after high dose methotrexate. Eur J Haematol 81(5):391–398. doi:10.1111/j.1600-0609.2008.01128.x
Karathanasis NV, Stiakaki E, Goulielmos GN, Kalmanti M (2011) The role of the methylenetetrahydrofolate reductase 677 and 1298 polymorphisms in Cretan children with acute lymphoblastic leukemia. Genet Test Mol Biomarkers 15(1–2):5–10. doi:10.1089/gtmb.2010.0083
Cheng L, Li M, Hu J, Ren W, Xie L, Sun ZP, Liu BR, Xu GX, Dong XL, Qian XP (2014) UGT1A1*6 polymorphisms are correlated with irinotecan-induced toxicity: a system review and meta-analysis in Asians. Cancer Chemother Pharmacol 73(3):551–560
Campbell JM, Peters MDJ (2014) The association of chemotherapy-induced toxicities with germline polymorphisms: an umbrella review of systematic reviews and meta-analyses. JBISRIR 12(10):40–46
Yang L, Hu X, Xu L (2012) Impact of methylenetetrahydrofolate reductase (MTHFR) polymorphisms on methotrexate-induced toxicities in acute lymphoblastic leukemia: a meta-analysis. Tumour Biol 33(5):1445–1454. doi:10.1007/s13277-012-0395-2
Lopez-Lopez E, Martin-Guerrero I, Ballesteros J, Garcia-Orad A (2013) A systematic review and meta-analysis of MTHFR polymorphisms in methotrexate toxicity prediction in pediatric acute lymphoblastic leukemia. Pharmacogenomics J 13(6):498–506
Hagleitner MM, Coenen MJH, Aplenc R, Patino-Garcia A, Chiusolo P, Gemmati D, De Mattei M, Ongaro A, Krajinovic M, Hoogerbrugge PM, Vermeulen SHHM, Te Loo DMWM (2014) The role of the MTHFR 677C>T polymorphism in methotrexate-induced liver toxicity: a meta-analysis in patients with cancer. Pharmacogenomics J 14(2):115–119
Ojha RP, Gurney JG (2014) Methylenetetrahydrofolate reductase C677T and overall survival in pediatric acute lymphoblastic leukemia: a systematic review. Leuk Lymphoma 55(1):67–73. doi:10.3109/10428194.2013.792336
Sepe DM, McWilliams T, Chen J, Kershenbaum A, Zhao H, La M, Devidas M, Lange B, Rebbeck TR, Aplenc R (2012) Germline genetic variation and treatment response on CCG-1891. Pediatr Blood Cancer 58(5):695–700. doi:10.1002/pbc.23192
Matloub Y, Bostrom BC, Hunger SP, Stork LC, Angiolillo A, Sather H, La M, Gastier-Foster JM, Heerema NA, Sailer S, Buckley PJ, Thomson B, Cole C, Nachman JB, Reaman G, Winick N, Carroll WL, Devidas M, Gaynon PS (2011) Escalating intravenous methotrexate improves event-free survival in children with standard-risk acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Blood 118(2):243–251. doi:10.1182/blood-2010-12-322909
Salazar J, Altes A, del Rio E, Estella J, Rives S, Tasso M, Navajas A, Molina J, Villa M, Vivanco JL, Torrent M, Baiget M, Badell I (2012) Methotrexate consolidation treatment according to pharmacogenetics of MTHFR ameliorates event-free survival in childhood acute lymphoblastic leukaemia. Pharmacogenomics J 12(5):379–385. doi:10.1038/tpj.2011.25
Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, Gluud C, Martin RM, Wood AJG, Sterne JAC (2008) Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. Br Med J 336(7644):601–605. doi:10.1136/bmj.39465.451748.AD
Balk EM, Bonis PAL, Moskowitz H, Schmid CH, Ioannidis JPA, Wang CC, Lau J (2002) Correlation of quality measures with estimates of treatment effect in meta-analyses of randomized controlled trials. Jama-J Am Med Assoc 287(22):2973–2982. doi:10.1001/jama.287.22.2973
Campbell JM, Bateman E, Peters M, Bowen JM, Keefe DM, Stephenson MD (2016) Fluoropyrimidine and platinum toxicity pharmacogenetics: an umbrella review of systematic reviews and meta-analyses. Pharmacogenomics 17(4):435–451. doi:10.2217/pgs.15.180
Robert J, Le Morvan V, Giovannetti E, Peters GJ, EORTC PG (2014) On the use of pharmacogenetics in cancer treatment and clinical trials. Eur J Cancer 50(15):2532–2543. doi:10.1016/j.ejca.2014.07.013
D’Angelo V, Ramaglia M, Iannotta A, Crisci S, Indolfi P, Francese M, Affinita MC, Pecoraro G, Napolitano A, Fusco C, Oreste M, Indolfi C, Casale F (2011) Methotrexate toxicity and efficacy during the consolidation phase in paediatric acute lymphoblastic leukaemia and MTHFR polymorphisms as pharmacogenetic determinants. Cancer Chemother Pharmacol 68(5):1339–1346. doi:10.1007/s00280-011-1665-1
Chiusolo P, Giammarco S, Bellesi S, Metafuni E, Piccirillo N, De Ritis D, Marietti S, Federica S, Laurenti L, Fianchi L, Hohaus S, Giuseppe L, Sica S (2012) The role of MTHFR and RFC1 polymorphisms on toxicity and outcome of adult patients with hematological malignancies treated with high-dose methotrexate followed by leucovorin rescue. Cancer Chemother Pharmacol 69(3):691–696. doi:10.1007/s00280-011-1751-4
Erculj N, Kotnik BF, Debeljak M, Jazbec J, Dolzan V (2012) Influence of folate pathway polymorphisms on high-dose methotrexate-related toxicity and survival in childhood acute lymphoblastic leukemia. Leuk Lymphoma 53(6):1096–1104. doi:10.3109/10428194.2011.639880
Jabeen S, Holmboe L, Alnaes GI, Andersen AM, Hall KS, Kristensen VN (2015) Impact of genetic variants of RFC1, DHFR and MTHFR in osteosarcoma patients treated with high-dose methotrexate. Pharmacogenomics J 15(5):385–390. doi:10.1038/tpj.2015.11
Acknowledgments
No grants or other funding were received for this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Campbell, J.M., Bateman, E., Stephenson, M.D. et al. Methotrexate-induced toxicity pharmacogenetics: an umbrella review of systematic reviews and meta-analyses. Cancer Chemother Pharmacol 78, 27–39 (2016). https://doi.org/10.1007/s00280-016-3043-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3043-5