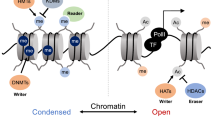
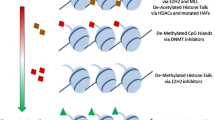
Abstract
Lymphomas, complex and heterogeneous malignant tumors, originate from the lymphopoietic system. These tumors are notorious for their high recurrence rates and resistance to treatment, which leads to poor prognoses. As ongoing research has shown, epigenetic modifications like DNA methylation, histone modifications, non-coding RNA regulation, and RNA modifications play crucial roles in lymphoma pathogenesis. Epigenetic modification–targeting drugs have exhibited therapeutic efficacy and tolerability in both monotherapy and combination lymphoma therapy. This review discusses pathogenic mechanisms and potential epigenetic therapeutic targets in common lymphomas, offering new avenues for lymphoma diagnosis and treatment. We also discuss the shortcomings of current lymphoma treatments, while suggesting potential areas for future research, in order to improve the prediction and prognosis of lymphoma.

Similar content being viewed by others
Data Availability
The datasets analysed during the current study are available to download from https://clinicaltrials.gov/.
Abbreviations
- HL:
-
Hodgkin’s lymphoma
- NHL:
-
Non-Hodgkin’s lymphoma
- DLBCL:
-
Diffuse large B cell lymphoma
- CAR-T:
-
Chimeric antigen receptor-modified T cell
- CHOP:
-
Cyclophosphamide, doxorubicin, vincristine, and prednisone
- CHOEP:
-
CHOP-like regimen
- SCT:
-
Stem cell transplantation
- DNMT:
-
DNA methyltransferases
- 5mC:
-
5-Methylcytosine
- EBV:
-
Epstein-Barr virus
- BL:
-
Burkitt lymphoma
- UHRF1:
-
Ubiquitin-like, containing PHD and RING finger domains, 1
- AZA:
-
5-Azacytidine
- ROMI:
-
Romidepsin
- CTAs:
-
Cancer testis antigens
- PTCL:
-
Peripheral T cell lymphoma
- TN:
-
Treatment-naïve
- TFH:
-
T follicular helper cell
- GC:
-
Germinal center
- PRC2:
-
Polycomb repressor complex 2
- HDAC:
-
Histone deacetylase
- HDACi:
-
Histone deacetylase inhibitors
- MCL:
-
Mantle cell lymphoma
- ADCC:
-
Antibody-dependent cell-mediated cytotoxicity
- MTCL:
-
Mature T cell lymphoma
- CTCL:
-
Cutaneous T cell lymphoma
- mTOR:
-
Mammalian target of rapamycin
- AAK:
-
Aurora A kinase
- ABK:
-
Aurora B kinase
- AAKi:
-
Aurora A kinase inhibitors
- JAKi:
-
Janus kinase inhibitors
- C:
-
Completed
- R:
-
Recruiting
- ANR:
-
Active, not recruiting
- DLT:
-
Dose-limiting toxicity
- CRR:
-
Complete response rate
- MTD:
-
Maximum tolerable dose
- ORR:
-
Objective response rate
- PRR:
-
Partial response rate
- PFS:
-
Progression-free survival
- PTCL:
-
Previously untreated peripheral T cell lymphoma
- R/R:
-
Relapsed/refractory
- ENKTCL:
-
Extranodal natural killer cell/T cell lymphoma
- CTL:
-
Cutaneous T cell lymphoma
- ATCL:
-
Angioimmunoblastic T cell lymphoma
- TCL:
-
T cell lymphoma
- MCL:
-
Mature cell lymphoma
- AITL:
-
Angioimmunoblastic T cell lymphoma
- CFDA:
-
The China Food and Drug Administration
- IPI:
-
International prognostic index
- CPET regimen:
-
Prednisone, etoposide, and thalidomide
- PEL regimen:
-
Prednisone, etoposide, lenalidomide
- PD-1:
-
Programmed death-1
- PD-L1:
-
Anti-PD1-ligand 1
- ICIs:
-
Immune checkpoint inhibitors
- ncRNAs:
-
Non-coding RNAs
- sncRNA:
-
Small non-coding RNAs
- lncRNA:
-
Long-stranded non-coding RNAs
- PTCL-NOS:
-
PTCL-not otherwise specified
- BCL:
-
B cell lymphoma
- m7G:
-
N7-methyluracil
- m6A:
-
N6-methyladenosine
- m5C:
-
5-Methylcytosine
- METTL3:
-
Methyltransferase 3, N6-adenosine-methyltransferase complex catalytic subunit
- PEDF:
-
Pigment epithelium-derived factor
- WTAP:
-
WT1-associated protein
References
Matasar MJ, Zelenetz AD (2008) Overview of lymphoma diagnosis and management. Radiol Clin North Am 46(175–198):vii. https://doi.org/10.1016/j.rcl.2008.03.005
Mugnaini EN, Ghosh N (2016) Lymphoma. Prim Care 43:661–675. https://doi.org/10.1016/j.pop.2016.07.012
Jaffe ES (2019) Diagnosis and classification of lymphoma: impact of technical advances. Semin Hematol 56:30–36. https://doi.org/10.1053/j.seminhematol.2018.05.007
Shi Y, Han Y, Yang J, Liu P, He X, Zhang C, Zhou S, Zhou L, Qin Y, Song Y et al (2019) Clinical features and outcomes of diffuse large B-cell lymphoma based on nodal or extranodal primary sites of origin: analysis of 1,085 WHO classified cases in a single institution in China. Chin J Cancer Res 31:152–161. https://doi.org/10.21147/j.issn.1000-9604.2019.01.10
Wang XM, Bassig BA, Wen JJ, Li GD, Liu ZB, Yao WX, Hu W, Wang Y, Li JM, Wang XD et al (2016) Clinical analysis of 1629 newly diagnosed malignant lymphomas in current residents of Sichuan province, China. Hematol Oncol 34:193–199. https://doi.org/10.1002/hon.2202
China Anti-Cancer Association Lymphoma Committee, Chinese Association for Clinical Oncologists, Medical Oncology Branch of Chinese International Exchange and Promotion Association for Medical and Healthcare (2021) Clinical practice guideline for multi-disciplinary treatment strategy of lymphoma in China. Chin J Oncol 43(02):163–166. https://doi.org/10.3760/cma.j.cn112152-20201109-00971
Bakhshi TJ, Georgel PT (2020) Genetic and epigenetic determinants of diffuse large B-cell lymphoma. Blood Cancer J 10:123. https://doi.org/10.1038/s41408-020-00389-w
China Anti-cancer Association Lymphoma Committee, Chinese Association for Clinical Oncologists, Medical Oncology Branch of Chinese International Exchange and Promotion Association for Medical and Healthcare (2021) Clinical practice guideline for lymphoma in China (2021 Edition). Chin J Oncol 43(07):707–735. https://doi.org/10.3760/cma.j.cn112152-20210516-00382
Miao Zhaoyi, Zhao Zhigang (2020) Advances in epigenetic modulation-based therapies for lymphoma. Chinese J. Clin. Oncol. 47(7):359–364. https://doi.org/10.3969/j.issn.1000-8179.2020.07.330
Chung C (2019) Current targeted therapies in lymphomas. Am J Health Syst Pharm 76:1825–1834. https://doi.org/10.1093/ajhp/zxz202
Hopken UE, Rehm A (2019) Targeting the tumor microenvironment of leukemia and lymphoma. Trends Cancer 5:351–364. https://doi.org/10.1016/j.trecan.2019.05.001
Godfrey J, Leukam MJ, Smith SM (2018) An update in treating transformed lymphoma. Best Pract Res Clin Haematol 31:251–261. https://doi.org/10.1016/j.beha.2018.07.008
Melani C, Wilson WH (2022) Front-Line treatment of diffuse large B-cell lymphoma in patients with cardiovascular comorbidities; omission of anthracycline reduces cure. Leuk Lymphoma 63:511–513. https://doi.org/10.1080/10428194.2021.2002323
Bhatt VR, Vose JM (2014) Hematopoietic stem cell transplantation for non-Hodgkin lymphoma. Hematol Oncol Clin North Am 28:1073–1095. https://doi.org/10.1016/j.hoc.2014.08.015
Yang H, Green MR (2020) Harnessing lymphoma epigenetics to improve therapies. Hematol Am Soc Hematol Educ Program 2020:95–100. https://doi.org/10.1182/hematology.2020006908
Falchi L, Ma H, Klein S, Lue JK, Montanari F, Marchi E, Deng C, Kim HA, Rada A, Jacob AT et al (2021) Combined oral 5-azacytidine and romidepsin are highly effective in patients with PTCL: a multicenter phase 2 study. Blood 137:2161–2170. https://doi.org/10.1182/blood.2020009004
Nieto Y, Valdez BC, Thall PF, Jones RB, Wei W, Myers A, Hosing C, Ahmed S, Popat U, Shpall EJ et al (2016) Double epigenetic modulation of high-dose chemotherapy with azacitidine and vorinostat for patients with refractory or poor-risk relapsed lymphoma. Cancer 122:2680–2688. https://doi.org/10.1002/cncr.30100
Morschhauser F, Tilly H, Chaidos A, McKay P, Phillips T, Assouline S, Batlevi CL, Campbell P, Ribrag V, Damaj GL et al (2020) Tazemetostat for patients with relapsed or refractory follicular lymphoma: an open-label, single-arm, multicentre, phase 2 trial. Lancet Oncol 21:1433–1442. https://doi.org/10.1016/S1470-2045(20)30441-1
Maruyama D, Tobinai K, Makita S, Ishida T, Kusumoto S, Ishitsuka K, Yoshimitsu M, Imaizumi Y, Sawayama Y, Takeuchi S et al (2017) First-in-human study of the EZH1/2 dual inhibitor DS-3201b in patients with relapsed or refractory non-Hodgkin lymphomas — preliminary results. Blood 130:4070–4070. https://doi.org/10.1182/blood.V130.Suppl_1.4070.4070
Izutsu K, Makita S, Nosaka K, Yoshimitsu M, Utsunomiya A, Kusumoto S, Morishima S, Tsukasaki K, Kawamata T, Ono T et al (2023) An open-label, single-arm phase 2 trial of valemetostat for relapsed or refractory adult T-cell leukemia/lymphoma. Blood 141:1159–1168. https://doi.org/10.1182/blood.2022016862
Amorim S, Stathis A, Gleeson M, Iyengar S, Magarotto V, Leleu X, Morschhauser F, Karlin L, Broussais F, Rezai K et al (2016) Bromodomain inhibitor OTX015 in patients with lymphoma or multiple myeloma: a dose-escalation, open-label, pharmacokinetic, phase 1 study. Lancet Haematol 3:e196-204. https://doi.org/10.1016/S2352-3026(16)00021-1
Liu W, Zhao D, Liu T, Niu T, Song Y, Xu W, Jin J, Cai Q, Huang H, Li Z et al (2021) A multi-center, real-world study of chidamide for patients with relapsed or refractory peripheral T-cell lymphomas in China. Front Oncol 11:750323. https://doi.org/10.3389/fonc.2021.750323
Bachy E, Camus V, Thieblemont C, Sibon D, Casasnovas RO, Ysebaert L, Damaj G, Guidez S, Pica GM, Kim WS et al (2022) Romidepsin plus CHOP versus CHOP in patients with previously untreated peripheral T-cell lymphoma: results of the Ro-CHOP Phase III study (conducted by LYSA). J Clin Oncol 40:242–251. https://doi.org/10.1200/JCO.21.01815
Batlevi CL, Crump M, Andreadis C, Rizzieri D, Assouline SE, Fox S, van der Jagt RHC, Copeland A, Potvin D, Chao R et al (2017) A phase 2 study of mocetinostat, a histone deacetylase inhibitor, in relapsed or refractory lymphoma. Br J Haematol 178:434–441. https://doi.org/10.1111/bjh.14698
Campbell P, Thomas CM (2017) Belinostat for the treatment of relapsed or refractory peripheral T-cell lymphoma. J Oncol Pharm Pract 23:143–147. https://doi.org/10.1177/1078155216634178
Gui L, Cao J, Ji D, Zhang H, Fan Q, Zhu J, Song Y, Jiang S, Ning Z, Yu J et al (2021) Chidamide combined with cyclophosphamide, doxorubicin, vincristine and prednisone in previously untreated patients with peripheral T-cell lymphoma. Chin J Cancer Res 33:616–626. https://doi.org/10.21147/j.issn.1000-9604.2021.05.08
Amengual JE, Lichtenstein R, Lue J, Sawas A, Deng C, Lichtenstein E, Khan K, Atkins L, Rada A, Kim HA et al (2018) A phase 1 study of romidepsin and pralatrexate reveals marked activity in relapsed and refractory T-cell lymphoma. Blood 131:397–407. https://doi.org/10.1182/blood-2017-09-806737
Jeffries MA (2020) The development of epigenetics in the study of disease pathogenesis. Adv Exp Med Biol 1253:57–94. https://doi.org/10.1007/978-981-15-3449-2_2
Zhang L, Lu Q, Chang C (2020) Epigenetics in health and disease. Adv Exp Med Biol 1253:3–55. https://doi.org/10.1007/978-981-15-3449-2_1
Werner RJ, Kelly AD, Issa JJ (2017) Epigenetics and precision oncology. Cancer J 23:262–269. https://doi.org/10.1097/PPO.0000000000000281
Villanueva L, Alvarez-Errico D, Esteller M (2020) The contribution of epigenetics to cancer immunotherapy. Trends Immunol 41:676–691. https://doi.org/10.1016/j.it.2020.06.002
Miranda Furtado CL, Dos Santos Luciano MC, Silva Santos RD, Furtado GP, Moraes MO, Pessoa C (2019) Epidrugs: targeting epigenetic marks in cancer treatment. Epigenetics 14:1164–1176. https://doi.org/10.1080/15592294.2019.1640546
Garsuault D, Bouyer C, Nguyen E, Kandhari R, Prochazkova-Carlotti M, Chevret E, Forgez P, Segal-Bendirdjian E (2020) Complex context relationships between DNA methylation and accessibility, histone marks, and hTERT gene expression in acute promyelocytic leukemia cells: perspectives for all-trans retinoic acid in cancer therapy. Mol Oncol 14:1310–1326. https://doi.org/10.1002/1878-0261.12681
Zhang H, Ying H, Wang X (2020) Methyltransferase DNMT3B in leukemia. Leuk Lymphoma 61:263–273. https://doi.org/10.1080/10428194.2019.1666377
Kulis M, Esteller M (2010) DNA methylation and cancer. Adv Genet 70:27–56. https://doi.org/10.1016/B978-0-12-380866-0.60002-2
Moore LD, Le T, Fan G (2013) DNA methylation and its basic function. Neuropsychopharmacology 38:23–38. https://doi.org/10.1038/npp.2012.112
Mervis JS, McGee JS (2020) DNA methylation and inflammatory skin diseases. Arch Dermatol Res 312:461–466. https://doi.org/10.1007/s00403-019-02005-9
Leoni C, Montagner S, Rinaldi A, Bertoni F, Polletti S, Balestrieri C, Monticelli S (2017) Dnmt3a restrains mast cell inflammatory responses. Proc Natl Acad Sci U S A 114:E1490–E1499. https://doi.org/10.1073/pnas.1616420114
Sinclair AJ (2021) Could changing the DNA methylation landscape promote the destruction of Epstein-Barr virus-associated cancers? Front Cell Infect Microbiol 11:695093. https://doi.org/10.3389/fcimb.2021.695093
Dalton T, Doubrovina E, Pankov D, Reynolds R, Scholze H, Selvakumar A, Vizconde T, Savalia B, Dyomin V, Weigel C et al (2020) Epigenetic reprogramming sensitizes immunologically silent EBV+ lymphomas to virus-directed immunotherapy. Blood 135:1870–1881. https://doi.org/10.1182/blood.2019004126
Guo R, Zhang Y, Teng M, Jiang C, Schineller M, Zhao B, Doench JG, O’Reilly RJ, Cesarman E, Giulino-Roth L et al (2020) DNA methylation enzymes and PRC1 restrict B-cell Epstein-Barr virus oncoprotein expression. Nat Microbiol 5:1051–1063. https://doi.org/10.1038/s41564-020-0724-y
Zhang Y, Sun Z, Jia J, Du T, Zhang N, Tang Y, Fang Y, Fang D (2021) Overview of histone modification. Adv Exp Med Biol 1283:1–16. https://doi.org/10.1007/978-981-15-8104-5_1
Hyun K, Jeon J, Park K, Kim J (2017) Writing, erasing and reading histone lysine methylations. Exp Mol Med 49:e324. https://doi.org/10.1038/emm.2017.11
Tan M, Luo H, Lee S, Jin F, Yang JS, Montellier E, Buchou T, Cheng Z, Rousseaux S, Rajagopal N et al (2011) Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell 146:1016–1028. https://doi.org/10.1016/j.cell.2011.08.008
Allis CD, Jenuwein T (2016) The molecular hallmarks of epigenetic control. Nat Rev Genet 17:487–500. https://doi.org/10.1038/nrg.2016.59
Morin RD, Mendez-Lago M, Mungall AJ, Goya R, Mungall KL, Corbett RD, Johnson NA, Severson TM, Chiu R, Field M et al (2011) Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature 476:298–303. https://doi.org/10.1038/nature10351
Jansch N, Sugiarto WO, Muth M, Kopranovic A, Desczyk C, Ballweg M, Kirschhofer F, Brenner-Weiss G, Meyer-Almes FJ (2020) Switching the switch: ligand induced disulfide formation in HDAC8. Chemistry 26:13249–13255. https://doi.org/10.1002/chem.202001712
Kim JJ, Lee SY, Miller KM (2019) Preserving genome integrity and function: the DNA damage response and histone modifications. Crit Rev Biochem Mol Biol 54:208–241. https://doi.org/10.1080/10409238.2019.1620676
Nichol JN, Dupere-Richer D, Ezponda T, Licht JD, Miller WH Jr (2016) H3K27 Methylation: a focal point of epigenetic deregulation in cancer. Adv Cancer Res 131:59–95. https://doi.org/10.1016/bs.acr.2016.05.001
Lawrence M, Daujat S, Schneider R (2016) Lateral thinking: how histone modifications regulate gene expression. Trends Genet 32:42–56. https://doi.org/10.1016/j.tig.2015.10.007
Francis M, Gopinathan G, Foyle D, Fallah P, Gonzalez M, Luan X, Diekwisch TGH (2020) Histone methylation: Achilles heel and powerful mediator of periodontal homeostasis. J Dent Res 99:1332–1340. https://doi.org/10.1177/0022034520932491
Zhang J, Dominguez-Sola D, Hussein S, Lee JE, Holmes AB, Bansal M, Vlasevska S, Mo T, Tang H, Basso K et al (2015) Disruption of KMT2D perturbs germinal center B cell development and promotes lymphomagenesis. Nat Med 21:1190–1198. https://doi.org/10.1038/nm.3940
Ji MM, Huang YH, Huang JY, Wang ZF, Fu D, Liu H, Liu F, Leboeuf C, Wang L, Ye J et al (2018) Histone modifier gene mutations in peripheral T-cell lymphoma not otherwise specified. Haematologica 103:679–687. https://doi.org/10.3324/haematol.2017.182444
Baubec T, Colombo DF, Wirbelauer C, Schmidt J, Burger L, Krebs AR, Akalin A, Schubeler D (2015) Genomic profiling of DNA methyltransferases reveals a role for DNMT3B in genic methylation. Nature 520:243–247. https://doi.org/10.1038/nature14176
Aymard F, Bugler B, Schmidt CK, Guillou E, Caron P, Briois S, Iacovoni JS, Daburon V, Miller KM, Jackson SP et al (2014) Transcriptionally active chromatin recruits homologous recombination at DNA double-strand breaks. Nat Struct Mol Biol 21:366–374. https://doi.org/10.1038/nsmb.2796
Li F, Mao G, Tong D, Huang J, Gu L, Yang W, Li GM (2013) The histone mark H3K36me3 regulates human DNA mismatch repair through its interaction with MutSalpha. Cell 153:590–600. https://doi.org/10.1016/j.cell.2013.03.025
Leung W, Teater M, Durmaz C, Meydan C, Chivu AG, Chadburn A, Rice EJ, Muley A, Camarillo JM, Arivalagan J et al (2022) SETD2 haploinsufficiency enhances germinal center-associated AICDA somatic hypermutation to drive B-cell lymphomagenesis. Cancer Discov 12:1782–1803. https://doi.org/10.1158/2159-8290.CD-21-1514
McCabe MT, Ott HM, Ganji G, Korenchuk S, Thompson C, Van Aller GS, Liu Y, Graves AP, Della Pietra A 3rd, Diaz E et al (2012) EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature 492:108–112. https://doi.org/10.1038/nature11606
Pfister SX, Ashworth A (2017) Marked for death: targeting epigenetic changes in cancer. Nat Rev Drug Discov 16:241–263. https://doi.org/10.1038/nrd.2016.256
Morin RD, Arthur SE, Assouline S (2021) Treating lymphoma is now a bit EZ-er. Blood Adv 5:2256–2263. https://doi.org/10.1182/bloodadvances.2020002773
Mondello P, Ansell SM (2022) Tazemetostat: a treatment option for relapsed/refractory follicular lymphoma. Expert Opin Pharmacother 23:295–301. https://doi.org/10.1080/14656566.2021.2014815
Italiano A, Soria JC, Toulmonde M, Michot JM, Lucchesi C, Varga A, Coindre JM, Blakemore SJ, Clawson A, Suttle B et al (2018) Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol 19:649–659. https://doi.org/10.1016/S1470-2045(18)30145-1
Munakata W, Shirasugi Y, Tobinai K, Onizuka M, Makita S, Suzuki R, Maruyama D, Kawai H, Izutsu K, Nakanishi T et al (2021) Phase 1 study of tazemetostat in Japanese patients with relapsed or refractory B-cell lymphoma. Cancer Sci 112:1123–1131. https://doi.org/10.1111/cas.14822
Scholze H, Stephenson RE, Reynolds R, Shah S, Puri R, Butler SD, Trujillo-Alonso V, Teater MR, van Besien H, Gibbs-Curtis D et al (2020) Combined EZH2 and Bcl-2 inhibitors as precision therapy for genetically defined DLBCL subtypes. Blood Adv 4:5226–5231. https://doi.org/10.1182/bloodadvances.2020002580
Tong KI, Yoon S, Isaev K, Bakhtiari M, Lackraj T, He MY, Joynt J, Silva A, Xu MC, Prive GG et al (2021) Combined EZH2 inhibition and IKAROS degradation leads to enhanced antitumor activity in diffuse large B-cell lymphoma. Clin Cancer Res 27:5401–5414. https://doi.org/10.1158/1078-0432.CCR-20-4027
Palomba ML, Cartron G, Popplewell L, Ribrag V, Westin J, Huw LY, Agarwal S, Shivhare M, Hong WJ, Raval A et al (2022) Combination of atezolizumab and tazemetostat in patients with relapsed/refractory diffuse large B-cell lymphoma: results from a phase Ib study. Clin Lymphoma Myeloma Leuk 22:504–512. https://doi.org/10.1016/j.clml.2021.12.014
Sarkozy C, Morschhauser F, Dubois S, Molina T, Michot JM, Cullieres-Dartigues P, Suttle B, Karlin L, Le Gouill S, Picquenot JM et al (2020) A LYSA phase Ib study of tazemetostat (EPZ-6438) plus R-CHOP in patients with newly diagnosed diffuse large B-cell lymphoma (DLBCL) with poor prognosis features. Clin Cancer Res 26:3145–3153. https://doi.org/10.1158/1078-0432.CCR-19-3741
Yamagishi M, Hori M, Fujikawa D, Ohsugi T, Honma D, Adachi N, Katano H, Hishima T, Kobayashi S, Nakano K et al (2019) Targeting excessive EZH1 and EZH2 activities for abnormal histone methylation and transcription network in malignant lymphomas. Cell Rep 29:2321–2337. https://doi.org/10.1016/j.celrep.2019.10.083. e2327
Honma D, Kanno O, Watanabe J, Kinoshita J, Hirasawa M, Nosaka E, Shiroishi M, Takizawa T, Yasumatsu I, Horiuchi T et al (2017) Novel orally bioavailable EZH1/2 dual inhibitors with greater antitumor efficacy than an EZH2 selective inhibitor. Cancer Sci 108:2069–2078. https://doi.org/10.1111/cas.13326
Dou F, Tian Z, Yang X, Li J, Wang R, Gao J (2022) Valemetostat: first approval as a dual inhibitor of EZH1/2 to treat adult T-cell leukemia/lymphoma. Drug Discov Ther 16:297–299. https://doi.org/10.5582/ddt.2022.01085
Hashimoto H, Vertino PM, Cheng X (2010) Molecular coupling of DNA methylation and histone methylation. Epigenomics 2:657–669. https://doi.org/10.2217/epi.10.44
Khan O, La Thangue NB (2012) HDAC inhibitors in cancer biology: emerging mechanisms and clinical applications. Immunol Cell Biol 90:85–94. https://doi.org/10.1038/icb.2011.100
Watters JM, Wright G, Smith MA, Shah B, Wright KL (2021) Histone deacetylase 8 inhibition suppresses mantle cell lymphoma viability while preserving natural killer cell function. Biochem Biophys Res Commun 534:773–779. https://doi.org/10.1016/j.bbrc.2020.11.001
Fujii K, Idogawa M, Suzuki N, Iwatsuki K, Kanekura T (2021) Functional depletion of HSP72 by siRNA and quercetin enhances vorinostat-induced apoptosis in an HSP72-overexpressing cutaneous T-cell lymphoma cell line, Hut78. Int J Mol Sci 22 https://doi.org/10.3390/ijms222011258
Gammoh N, Lam D, Puente C, Ganley I, Marks PA, Jiang X (2012) Role of autophagy in histone deacetylase inhibitor-induced apoptotic and nonapoptotic cell death. Proc Natl Acad Sci U S A 109:6561–6565. https://doi.org/10.1073/pnas.1204429109
Strati P, Nastoupil LJ, Davis RE, Fayad LE, Fowler N, Hagemeister FB, Kwak L, Oki Y, Wang M, Westin J et al (2020) A phase 1 trial of alisertib and romidepsin for relapsed/refractory aggressive B-cell and T-cell lymphomas. Haematologica 105:e26–e28. https://doi.org/10.3324/haematol.2019.220012
Karagianni F, Piperi C, Mpakou V, Spathis A, Foukas PG, Dalamaga M, Pappa V, Papadavid E (2021) Ruxolitinib with resminostat exert synergistic antitumor effects in cutaneous T-cell lymphoma. PLoS One 16:e0248298. https://doi.org/10.1371/journal.pone.0248298
Karagianni F, Piperi C, Casar B, de la Fuente-Vivas D, Garcia-Gomez R, Lampadaki K, Pappa V, Papadavid E (2022) Combination of resminostat with ruxolitinib exerts antitumor effects in the chick embryo chorioallantoic membrane model for cutaneous T cell lymphoma. Cancers (Basel) 14 https://doi.org/10.3390/cancers14041070
Yazbeck VY, Grant S (2015) Romidepsin for the treatment of non-Hodgkin’s lymphoma. Expert Opin Investig Drugs 24:965–979. https://doi.org/10.1517/13543784.2015.1041586
Barbarotta L, Hurley K (2015) Romidepsin for the treatment of peripheral T-cell lymphoma. J Adv Pract Oncol 6:22–36
Ma H, Davarifar A, Amengual JE (2018) The future of combination therapies for peripheral T cell lymphoma (PTCL). Curr Hematol Malig Rep 13:13–24. https://doi.org/10.1007/s11899-018-0432-3
Nachmias B, Shaulov A, Lavie D, Goldschmidt N, Gural A, Saban R, Lebel E, Gatt ME (2019) Romidepsin-bendamustine combination for relapsed/refractory T cell lymphoma. Acta Haematol 141:216–221. https://doi.org/10.1159/000498905
O’Connor OA, Falchi L, Lue JK, Marchi E, Kinahan C, Sawas A, Deng C, Montanari F, Amengual JE, Kim HA et al (2019) Oral 5-azacytidine and romidepsin exhibit marked activity in patients with PTCL: a multicenter phase 1 study. Blood 134:1395–1405. https://doi.org/10.1182/blood.2019001285
Kim M, Thompson LA, Wenger SD, O’Bryant CL (2012) Romidepsin: a histone deacetylase inhibitor for refractory cutaneous T-cell lymphoma. Ann Pharmacother 46:1340–1348. https://doi.org/10.1345/aph.1R036
O’Connor OA, Horwitz S, Masszi T, Van Hoof A, Brown P, Doorduijn J, Hess G, Jurczak W, Knoblauch P, Chawla S et al (2015) Belinostat in patients with relapsed or refractory peripheral T-cell lymphoma: results of the pivotal phase II BELIEF (CLN-19) study. J Clin Oncol 33:2492–2499. https://doi.org/10.1200/JCO.2014.59.2782
Johnston PBCA, Nikolinakos PG, Beaven AW, Barta SK, Bhat G, Hasal SJ, De Vos S, Oki Y, Deng C, Foss FM (2021) Belinostat in combination with standard cyclophosphamide, doxorubicin, vincristine and prednisone as first-line treatment for patients with newly diagnosed peripheral T-cell lymphoma. Exp Hematol Oncol 10(1):15. https://doi.org/10.1186/s40164-021-00203-8
Fukutomi A, Hatake K, Matsui K, Sakajiri S, Hirashima T, Tanii H, Kobayashi K, Yamamoto N (2012) A phase I study of oral panobinostat (LBH589) in Japanese patients with advanced solid tumors. Invest New Drugs 30:1096–1106. https://doi.org/10.1007/s10637-011-9666-9
Oki Y, Younes A, Copeland A, Hagemeister F, Fayad LE, McLaughlin P, Shah J, Fowler N, Romaguera J, Kwak LW et al (2013) Phase I study of vorinostat in combination with standard CHOP in patients with newly diagnosed peripheral T-cell lymphoma. Br J Haematol 162:138–141. https://doi.org/10.1111/bjh.12326
Persky DO, Li H, Rimsza LM, Barr PM, Popplewell LL, Bane CL, Von Gehr A, LeBlanc M, Fisher RI, Smith SM et al (2018) A phase I/II trial of vorinostat (SAHA) in combination with rituximab-CHOP in patients with newly diagnosed advanced stage diffuse large B-cell lymphoma (DLBCL): SWOG S0806. Am J Hematol 93:486–493. https://doi.org/10.1002/ajh.25010
Zhou J, Zhang C, Sui X, Cao S, Tang F, Sun S, Wang S, Chen B (2018) Histone deacetylase inhibitor chidamide induces growth inhibition and apoptosis in NK/T lymphoma cells through ATM-Chk2-p53-p21 signalling pathway. Invest New Drugs 36:571–580. https://doi.org/10.1007/s10637-017-0552-y
Zhang H, Chi F, Qin K, Mu X, Wang L, Yang B, Wang Y, Bai M, Li Z, Su L, et al. (2021) Chidamide induces apoptosis in DLBCL cells by suppressing the HDACs/STAT3/Bcl2 pathway. Mol Med Rep 23 https://doi.org/10.3892/mmr.2021.11947
Qiu S, Liu Y, Gui A, Xia Z, Liu W, Gu JJ, Zuo J, Yang L, Zhang Q (2022) Deubiquitinase OTUD7B is a potential prognostic biomarker in diffuse large B-cell lymphoma. J Cancer 13:998–1004. https://doi.org/10.7150/jca.65835
Shi Y, Jia B, Xu W, Li W, Liu T, Liu P, Zhao W, Zhang H, Sun X, Yang H et al (2017) Chidamide in relapsed or refractory peripheral T cell lymphoma: a multicenter real-world study in China. J Hematol Oncol 10:69. https://doi.org/10.1186/s13045-017-0439-6
Wang J, Fang Y, Ma S, Su N, Zhang Y, Huang H, Li Z, Huang H, Tian X, Cai J et al (2021) Comparison of chidamide-contained treatment modalities versus chemotherapy in the second-line treatment for relapsed or refractory peripheral T-cell lymphoma. Leuk Res 111:106705. https://doi.org/10.1016/j.leukres.2021.106705
Wang Y, Zhang M, Song W, Cai Q, Zhang L, Sun X, Zou L, Zhang H, Wang L, Xue H (2022) Chidamide plus prednisone, etoposide, and thalidomide for untreated angioimmunoblastic T-cell lymphoma in a Chinese population: a multicenter phase II trial. Am J Hematol. https://doi.org/10.1002/ajh.26499
Wang Y, Xue H, Song W, Xiao S, Jing F, Dong T, Wang L (2022) Chidamide with PEL regimen (prednisone, etoposide, lenalidomide) for elderly or frail patients with relapsed/refractory diffuse large B-Cell lymphoma -results of a single center, retrospective cohort in China. Hematol Oncol https://doi.org/10.1002/hon.2979
Zhao LM, Zhang JH (2019) Histone deacetylase inhibitors in tumor immunotherapy. Curr Med Chem 26:2990–3008. https://doi.org/10.2174/0929867324666170801102124
Chen X, Pan X, Zhang W, Guo H, Cheng S, He Q, Yang B, Ding L (2020) Epigenetic strategies synergize with PD-L1/PD-1 targeted cancer immunotherapies to enhance antitumor responses. Acta Pharm Sin B 10:723–733. https://doi.org/10.1016/j.apsb.2019.09.006
Burke B, Eden C, Perez C, Belshoff A, Hart S, Plaza-Rojas L, Delos Reyes M, Prajapati K, Voelkel-Johnson C, Henry E et al (2020) Inhibition of histone deacetylase (HDAC) enhances checkpoint blockade efficacy by rendering bladder cancer cells visible for T cell-mediated destruction. Front Oncol 10:699. https://doi.org/10.3389/fonc.2020.00699
Cao Z, Kon N, Liu Y, Xu W, Wen J, Yao H, Zhang M, Wu Z, Yan X, Zhu WG, et al. (2021) An unexpected role for p53 in regulating cancer cell-intrinsic PD-1 by acetylation. Sci Adv 7 https://doi.org/10.1126/sciadv.abf4148
Yan Z, Yao S, Liu Y, Zhang J, Li P, Wang H, Chu J, Zhao S, Yao Z (2020) Durable response to sintilimab and chidamide in a patient with pegaspargase- and immunotherapy-resistant NK/T-cell lymphoma: case report and literature review. Front Oncol 10:608304. https://doi.org/10.3389/fonc.2020.608304
Chen C, Zhang W, Zhou D, Zhang Y (2021) Sintilimab and chidamide for refractory transformed diffuse large B cell lymphoma: a case report and a literature review. Front Oncol 11:757403. https://doi.org/10.3389/fonc.2021.757403
Zheng R, Chen X, Wang C, Qin P, Tan H, Luo X (2020) Triplet therapy with PD-1 blockade, histone deacetylase inhibitor, and DNA methyltransferase inhibitor achieves radiological response in refractory double-expressor diffuse large B-cell lymphoma with 17p deletion. Case Rep Hematol 2020:8879448. https://doi.org/10.1155/2020/8879448
Xu J, Xu X, Chen J, Wang J, Jiang C, Lv C, Chen B (2021) Sustained remission of multi-line relapsed extranodal NK/T-cell lymphoma, nasal type, following sintilimab and chidamide: a case report. Medicine (Baltimore) 100:e24824. https://doi.org/10.1097/MD.0000000000024824
Wang J, Gao YS, Xu K, Li XD (2022) Combination of atezolizumab and chidamide to maintain long-term remission in refractory metastatic extranodal natural killer/T-cell lymphoma: a case report. World J Clin Cases 10:1609–1616. https://doi.org/10.12998/wjcc.v10.i5.1609
Bissonnette RP, Cesario RM, Goodenow B, Shojaei F, Gillings M (2021) The epigenetic immunomodulator, HBI-8000, enhances the response and reverses resistance to checkpoint inhibitors. BMC Cancer 21:969. https://doi.org/10.1186/s12885-021-08702-x
Ye B, Shi J, Kang H, Oyebamiji O, Hill D, Yu H, Ness S, Ye F, Ping J, He J et al (2020) Advancing pan-cancer gene expression survial analysis by inclusion of non-coding RNA. RNA Biol 17:1666–1673. https://doi.org/10.1080/15476286.2019.1679585
Misso G, Zarone MR, Grimaldi A, Di Martino MT, Lombardi A, Kawasaki H, Stiuso P, Tassone P, Tagliaferri P, Caraglia M (2017) Non coding RNAs: a new avenue for the self-tailoring of blood cancer treatment. Curr Drug Targets 18:35–55. https://doi.org/10.2174/1389450117666160606104208
Esteller M (2011) Non-coding RNAs in human disease. Nat Rev Genet 12:861–874. https://doi.org/10.1038/nrg3074
Slack FJ, Chinnaiyan AM (2019) The role of non-coding RNAs in oncology. Cell 179:1033–1055. https://doi.org/10.1016/j.cell.2019.10.017
Benetatos L, Benetatou A, Vartholomatos G (2020) Long non-coding RNAs and MYC association in hematological malignancies. Ann Hematol 99:2231–2242. https://doi.org/10.1007/s00277-020-04166-4
Roisman A, Castellano G, Navarro A, Gonzalez-Farre B, Perez-Galan P, Esteve-Codina A, Dabad M, Heath S, Gut M, Bosio M et al (2019) Differential expression of long non-coding RNAs are related to proliferation and histological diversity in follicular lymphomas. Br J Haematol 184:373–383. https://doi.org/10.1111/bjh.15656
Li Y, Li G, Guo X, Yao H, Wang G, Li C (2020) Non-coding RNA in bladder cancer. Cancer Lett 485:38–44. https://doi.org/10.1016/j.canlet.2020.04.023
Fuchs S, Naderi J, Meggetto F (2019) Non-coding RNA networks in ALK-positive anaplastic-large cell lymphoma. Int J Mol Sci 20 https://doi.org/10.3390/ijms20092150
Dahl M, Kristensen LS, Gronbaek K (2018) Long non-coding RNAs guide the fine-tuning of gene regulation in B-cell development and malignancy. Int J Mol Sci 19 https://doi.org/10.3390/ijms19092475
Sole C, Larrea E, Di Pinto G, Tellaetxe M, Lawrie CH (2017) miRNAs in B-cell lymphoma: molecular mechanisms and biomarker potential. Cancer Lett 405:79–89. https://doi.org/10.1016/j.canlet.2017.07.020
Labi V, Schoeler K, Melamed D (2019) miR-17 approximately 92 in lymphocyte development and lymphomagenesis. Cancer Lett 446:73–80. https://doi.org/10.1016/j.canlet.2018.12.020
Zheng Z, Sun R, Zhao HJ, Fu D, Zhong HJ, Weng XQ, Qu B, Zhao Y, Wang L, Zhao WL (2019) MiR155 sensitized B-lymphoma cells to anti-PD-L1 antibody via PD-1/PD-L1-mediated lymphoma cell interaction with CD8+T cells. Mol Cancer 18:54. https://doi.org/10.1186/s12943-019-0977-3
Lin Y, Chen WM, Wang C, Chen XY (2017) MicroRNA profiling in peripheral T-cell lymphoma, not otherwise specified. Cancer Biomark 18:339–347. https://doi.org/10.3233/CBM-160126
Yan ZX, Wu LL, Xue K, Zhang QL, Guo Y, Romero M, Leboeuf C, Janin A, Chen SJ, Wang L et al (2014) MicroRNA187 overexpression is related to tumor progression and determines sensitivity to bortezomib in peripheral T-cell lymphoma. Leukemia 28:880–887. https://doi.org/10.1038/leu.2013.291
Xu Y, Liu Z, Lv L, Li P, Xiu B, Qian W, Liang A (2020) MiRNA-340-5p mediates the functional and infiltrative promotion of tumor-infiltrating CD8(+) T lymphocytes in human diffuse large B cell lymphoma. J Exp Clin Cancer Res 39:238. https://doi.org/10.1186/s13046-020-01752-2
Paczkowska J, Giefing M (2021) MicroRNA signature in classical Hodgkin lymphoma. J Appl Genet 62:281–288. https://doi.org/10.1007/s13353-021-00614-7
Sekar D, Hairul Islam VI, Thirugnanasambantham K, Saravanan S (2014) Relevance of miR-21 in HIV and non-HIV-related lymphomas. Tumour Biol 35:8387–8393. https://doi.org/10.1007/s13277-014-2068-9
Han BWS, Zhao H (2020) MicroRNA-21 and microRNA-155 promote the progression of Burkitt’s lymphoma by the PI3K/AKT signaling pathway. Int J Clin Exp Pathol 13:89–98
Sun R, Zhang PP, Weng XQ, Gao XD, Huang CX, Wang L, Hu XX, Xu PP, Cheng L, Jiang L et al (2022) Therapeutic targeting miR130b counteracts diffuse large B-cell lymphoma progression via OX40/OX40L-mediated interaction with Th17 cells. Signal Transduct Target Ther 7:80. https://doi.org/10.1038/s41392-022-00895-2
Chang Y, Cui M, Fu X, Zhang L, Li X, Li L, Wu J, Sun Z, Zhang X, Li Z et al (2019) MiRNA-155 regulates lymphangiogenesis in natural killer/T-cell lymphoma by targeting BRG1. Cancer Biol Ther 20:31–41. https://doi.org/10.1080/15384047.2018.1504721
Zheng X, Rui H, Liu Y, Dong J (2020) Proliferation and apoptosis of B-cell lymphoma cells under targeted regulation of FOXO3 by miR-155. Mediterr J Hematol Infect Dis 12:e2020073. https://doi.org/10.4084/MJHID.2020.073
Niu F, Dzikiewicz-Krawczyk A, Koerts J, de Jong D, Wijenberg L, Fernandez Hernandez M, Slezak-Prochazka I, Winkle M, Kooistra W, van der Sluis T, et al. (2020) MiR-378a-3p is critical for Burkitt lymphoma cell growth. Cancers (Basel) 12 https://doi.org/10.3390/cancers12123546
Wu W, Chen L, Chen C, Yu L, Zheng J (2021) miRNA-425-5p enhances diffuse large B cell lymphoma growth by targeting PTEN. Transl Cancer Res 10:4905–4913. https://doi.org/10.21037/tcr-21-2394
Wang CC, Han L, Hou YH, Ying XY (2020) MiRNA-584 suppresses the progression of NK/T-cell lymphoma by targeting FOXO1. Eur Rev Med Pharmacol Sci 24:4404–4411. https://doi.org/10.26355/eurrev_202004_21022
Lin L, Huang Y, Zhuang W, Lin P, Ma X (2020) miR-100 inhibits cell proliferation in mantle cell lymphoma by targeting mTOR. Exp Hematol Oncol 9:25. https://doi.org/10.1186/s40164-020-00182-2
Huang Y, Zou Y, Lin L, Ma X, Zheng R (2019) miR-101 regulates cell proliferation and apoptosis by targeting KDM1A in diffuse large B cell lymphoma. Cancer Manag Res 11:2739–2746. https://doi.org/10.2147/CMAR.S197744
Wei H, Liu R, Guo X, Zhou Y, Sun B, Wang J (2019) miRNA-135a regulates Hut78 cell proliferation via the GATA-3/TOX signaling pathway. Mol Med Rep 19:2361–2367. https://doi.org/10.3892/mmr.2019.9885
Gao Y, Ding X (2021) miR-145-5p exerts anti-tumor effects in diffuse large B-cell lymphoma by regulating S1PR1/STAT3/AKT pathway. Leuk Lymphoma 62:1884–1891. https://doi.org/10.1080/10428194.2021.1894642
Wang QM, Lian GY, Song Y, Peng ZD, Xu SH, Gong Y (2019) Downregulation of miR-152 contributes to DNMT1-mediated silencing of SOCS3/SHP-1 in non-Hodgkin lymphoma. Cancer Gene Ther 26:195–207. https://doi.org/10.1038/s41417-018-0057-7
Sun JR, Zhang X, Zhang Y (2019) MiR-214 prevents the progression of diffuse large B-cell lymphoma by targeting PD-L1. Cell Mol Biol Lett 24:68. https://doi.org/10.1186/s11658-019-0190-9
Tian Y, Wang L, Zhang Y, Li L, Fei Y, Zhang X, Lin G (2021) Association between miR-212-3p and SOX11, and the effects of miR-212-3p on cell proliferation and migration in mantle cell lymphoma. Oncol Lett 22:709. https://doi.org/10.3892/ol.2021.12970
Xia L, Wu L, Xia H, Bao J, Li Q, Chen X, Xia R (2019) miR-337 suppresses cutaneous T-cell lymphoma via the STAT3 pathway. Cell Cycle 18:1635–1645. https://doi.org/10.1080/15384101.2019.1629789
Xu B, Jiang L, Cui JL, Zhu XL, Bai YJ, Chen J, Diao YQ (2022) MiR-363 suppresses the tumor growth of natural killer/T-cell lymphoma via the SIRT6/PI3K/AKT axis. Ann Transl Med 10:1276. https://doi.org/10.21037/atm-22-5649
Zhou W, Xu Y, Zhang J, Zhang P, Yao Z, Yan Z, Wang H, Chu J, Yao S, Zhao S et al (2022) MiRNA-363-3p/DUSP10/JNK axis mediates chemoresistance by enhancing DNA damage repair in diffuse large B-cell lymphoma. Leukemia 36:1861–1869. https://doi.org/10.1038/s41375-022-01565-6
Jafarzadeh A, Noori M, Sarrafzadeh S, TamehriZadeh SS, Nemati M, Chatrabnous N, Jafarzadeh S, Hamblin MR, Jafari Najaf Abadi MH, Mirzaei H (2022) MicroRNA-383: a tumor suppressor miRNA in human cancer. Front Cell Dev Biol 10:955486. https://doi.org/10.3389/fcell.2022.955486
Chen LY, Han BQ, Zhang XM, Yu XB, Yao DD, Yu LQ (2021) MicroRNA-383-5p predicts favorable prognosis and inhibits the progression of diffuse large B-cell lymphoma. Oncol Lett 22:515. https://doi.org/10.3892/ol.2021.12776
Matsuda Y, Ikeda S, Abe F, Takahashi Y, Kitadate A, Takahashi N, Wakui H, Tagawa H (2022) Downregulation of miR-26 promotes invasion and metastasis via targeting interleukin-22 in cutaneous T-cell lymphoma. Cancer Sci 113:1208–1219. https://doi.org/10.1111/cas.15296
Liu Y, Li Q, Dai Y, Jiang T, Zhou Y (2020) miR-532-3p inhibits proliferation and promotes apoptosis of lymphoma cells by targeting beta-catenin. J Cancer 11:4762–4770. https://doi.org/10.7150/jca.45684
Wang Y, Guo D, Li B, Wang Y, Wang B, Wang Z, Wang M, Teng Q (2022) MiR-665 suppresses the progression of diffuse large B cell lymphoma (DLBCL) through targeting LIM and SH3 protein 1 (LASP1). Leuk Res 112:106769. https://doi.org/10.1016/j.leukres.2021.106769
Zhang MY, Wang LQ, Chim CS (2021) miR-1250-5p is a novel tumor suppressive intronic miRNA hypermethylated in non-Hodgkin’s lymphoma: novel targets with impact on ERK signaling and cell migration. Cell Commun Signal 19:62. https://doi.org/10.1186/s12964-021-00707-0
Dang W, Cao P, Yan Q, Yang L, Wang Y, Yang J, Xin S, Zhang J, Li J, Long S et al (2021) IGFBP7-AS1 is a p53-responsive long noncoding RNA downregulated by Epstein-Barr virus that contributes to viral tumorigenesis. Cancer Lett 523:135–147. https://doi.org/10.1016/j.canlet.2021.10.006
Senousy MA, El-Abd AM, Abdel-Malek RR, Rizk SM (2021) Circulating long non-coding RNAs HOTAIR, Linc-p21, GAS5 and XIST expression profiles in diffuse large B-cell lymphoma: association with R-CHOP responsiveness. Sci Rep 11:2095. https://doi.org/10.1038/s41598-021-81715-5
Zhu D, Fang C, Li X, Geng Y, Li R, Wu C, Jiang J, Wu C (2017) Predictive analysis of long non-coding RNA expression profiles in diffuse large B-cell lymphoma. Oncotarget 8:23228–23236. https://doi.org/10.18632/oncotarget.15571
Yu X, Li Z, Liu J (2015) MiRNAs in primary cutaneous lymphomas. Cell Prolif 48:271–277. https://doi.org/10.1111/cpr.12179
Zhang MY, Calin G, Deng MD, Au-Yeung RKH, Wang LQ, Chim CS (2022) Epigenetic silencing of tumor suppressor lncRNA NKILA: Implication on NF-kappaB Signaling in non-Hodgkin’s lymphoma. Genes (Basel) 13 https://doi.org/10.3390/genes13010128
Li Y, Lv Y, Wang J, Zhu X, Chen J, Zhang W, Wang C, Jiang L (2022) LncRNA NORAD mediates the proliferation and apoptosis of diffuse large-B-cell lymphoma via regulation of miR-345–3p/TRAF6 axis. Arch Med Res https://doi.org/10.1016/j.arcmed.2022.01.004
Xing X, Xu T, Liu B, Guo Q (2022) LncRNA SNHG5 can regulate the proliferation and migration of diffuse large B cell lymphoma progression via targeting miR-181-5p/XIAP. J Cancer 13:784–792. https://doi.org/10.7150/jca.60521
Zhao L, Liu Y, Zhang J, Liu Y, Qi Q (2019) LncRNA SNHG14/miR-5590-3p/ZEB1 positive feedback loop promoted diffuse large B cell lymphoma progression and immune evasion through regulating PD-1/PD-L1 checkpoint. Cell Death Dis 10:731. https://doi.org/10.1038/s41419-019-1886-5
Zhao CC, Jiao Y, Zhang YY, Ning J, Zhang YR, Xu J, Wei W, Kang-Sheng G (2019) Lnc SMAD5-AS1 as ceRNA inhibit proliferation of diffuse large B cell lymphoma via Wnt/beta-catenin pathway by sponging miR-135b-5p to elevate expression of APC. Cell Death Dis 10:252. https://doi.org/10.1038/s41419-019-1479-3
Zhao J, Su L, Jiang J (2020) Long non-coding RNA paternally expressed imprinted gene 10 (PEG10) elevates diffuse large B-cell lymphoma progression by regulating kinesin family member 2A (KIF2A) via targeting MiR-101–3p. Med Sci Monit 26:e922810. https://doi.org/10.12659/MSM.922810
Song W, Fei F, Qiao F, Weng Z, Yang Y, Cao B, Yue J, Xu J, Zheng M, Li J (2022) ALKBH5-mediated N(6)-methyladenosine modification of TRERNA1 promotes DLBCL proliferation via p21 downregulation. Cell Death Discov 8:25. https://doi.org/10.1038/s41420-022-00819-7
Li J, Chen Y, Guo X, Bai X, Xu X, Han T, Tan A, Liu N, Xia Y, Sun Q et al (2022) lncNBAT1/APOBEC3A is a mediator of HBX-induced chemoresistance in diffuse large B cell lymphoma cells. Mol Ther Nucleic Acids 27:1064–1077. https://doi.org/10.1016/j.omtn.2022.01.015
Jathar S, Kumar V, Srivastava J, Tripathi V (2017) Technological developments in lncRNA biology. Adv Exp Med Biol 1008:283–323. https://doi.org/10.1007/978-981-10-5203-3_10
Wang Y, Wang L, Sui M (2019) Long non-coding RNA H19 promotes proliferation of Hodgkin’s lymphoma via AKT pathway. J BUON 24:763–769
Fan CB, Yan XH, Tian M, Zhang S, Liu JL, Sheng YX, Dong L, Zhang WL (2020) Long non-coding RNA NEAT1 regulates Hodgkin’s lymphoma cell proliferation and invasion via miR-448 mediated regulation of DCLK1. Eur Rev Med Pharmacol Sci 24:6219–6227. https://doi.org/10.26355/eurrev_202006_21518
Wang L, Yang J, Wang HN, Fu RY, Liu XD, Piao YS, Wei LQ, Wang JW, Zhang L (2021) LncRNA BCYRN1-induced autophagy enhances asparaginase resistance in extranodal NK/T-cell lymphoma. Theranostics 11:925–940. https://doi.org/10.7150/thno.46655
Zhang H, Huang Q (2021) TP73-AS1 promotes malignant progression of NK/T cell lymphoma by regulating DKK1 methylation. J Buon 26:1530–1535
Roundtree IA, Evans ME, Pan T, He C (2017) Dynamic RNA modifications in gene expression regulation. Cell 169:1187–1200. https://doi.org/10.1016/j.cell.2017.05.045
Jonkhout N, Tran J, Smith MA, Schonrock N, Mattick JS, Novoa EM (2017) The RNA modification landscape in human disease. RNA 23:1754–1769. https://doi.org/10.1261/rna.063503.117
Barbieri I, Kouzarides T (2020) Role of RNA modifications in cancer. Nat Rev Cancer 20:303–322. https://doi.org/10.1038/s41568-020-0253-2
Zheng HX, Zhang XS, Sui N (2020) Advances in the profiling of N(6)-methyladenosine (m(6)A) modifications. Biotechnol Adv 45:107656. https://doi.org/10.1016/j.biotechadv.2020.107656
Coker H, Wei G, Brockdorff N (2019) m6A modification of non-coding RNA and the control of mammalian gene expression. Biochim Biophys Acta Gene Regul Mech 1862:310–318. https://doi.org/10.1016/j.bbagrm.2018.12.002
Zhu ZM, Huo FC, Pei DS (2020) Function and evolution of RNA N6-methyladenosine modification. Int J Biol Sci 16:1929–1940. https://doi.org/10.7150/ijbs.45231
Ma S, Chen C, Ji X, Liu J, Zhou Q, Wang G, Yuan W, Kan Q, Sun Z (2019) The interplay between m6A RNA methylation and noncoding RNA in cancer. J Hematol Oncol 12:121. https://doi.org/10.1186/s13045-019-0805-7
Han H, Fan G, Song S, Jiang Y, Qian C, Zhang W, Su Q, Xue X, Zhuang W, Li B (2021) piRNA-30473 contributes to tumorigenesis and poor prognosis by regulating m6A RNA methylation in DLBCL. Blood 137:1603–1614. https://doi.org/10.1182/blood.2019003764
Xia TL, Li X, Wang X, Zhu YJ, Zhang H, Cheng W, Chen ML, Ye Y, Li Y, Zhang A et al (2021) N(6)-methyladenosine-binding protein YTHDF1 suppresses EBV replication and promotes EBV RNA decay. EMBO Rep 22:e50128. https://doi.org/10.15252/embr.202050128
Huang H, Weng H, Zhou K, Wu T, Zhao BS, Sun M, Chen Z, Deng X, Xiao G, Auer F et al (2019) Histone H3 trimethylation at lysine 36 guides m(6)A RNA modification co-transcriptionally. Nature 567:414–419. https://doi.org/10.1038/s41586-019-1016-7
Cheng Y, Fu Y, Wang Y, Wang J (2020) The m6A methyltransferase METTL3 is functionally implicated in DLBCL development by regulating m6A modification in PEDF. Front Genet 11:955. https://doi.org/10.3389/fgene.2020.00955
Ma H, Shen L, Yang H, Gong H, Du X, Li J (2021) m6A methyltransferase Wilms’ tumor 1-associated protein facilitates cell proliferation and cisplatin resistance in NK/T cell lymphoma by regulating dual-specificity phosphatases 6 expression via m6A RNA methylation. IUBMB Life 73:108–117. https://doi.org/10.1002/iub.2410
Bohnsack KE, Hobartner C, Bohnsack MT (2019) Eukaryotic 5-methylcytosine (m(5)C) RNA methyltransferases: mechanisms, cellular functions, and links to disease. Genes (Basel) 10 https://doi.org/10.3390/genes10020102
Jeltsch A, Ehrenhofer-Murray A, Jurkowski TP, Lyko F, Reuter G, Ankri S, Nellen W, Schaefer M, Helm M (2017) Mechanism and biological role of Dnmt2 in nucleic acid methylation. RNA Biol 14:1108–1123. https://doi.org/10.1080/15476286.2016.1191737
Li Q, Li X, Tang H, Jiang B, Dou Y, Gorospe M, Wang W (2017) NSUN2-mediated m5C methylation and METTL3/METTL14-mediated m6A methylation cooperatively enhance p21 translation. J Cell Biochem 118:2587–2598. https://doi.org/10.1002/jcb.25957
Chellamuthu A, Gray SG (2020) The RNA methyltransferase NSUN2 and its potential roles in cancer. Cells 9 https://doi.org/10.3390/cells9081758
Okamoto M, Fujiwara M, Hori M, Okada K, Yazama F, Konishi H, Xiao Y, Qi G, Shimamoto F, Ota T et al (2014) tRNA modifying enzymes, NSUN2 and METTL1, determine sensitivity to 5-fluorouracil in HeLa cells. PLoS Genet 10:e1004639. https://doi.org/10.1371/journal.pgen.1004639
Acknowledgements
The work was supported by the Department of Hematology of Linyi People’s Hospital and Weifang Medical University, and I would like to show great gratitude to them all.
Funding
This study was supported by Shandong Provincial Postdoctoral Innovation Project (201903077) and Shandong Provincial Natural Science Foundation (ZR2018PH014).
Author information
Authors and Affiliations
Contributions
All authors searched for the literature and wrote and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhuang, S., Yang, Z., Cui, Z. et al. Epigenetic alterations and advancement of lymphoma treatment. Ann Hematol 103, 1435–1454 (2024). https://doi.org/10.1007/s00277-023-05395-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05395-z