Abstract
Purpose
To determine the pharmacokinetics of the p110α-selective inhibitor alpelisib (BYL719) in humans, to identify metabolites in plasma and excreta, and to characterize pathways of biotransformation.
Methods
Four healthy male volunteers received a single oral dose of [14C]-labeled alpelisib (400 mg, 2.78 MBq). Blood, urine, and feces samples were collected throughout the study. Total radioactivity was measured by liquid scintillation counting, and metabolites were quantified and identified by radiometry and mass spectrometry. Complementary in vitro experiments characterized the hydrolytic, oxidative, and conjugative enzymes involved in metabolite formation.
Results
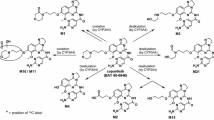
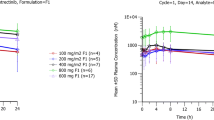
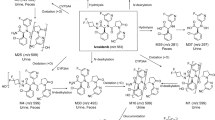
Over 50 % of [14C] alpelisib was absorbed, with a T max of 2 h and an elimination half-life from plasma of 13.7 h. Over the first 12 h, exposure to alpelisib and the primary metabolite M4 was 67.9 and 26.7 % of total drug-related material in circulation, respectively. Mass balance was achieved, with 94.5 % of administered radioactivity recovered in excreta. In total, 38.2 % of alpelisib was excreted unchanged, while 39.5 % was excreted as M4. Based on the excreta pools analyzed, excretion occurred mainly via feces (79.8 % of administered dose); 13.1 % was excreted via urine. In vitro experiments showed that spontaneous and enzymatic hydrolysis contributed to M4 formation, while CYP3A4-mediated oxidation and UGT1A9-mediated glucuronidation formed minor metabolites. Alpelisib was well tolerated, and no new safety concerns were raised during this study.
Conclusions
Alpelisib was rapidly absorbed and cleared by multiple metabolic pathways; the primary metabolite M4 is pharmacologically inactive. Alpelisib has limited potential for drug–drug interactions and is therefore a promising candidate for combination therapy.



Similar content being viewed by others
References
Liu P, Cheng H, Roberts TM, Zhao JJ (2009) Targeting the phosphoinositide 3-kinase (PI3K) pathway in cancer. Nat Rev Drug Discov 8:627–644. doi:10.1038/nrd2926
Dienstmann R, Rodon J, Serra V, Tabernero J (2014) Picking the point of inhibition: a comparative review of PI3K/AKT/mTOR pathway inhibitors. Mol Cancer Ther 13:1021–1031. doi:10.1158/1535-7163.MCT-13-0639
Engelman JA (2009) Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer 9:550–562. doi:10.1038/nrc2664
Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B (2010) The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol 11:329–341. doi:10.1038/nrm2882
Furet P, Guagnano V, Fairhurst RA et al (2013) Discovery of NVP-BYL719 a potent and selective phosphatidylinositol-3 kinase alpha inhibitor selected for clinical evaluation. Bioorg Med Chem Lett 23:3741–3748. doi:10.1016/j.bmcl.2013.05.007
Fritsch C, Huang A, Chatenay-Rivauday C et al (2014) Characterization of the novel and specific PI3Kα inhibitor NVP-BYL719 and development of the patient stratification strategy for clinical trials. Mol Cancer Ther 13:1117–1129. doi:10.1158/1535-7163.MCT-13-0865
Razak ARA, Ahn M-J, Yen C-J et al (2014) Phase Ib/II study of the PI3Kα inhibitor BYL719 in combination with cetuximab in recurrent/metastatic squamous cell cancer of the head and neck (SCCHN). J Clin Oncol 32:5s:abstr 6044
Juric D, Burris H, Schuler M et al (2014) Phase I study of the PI3kα inhibitor BYL719, as a single agent in patients with advanced solid tumors (aST). Ann Oncol 25:abstr 451PD. doi:10.1093/annonc/mdu331.11
Juric D, Gonzalez-Angulo AM, Burris HA et al (2013) Preliminary safety, pharmacokinetics and anti-tumor activity of BYL719, an alpha-specific PI3K inhibitor in combination with fulvestrant: results from a phase I study. Cancer Res 73:abstr P2-16-14. doi:10.1158/0008-5472.SABCS13-P2-16-14
Beumer DJH, Beijnen JH, Schellens JHM (2006) Mass balance studies, with a focus on anticancer drugs. Clin Pharmacokinet 45:33–58. doi:10.2165/00003088-200645010-00003
Data on file at Novartis Institutes for Biomedical Research. Drug metabolism and Pharmacokinetics (DMPK). Europe, Basel, Switzerland
Gonzalez-Angulo A, Juric D, Argilés G et al (2013) Safety, pharmacokinetics, and preliminary activity of the α-specific PI3K inhibitor BYL719: results from the first-in-human study. J Clin Oncol 31:abstr 2531
De Buck SS, Jakab A, Boehm M, Bootle D, Juric D, Quadt C, Goggin TK (2014) Population pharmacokinetics and pharmacodynamics of BYL719, a phosphoinositide 3-kinase antagonist, in adult patients with advanced solid malignancies. Br J Clin Pharmacol 78:543–555. doi:10.1111/bcp.12378
ICRP (1991) 1990 Recommendations of the international commission on radiological protection. ICRP Publication 60—Ann ICRP 21(1–3)
Dressman JB, Berardi RR, Dermentzoglou LC, Russell TL, Schmaltz SP, Barnett JL, Jarvenpaa KM (1990) Upper gastrointestinal (GI) pH in young, healthy men and women. Pharm Res 7:756–761. doi:10.1023/A:1015827908309
Russell TL, Berardi RR, Barnett JL, Dermentzoglou LC, Jarvenpaa KM, Schmaltz SP, Dressman JB (1993) Upper gastrointestinal pH in seventy-nine healthy, Elderly, North American men and women. Pharm Res 10:187–196. doi:10.1023/A:1018970323716
Rowland Yeo K, Rostami-Hodjegan A, Tucker G (2004) Abundance of cytochrome P450 in human liver: a meta-analysis. Br J Clin Pharmacol 57:687–688
Proctor NJ, Tucker GT, Rostami-Hodjegan A (2004) Predicting drug clearance from recombinantly expressed CYPs: intersystem extrapolation factors. Xenobiotica 34:151–178. doi:10.1080/00498250310001646353
Acknowledgments
We acknowledge Thomas Moenius, Albrecht Glaenzel, and the Novartis Isotope Lab (Synthesis and Analytical Groups) for the synthesis and certification of [14C]BYL719; Hubert Borell and Veronique Pflimlin-Fritschy for performing the in vitro experiments; Francis Ehrhart for the analysis of alpelisib in human plasma by a validated bioanalytical method; Christina Coughlin for her contributions to the manuscript; and PRA Health Sciences, Zuidlaren, the Netherlands, for execution of the study. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. We thank Nirmal Jethwa PhD for medical editorial assistance with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors are employees of Novartis Pharmaceuticals Corporation, and LB holds shares in Novartis.
Human and animal rights
Informed consent was obtained from all individual participants included in the study. The study followed the ethical principles of the Declaration of Helsinki and the ICH Harmonized Tripartite Guidelines for Good Clinical Practice and applicable local regulations (European Directive 2001/20/EC and US Code of Federal Regulations Title 21) and was approved by an independent ethics committee prior to site initiation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
James, A., Blumenstein, L., Glaenzel, U. et al. Absorption, distribution, metabolism, and excretion of [14C]BYL719 (alpelisib) in healthy male volunteers. Cancer Chemother Pharmacol 76, 751–760 (2015). https://doi.org/10.1007/s00280-015-2842-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2842-4