Abstract
Purpose
The metabolism and disposition of the first-in-class DOT1L inhibitor, EPZ-5676 (pinometostat), was investigated in rat and dog. Metabolite profiles were compared with those from adult patients in the first-in-man phase 1 study as well as the cross-species metabolism observed in vitro.
Methods
EPZ-5676 was administered to rat and dog as a 24-h IV infusion of [14C]-EPZ-5676 for determination of pharmacokinetics, mass balance, metabolite profiling and biodistribution by quantitative whole-body autoradiography (QWBA). Metabolite profiling and identification was performed by radiometric and LC–MS/MS analysis.
Results
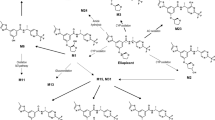
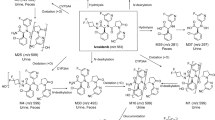
Fecal excretion was the major route of elimination, representing 79 and 81 % of the total dose in and rat and dog, respectively. QWBA in rats showed that the radioactivity was well distributed in the body, except for the central nervous system, and the majority of radioactivity was eliminated from most tissues by 168 h. Fecal recovery of dose-related material in bile duct-cannulated animals as well as higher radioactivity concentrations in the wall of the large intestine relative to liver implicated intestinal secretion as well as biliary elimination. EPZ-5676 underwent extensive oxidative metabolism with the major metabolic pathways being hydroxylation of the t-butyl group (EPZ007769) and N-dealkylation of the central nitrogen. Loss of adenine from parent EPZ-5676 (M7) was observed only in rat and dog feces, suggesting the involvement of gut microbiota. In rat and dog, steady-state plasma levels of total radioactivity and parent EPZ-5676 were attained rapidly and maintained through the infusion period before declining rapidly on cessation of dosing. Unchanged EPZ-5676 was the predominant circulating species in rat, dog and man.
Conclusions
The excretory and metabolic pathways for EPZ-5676 were very similar across species. Renal excretion of both parent EPZ-5676 and EPZ-5676-related material was low, and in preclinical species fecal excretion of parent EPZ-5676 and EPZ007769 accounted for the majority of drug-related elimination.








Similar content being viewed by others
Abbreviations
- BDC:
-
Bile duct cannulated
- HMT:
-
Histone methyltransferase
- HPLC:
-
High performance liquid chromatography
- IV:
-
Intravenous
- LLOQ:
-
Lower limit of quantitation
- MS:
-
Mass spectrometry
- SOI:
-
Start of infusion
References
Hess JL (2004) MLL: a histone methyltransferase disrupted in leukemia. Trends Mol Med 10:500–507
Krivtsov AV, Armstrong SA (2007) MLL translocations, histone modifications and leukaemia stem-cell development. Nat Rev Cancer 7:823–833
Muntean AG, Hess JL (2012) The pathogenesis of mixed-lineage leukemia. Annu Rev Pathol 7:283–301
Tamai H, Inokuchi K (2010) 11q23/MLL acute leukemia: update of clinical aspects. J Clin Exp Hematop 50:91–98
Biswas D, Milne TA, Basrur V, Kima J, Elenitoba-Johnson KSJ, Allisb CD, Roedera RG (2011) Function of leukemogenic mixed lineage leukemia 1 (MLL) fusion proteins through distinct partner protein complexes. Proc Natl Acad Sci USA 108:15751–15756
Bernt KM, Zhu N, Sinha AU, Vempati S, Faber J, Krivtsov AV, Feng Z, Punt N, Daigle A, Bullinger L, Pollock RM, Richon VM, Kung AL, Armstrong SA (2011) MLL-rearranged leukemia is dependent on aberrant H3K79 methylation by DOT1L. Cancer Cell 20:66–78
Chen L, Deshpande AJ, Banka D, Bernt KM, Dias S, Buske C, Olhava EJ, Daigle SR, Richon VM, Pollock RM, Armstrong SA (2012) Abrogation of MLL-AF10 and CALM-AF10- mediated transformation through genetic inactivation or pharmacological inhibition of the H3K79 methyltransferase Dot1l. Leukemia 27:813–822
Daigle SR, Olhava EJ, Therkelsen CA, Majer CR, Sneeringer CJ, Song J, Johnston LD, Scott MP, Smith JJ, Xiao Y, Jin L, Kuntz KW, Chesworth R, Moyer MP, Bernt KM, Tseng JC, Kung AL, Armstrong SA, Copeland RA, Richon VM, Pollock RM (2011) Selective killing of mixed lineage leukemia cells by a potent small-molecule DOT1L inhibitor. Cancer Cell 20:53–65
Yu W, Chory EJ, Wernimont AK, Tempel W, Scopton A, Federation A, Marineau JJ, Qi J, Barsyte-Lovejoy D, Yi J, Marcellus R, Iacob RE, Engen JR, Griffin C, Aman A, Wienholds E, Li F, Pineda J, Estiu G, Shatseva T, Hajian T, Al-Awar R, Dick JE, Vedadi M, Brown PJ, Arrowsmith CH, Bradner JE, Schapira M (2012) Catalytic site remodelling of the DOT1L methyltransferase by selective inhibitors. Nat Commun 3:1288
Daigle SR, Olhava EJ, Therkelsen CA, Basavapathruni A, Jin L, Boriack-Sjodin PA, Allain CJ, Klaus CR, Raimondi A, Scott MP, Waters NJ, Chesworth R, Moyer MP, Copeland RA, Richon VM, Pollock RM (2013) Potent inhibition of DOT1L as treatment for MLL-fusion leukemia. Blood 122:1017–1025
Chesworth R, Olhava EJ, Kuntz KW, Basavapathruni A, Majer CR, Sneeringer CJ, Allain CJ, Raimondi A, Klaus CR, Scott MP, Therkelsen CA, Daigle SR, Pollock RM, Richon VM, Copeland RA, Boriack-Sjodin PA, Jin L, Waters NJ, Arnold L, Patane M, Pearson P, Sacks J, Moyer MP (2013) From protein to candidate: discovery of EPZ-5676, a potent and selective inhibitor of the histone methyltransferase DOT1L. Abstracts of papers, 246th ACS national meeting & exposition, Indianapolis, IN, United States, 8–12 Sep 2013
Stein E, Garcia-Manero G, Rizzieri D, Savona MR, Tibes R, Altman J, Jongen-Lavrencic M, Döhner H, Armstrong S, Pollock RM, Waters NJ, Legler M, Thomson B, Daigle SR, McDonald A, Campbell C, Olhava E, Hedrick E, Copeland RA, Tallman M (2014) The DOT1L inhibitor EPZ-5676: safety and activity in relapsed/refractory patients with MLL-rearranged leukemia. Blood 124:387
Waters NJ, Thomson B, Gardner I, Johnson TN, Olhava EJ, Pollock RM, Legler M, Copeland RA, Hedrick E (2014) Pediatric dose determinations for the Phase I study of the DOT1L inhibitor, EPZ-5676, in MLL-r acute leukemia: leveraging early clinical data in adults through physiologically-based pharmacokinetic modeling. Blood 124:3619
Basavapathruni A, Olhava EJ, Daigle SR, Therkelsen CA, Jin L, Boriack-Sjodin PA, Allain CJ, Klaus CR, Raimondi A, Scott MP, Dovletoglou A, Richon VM, Pollock RM, Copeland RA, Moyer MP, Chesworth R, Pearson PG, Waters NJ (2014) Nonclinical pharmacokinetics and metabolism of EPZ-5676, a novel DOT1L histone methyltransferase inhibitor. Biopharm Drug Dispos 35:237–252
Roffey SJ, Obach RS, Gedge JI, Smith DA (2007) What is the objective of the mass balance study? A retrospective analysis of data in animal and human excretion studies employing radiolabeled drugs. Drug Metab Rev 39:17–43
Laffont CM, Toutain P, Alvinerie M, Bousquet-Melou A (2002) Intestinal secretion is a major route for parent ivermectin elimination. Drug Metab Dispos 30:626–630
Arimori K, Miyamoto S, Fukuda K, Nakamura C, Nakano M (1998) Characteristic difference in gastrointestinal excretion of clarithromycin and roxithromycin. Biopharm Drug Dispos 19:433–438
Dautrey S, Felice K, Petiet A, Lacour B, Carbon C, Farinotti R (1999) Active intestinal elimination of ciprofloxacin in rats: modulation by different substrates. Br J Pharmacol 127:1728–1734
Van Asperen J, Van Tellingen O, Beijnen JH (2000) The role of mdr1a P-glycoprotein in the biliary and intestinal secretion of doxorubicin and vinblastine in mice. Drug Metab Dispos 28:264–267
Prakash C, Wang W, O’Connell T, Johnson KA (2008) CYP2C8- and CYP3A-mediated C-demethylation of (3-{[(4-tertButylbenzyl)-(pyridine-3-sulfonyl)-amino]-methyl}-phenoxy)-acetic acid (CP-533,536), an EP2 receptor-selective prostaglandin E2 agonist: characterization of metabolites by high-resolution liquid chromatography–tandem mass spectrometry and liquid chromatography/mass spectrometry–nuclear magnetic resonance. Drug Metab Dispos 36:2093–2103
Hutzler JM, Obach RS, Dalvie D, Zientek MA (2013) Strategies for a comprehensive understanding of metabolism by aldehyde oxidase. Expert Opin Drug Metab Toxicol 9:153–168
Wang TP, Lampen JO (1951) The cleavage of adenosine, cytidine and xanthosine by Lactobacillus pentosus. J Biol Chem 192:339–347
Acknowledgments
The authors thank Jessica Shoultz and Shelby Anderson at Q2 Solutions, and Steve Madden, Alda Pombo-Gentile and Sandra Dennis at Charles River Labs, for their technical and scientific support to these studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
NJW, SS, EJO, RMP, MPM and RC are all previous or current employees of Epizyme and hold stock in Epizyme.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Waters, N.J., Smith, S.A., Olhava, E.J. et al. Metabolism and disposition of the DOT1L inhibitor, pinometostat (EPZ-5676), in rat, dog and human. Cancer Chemother Pharmacol 77, 43–62 (2016). https://doi.org/10.1007/s00280-015-2929-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2929-y