Abstract
Purpose
We conducted a phase 1 study to determine the maximum tolerated dose and the recommended dose of gemcitabine/nab-paclitaxel/S-1 combination chemotherapy in patients with unresectable pancreatic cancer.
Methods
We enrolled patients aged 20 years or older with unresectable pancreatic cancer and who had not been treated with chemotherapy or radiation therapy. Gemcitabine and nab-paclitaxel were administered on days 1 and 8, and S-1 was administered orally twice daily for 2 weeks, repeated every 3 weeks. The starting dose was level 0 [gemcitabine 700 mg/m2, nab-paclitaxel 90 mg/m2, S-1 60/80/100 mg/day (< 1.25 m2/1.25–1.50 m2/ > 1.5 m2)]. Dose-limiting toxicities were determined during the first course, and a classical 3 + 3 dose finding design was planned.
Results
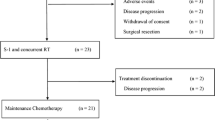
From March 2018 to October 2019, 20 patients were enrolled. At dose level 0, three of six patients experienced dose-limiting toxicities; one grade 3 skin rash on day 8, and two grade 3 or 4 neutropenia on day 8. At dose level-1 (gemcitabine 600 mg/m2, nab-paclitaxel 90 mg/m2, and S-1 50/70/80 mg/day), two of twelve patients experienced dose-limiting toxicities, all of which were grade 3 neutropenia on day 8. The most frequently observed toxicity during eight courses was neutropenia. Other treatment-related adverse events were mild. Eleven out of 19 (58%) patients achieved partial response.
Conclusion
We defined the maximum tolerated dose and the recommended dose for combination therapy with gemcitabine/nab-paclitaxel/S-1 as dose level-1. Considering the observed response rate, further studies are warranted in order to determine the efficacy of this regimen (UMIN-CTR 000030007).

Similar content being viewed by others
References
Hidalgo M (2010) Pancreatic cancer. N Engl J Med 362:1605–1617
Matsuno S, Egawa S, Fukuyama S et al (2004) Pancreatic Cancer Registry in Japan: 20 years of experience. Pancreas 28:219–230
llic M, llic I, (2016) Epidemiology of pancreatic cancer. World J Gastroenterol 22:9694–9705
Von Hoff DD, Ervin T, Arena FP et al (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369:1691–1703
Thierry C, Francoise D, Marc Y et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825
Ueno H, Ioka T, Ikeda M et al (2013) Randomized phase III study of gemcitabine plus s-1, s-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 31:1640–1648
Okusaka T, Ikeda M, Fukutomi A et al (2014) Phase II study of FOLFIRINOX for chemotherapy-naive Japanese patients with metastatic pancreatic cancer. Cancer Sci 105:1321–1326
Sasaki Y, Hamada K, Kaneta T et al (2015) Is repeating FOLFIRINOX in the original dosage and treatment schedule tolerable in Japanese patients with pancreatic cancer? Cancer Sci 106:1100
Ueno H, Ikeda M, Ueno M et al (2016) Phase I/II study of nab- paclitaxel plus gemcitabine for chemotherapy-naive Japanese patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol 77:595–603
Yan S, Sui Z, Quanli H et al (2017) Nab-paclitaxel plus S-1 in advanced pancreatic adenocarcinoma (NPSPAC): a single arm, single center, phase II trial. Oncotarget 8:92401–92410
Wen Z, Chunxia D, Yongkun S et al (2018) Nab-paclitaxel plus S-1 as first-line followed by S-1 maintenance for advanced pancreatic adenocarcinoma: a single-arm phase II trial. Cancer Chemother Pharmacol 82:655–660
Morizane C, Okusaka T, Mizusawa J et al (2019) Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: the FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann Oncol 30:1950–1958
Kondo N, Murakami Y, Uemura K et al (2017) A phase 1 study of gemcitabine/nab-paclitaxel/S-1 (GAS) combination neoadjuvant chemotherapy for patients with locally advanced pancreatic adenocarcinoma. Cancer Chemother Pharmacol 79:775–781
Tanaka H, Kanda M, Morita S et al (2017) Randomized phase II study of daily and alternate-day administration of S-1 for advanced gastric cancer (JFMC43-1003). Int J Clin Oncol 22:1052–1059
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
All authors have contributed significantly, and all authors are in agreement with the content of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Masanori Toyoda has received a honoraria from Taiho Pharmaceutical Co., Ltd. Hironaga Satake has received a research grant from Taiho Pharmaceutical Co., Ltd, a honoraria from Eli Lilly Japan Co., Ltd. and Taiho Pharmaceutical Co., Ltd. Hisateru Yasui has received a honoraria from Eli Lilly Japan Co., Ltd. and Taiho Pharmaceutical Co., Ltd. Hironobu Minami has received a research grant and personal fees from Eli Lilly Japan Co., Ltd. and Taiho Pharmaceutical Co., Ltd. The remaining authors have no conflict of interest to declare.
Ethical approval
This study was conducted in compliance with the ethical principles of the Declaration of Helsinki, the principles of Good Clinical Practice and all applicable regulations. The study protocol was approved by the institutional review board of each participating institution, and written informed consent was obtained from all participants.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding publishing their data.
Availability of data and material
The content of this manuscript has not been published or submitted for publication elsewhere and is not under consideration elsewhere.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sai, S., Toyoda, M., Tobimatsu, K. et al. Phase 1 study of Gemcitabine/Nab-paclitaxel/S-1 in patients with unresectable pancreatic cancer (GeNeS1S trial). Cancer Chemother Pharmacol 87, 65–71 (2021). https://doi.org/10.1007/s00280-020-04174-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-020-04174-1