Abstract
Plasticity is a key trait when an individual’s role in the social environment, and hence its optimum phenotype, fluctuates unpredictably. Plasticity is especially important in primitively eusocial insects where small colony sizes and little morphological caste differentiation mean that individuals may find themselves switching from non-reproductive to reproductive roles. To understand the scope of this plasticity, workers of the primitively eusocial sweat bee Lasioglossum malachurum were experimentally promoted to the reproductive role (worker-queens) and their performance compared with foundress-queens. We focussed on how their developmental trajectory as workers influenced three key traits: group productivity, monopolisation of reproduction, and social control of foraging nest-mates. No significant difference was found between the number of offspring produced by worker-queens and foundress-queens. Genotyping of larvae showed that worker-queens monopolised reproduction in their nests to the same extent as foundress queens. However, non-reproductives foraged less and produced a smaller total offspring biomass when the reproductive was a promoted worker: offspring of worker-queens were all males, which are the cheaper sex to produce. Greater investment in each offspring as the number of foragers increased suggests a limit to both worker-queen and foundress-queen offspring production when a greater quantity of pollen arrives at the nest. The data presented here suggest a remarkable level of plasticity and represent one of the first quantitative studies of worker reproductive plasticity in a non-model primitively eusocial species.
Significance statement
The ability of workers to take on a reproductive role and produce offspring is expected to relate strongly to the size of their colony. Workers in species with smaller colony sizes should have greater reproductive potential to insure against the death of the queen. We quantified the reproductive plasticity of workers in small colonies of sweat bees by removing the queen and allowing the workers to control the reproductive output of the nest. A single worker then took on the reproductive role and hence prevented her fellow workers from producing offspring of their own. These worker-queens produced as many offspring as control queens, demonstrating remarkable worker plasticity in a primitively eusocial species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Individuals in cooperatively breeding and eusocial societies have two fundamental sources of fitness: direct and indirect. Raising the offspring of genetic relatives allows helpers or workers to gain indirect fitness benefits while foregoing reproduction, whereas breeders or reproductives reproduce directly. Helpers typically retain the ability for direct reproduction, but are inhibited by a mixture of social control and self-restraint (Arévalo et al. 1998; Bourke 1999; Cant 2000; Clarke et al. 2001; Saigo and Tsuchida 2004; Wenseleers et al. 2004; De Souza et al. 2008; Moore and Liebig 2013; Maruska 2014). However, if the opportunity arises, helpers in many species may switch roles and become reproductives. This flexibility in trait expression is an example of phenotypic plasticity, the ability of a single genotype to express multiple phenotypes depending on environmental stimuli (West-Eberhard 2005; Richards et al. 2006; Smith 2010). Plasticity is key to how organisms survive in unpredictably changing environments (Clarke and Schluter 2011; Stevens et al. 2013).
In cooperatively breeding/eusocial taxa, retaining the ability to switch between non-reproductive and reproductive roles has several potential fitness benefits. First, although primarily reproducing indirectly, helpers may also produce occasional offspring of their own (Richards et al. 2005; Leadbeater et al. 2011). Second, reproductives may die (Lopez-Vaamonde et al. 2009), leaving helpers to take over the reproductive role (Leadbeater et al. 2011; Van Der Westhuizen et al. 2013; Friend and Bourke 2014). Third, if the reproductive’s productivity falls, then the worker’s own direct fitness may take priority (Almond et al. 2019). Helpers’ optimal strategy may then be to remove the reproductive through worker matricide (Bourke 1994; Lopez-Vaamonde et al. 2009). Replacement of a reproductive may involve conflict: multiple non-reproductives might compete for the role (Cant et al. 2006; Bridge and Field 2007; Zanette and Field 2009). Following the resolution of this conflict, the new reproductive may need to exert the same social control over group mates that it experienced itself as a helper, in order to maximize its fitness.
If phenotypes were completely reversible and plasticity was cost free, the optimal response to a variable environment would be perfect adaptive plasticity. Organisms would interpret their current environment and respond to it with the appropriate phenotype at all stages of development. However, this is limited by constraints on plasticity, revealed when organisms fail to exhibit the optimum trait value as expressed by non-plastic specialists (DeWitt et al. 1998). Understanding how individuals prioritise plasticity over role specialisation, and the limits of this plasticity, is key to understanding the interplay between direct and indirect fitness in social organisms where both remain an option. Investment in indirect fitness while a helper will trade off against future direct fitness via two main mechanisms. First, helping often involves an increased mortality risk while foraging away from the safety of the nest (Cant and Field 2001, 2005): helpers may not survive long enough to inherit breeding positions. Second, helpers that do successfully attain reproductive roles may have reduced productivity in comparison with lifelong reproductives: their investment in helping may have lowered their potential productivity through changes in traits such as fat reserves (Toth and Robinson 2005) and immune response (Lourenço et al. 2019). In addition, however, irreversible differences between queens and workers of eusocial insects usually arise during immature development. Individuals that are pre-destined to become helpers may be morphologically differentiated because they receive different quantities and qualities of nutrition as larvae, are reared in different environments, and respond to different environmental cues compared with individuals destined to be reproductives. The extent of this queen-worker differentiation varies widely in the Hymenoptera, probably as an evolutionary response to variation in the chance that helpers will eventually reproduce directly (Bourke 1999). In advanced eusocial taxa such as honeybees and ants, there is often extreme morphological differentiation, sometimes including irreversible helper sterility (Bourke and Franks 1995; Peeters and Molet 2010). Closer to the origin of eusociality, however, in so-called primitively eusocial taxa, there may primarily be small or even zero size differences (Field and Foster 1999; Field et al. 2010; Kapheim et al. 2012; Pennell and Field 2021). It is plasticity in the face of these developmental effects that we focus on in this paper.
In primitively eusocial taxa, an initial investment in indirect fitness does not preclude a future switch to obtaining direct fitness in a reproductive role (Leadbeater et al. 2011; Friend and Bourke 2014; Rehan et al. 2014). This implies that potential direct fitness through retaining plasticity must be greater than indirect fitness gains if plasticity was lost and investment was targeted entirely at the worker role. In such taxa, death of reproductives is relatively common and worker plasticity insures against this loss for both queens and workers (Yanega 1989; Boomsma 1991; Mueller 1991; Field and Toyoizumi 2020). Small group sizes mean that direct fitness remains a constant option for each individual (Shreeves and Field 2002), and worker ovary development and egg laying can be common even in queenright colonies (Strohm and Bordon-Hauser 2003; Wyman and Richards 2003; Richards et al. 2005). In Hymenoptera, a focal worker is less closely related to her nieces and nephews (r = 0.375) — the offspring of other workers — than to her own offspring (r = 0.5). Thus, if a worker inherits the queen position, she may prefer to exert a similar level of social control as the original queen in order to maximize her fitness by monopolising reproduction. However, the remaining workers in the colony may oppose control by the new worker-queen and attempt to produce offspring of their own. This could be either through preparation for the death of the new worker-queen or by developing their ovaries and producing offspring alongside the new primary reproductive. Between colony differences in relatedness asymmetry could potentially govern the extent to which worker interests align with those of the queen. Multiple paternity and unrelated alien workers are both found in primitively eusocial taxa (e.g. Pennell and Field 2021), altering worker-brood relatedness. However, these differences may be too subtle for workers to detect (Soro et al. 2011) and the obvious change in size between the foundress and replacement queen may provide a better cue.
In some species, there is an age-based convention governing inheritance of the reproductive role that can minimise costs arising from conflict between nest mates (Bridge and Field 2007; Taylor et al. 2020). Even in primitively eusocial taxa, however, workers are usually smaller than reproductives, albeit to a lesser extent than in advanced eusocial taxa, so that the extent of their plasticity may be constrained. Reproductives may strategically produce lower quality worker offspring in order to reduce queen-worker conflict and place offspring in a position where helping is their best option (Brand and Chapuisat 2012; Kapheim et al. 2015; Couchoux and Field 2019). In our study species, the primitively eusocial sweat bee Lasioglossum malachurum (Hymenoptera: Apoidea, Halictidae), the original queen dies before the end of the season in approximately one-third of nests (Pennell and Field 2021). Hence, from the viewpoint of the reproductive, there may be a trade-off between producing a socially controllable worker versus a successful future reproductive. In this paper, we carry out a rare quantitative test of worker phenotypic plasticity by investigating offspring production, monopolisation of reproduction and social control of foraging by workers that take over the reproductive role after experimental queen removal.
Methods
Outline
Foundress-queens were experimentally removed from nests at two time points around worker emergence; then, nests were filmed to record foraging effort in comparison with controls (n = 54 nests). Worker plasticity was first explored by comparing productivity and reproductive skew in nests headed by worker-queens versus foundress-queens. Second, foraging behaviour was examined to investigate how effective the remaining workers were in their role. Following a period of foraging, nests were excavated and their contents catalogued. Microsatellite genotyping was used to determine reproductive success. To estimate investment in male versus female offspring, reproductives of both sexes were weighed soon after they matured as adults.
Study population
The study population of L. malachurum was at a site in West Sussex, UK (Knepp rewilding estate, 50°58′N, 0°22′W). The site was a track way used by people, wildlife, and occasionally vehicles which left the soil bare. This species forms large aggregations of underground nests in patches of bare soil. In early spring, mated females (foundresses) emerge from hibernation and work by themselves to excavate a small nest burrow, comprising a central tunnel and individual cells. Foundresses forage to produce a few cells each containing a single female offspring (B1). L. malachurum is a mass provisioning species. Each egg is laid on a pollen ball which has all of the nutrition needed to reach adulthood. Once the B1 generation reach adulthood, they take over the foraging role, and thereafter, the queen rarely leaves the nest. B1 females emerge in early summer and provision the B2 generation of reproductive offspring (Packer and Knerer 1985; Schwarz et al. 2007). In a third of colonies in our study population, the foundress dies during the B2 provisioning stage (Pennell and Field 2021). The B2 generation mate upon emerging from the nest. Males die soon after the mating period ends and mated females overwinter to restart the nest cycle the following year. In February 2018, before foundresses emerged from overwintering, 14-L plastic buckets were embedded in the ground and filled with excavated soil. To aid drainage, buckets had four circular holes cut into the base, and a fine mesh gauze placed in the bottom before they were filled with soil. From April 2018, the middle of the foundress foraging stage, nests were marked with a numbered nail either side of the nest entrance. A total of 750 nests at the site were initially tracked and these nests were randomly assigned to control (two thirds of nests) and manipulated (one third) treatments.
Queen removals
In the last few days of foundress foraging for B1 production, a total of 63 foundresses were captured as they exited their nests to forage. They were caught in glass jars placed over the nest entrance to ensure it was known which nest was theirs. As nest entrances are normally sealed overnight, the nest entrances were blocked with a flathead nail following foundress removal, to prevent a new queen taking over the nest or the B1 offspring being predated. Following B1 emergence, a further 50 foundresses were removed from their nests using a pooter. B1 offspring had undertaken 1.55 ± 0.41 days of foraging prior to this second set of foundress removals. Foundresses could be identified from their swollen abdomens and their greater wing wear, the latter reflecting the foraging they had carried out prior to workers reaching adulthood. Of these nests, 20 were successful and tracked across all required fields: foraging data, offspring excavations, and genotyping (queen removal pre-emergence: 3 of 63, post-emergence: 17 of 50).
Group size and foraging
Following foundress removals, a camera (Sony HDR-CX625) mounted on a tripod was pointed at each nest entrance where workers were seen to be foraging. Nests were filmed from 9 a.m. to 6 p.m. encompassing the full extent of daily foraging. Nest entrances were filmed on all sunny days in batches of 50 nests per day, again divided 2:1, control:foundress-removed nests. The number of nests involved in the study precluded filming each nest every day, so that they were filmed in rotation. To minimise disturbance, cameras were set up early each morning, prior to helpers leaving the nest and orienting themselves. During the filming period (9 to 30 June), every nest was observed each day to note whether the entrance was open or closed. This allowed the total number of days on which foraging occurred to be tracked, even though each nest could not be filmed every day. Thus, for each nest, we determined the total number of active foraging days, with a subset of those days filmed to gain a measure of foraging effort and group size. Nests were excluded from analysis if their filming days did not match other nests in the study. In total, there were 54 nests where foraging data were examined (n = 34 foundress-queen nests, n = 20 worker-queen). Two days of video footage were analysed for each nest, except two nests in the worker-queen group that had only one day. Video data from 11 different days were analysed, with the 2 days for each nest chosen so that a sample of both treatments was used on each day.
The footage for each nest was reviewed to note every time a bee entered or exited the nest. Each entrance was taken as one successful foraging trip. Each exit was taken as the start of a foraging trip unless the bee was seen to re-enter the nest without leaving the frame of the camera. The group size was taken as the maximum number of bees that were away from the nest in a given day. In a separate study in 2021, this method was applied to multiple days of foraging at 10 nests where, because all individuals had been marked, the true group size was known. It was discovered that the above method can underestimate group size by one, as one bee typically stays in the nest entrance to guard. Hence, we will refer to the total number of ‘foraging workers’ when discussing foraging effort. Each nest had a single worker removed as it departed the nest as part of a different study, after 6.08 ± 0.43 days of the worker phase. These removed workers were accounted for when calculating maximum group size, i.e. if they had been removed prior to both filming days, then the maximum group size was recorded as the larger number of bees seen across the two days plus one. Using the number of bees away from the nest at each time point, a total foraging time per nest was calculated. It was not possible to score video data blind because each video started with the recorder stating what was being filmed. This was needed to allow each video to be individually identified when cataloguing all collected data.
Total offspring production
To obtain the productivity of each group, the immature B2 offspring were collected. Buckets were removed from the ground after 14 ± 0.4 (mean ± SE) days of worker activity. B1 foraging had finished at 39% of nests before they were excavated. Each bucket was removed early in the morning before worker activity had begun and the nest entrances were plugged with a flat headed nail to prevent any adults from leaving the nest as excavations occurred. Burrows were tracked downwards from their entrances using coloured talcum powder. All offspring and adults (B1 and foundresses) found were preserved for later genotyping (adults in RNAlater, stored at − 80 °C; offspring in 100% ethanol stored at 4 °C). Some nests were immediately adjacent to others such that the source of the offspring and adults could not be confidently assigned to either nest. In this case, all those of unknown origin were collected for later genotyping to determine the true count of offspring for nests of interest.
Genotyping and relatedness
Genotyping had three aims: (1) to check that the foundress had been removed and a worker had taken over reproduction in the manipulated nests, (2) to eliminate any offspring collected from the wrong nest, and (3) to measure the reproductive skew in each nest. Genomic DNA was extracted from a leg of adults, from the body wall tissue of the larvae and from eggs in their entirety using an ammonium acetate extraction (Nicholls et al. 2000; Richardson et al. 2001). Each individual was genotyped for 9 microsatellite markers: Mala 09, Lma 14, Lma 51, Lma 52, Lma 36, Mala 01, Lma 2, Lma 53, and LmA 29 (Paxton et al. 2003; Parsons et al. 2017). PCR was performed using the following protocol: each 5 µl reaction consisted of 1 µl DNA and 4 µl of a mastermix made of 0.75 µl 0.2 µM of each primer, 80 µl ultrapure water, and 250 µl QIAGEN Multiplex PCR mix (QIAGEN Inc. Cat. No. 20614). The following PCR profile was used: 95 °C for 15 min, followed by 44 cycles of 94 °C for 30 s, 57 °C for 90 s, 72 °C for 90 s, and finally 60 °C for 30 min. PCR amplification was performed using a DNA Engine Tetrad ®Thermal Cycler (MJ Research, Bio-Rad, Hemel Hempstead, Herts, UK). PCR products were genotyped on an ABI 3730 48-well capillary DNA Analyser using the LIZ size standard (Applied Biosystems Inc. Cat. No. 4322682). Alleles were scored using Geneious v9.1.5 (Biomatters Ltd). Individuals could be sexed by the number of peaks present for each locus. Individuals with a single peak across all loci were assigned as male and those with double peaks at any loci were assigned as female. Relatedness 5.0.8 Software (Queller and Goodnight 1989) was used to estimate genetic relatedness, and Kinship (Goodnight and Queller 1999) was used to test for mother–offspring relationships between adults and larvae within each nest. Together these were used to determine the reproductive skew of each nest.
Previous work in our study population has found that 25% of workers are unrelated to the foundress (Pennell and Field 2021), and this matched with the 27% found here (22% and 30% in worker-queen nests and foundress-queen nests respectively). Unless the origin of an adult was unclear upon excavation, unrelated adults were treated as workers from the target nest. Relatedness 5.0.8 was used to estimate within group genetic relatedness of the workers in each colony, and to examine their relatedness to the brood. Larvae were compared with all adults collected from the nest and removed from analysis if their genotypes meant that none of the adults could be their mother, suggesting that they originated from a spatially adjacent nest. There was therefore the potential to remove the offspring of unrelated workers that died prior to excavation from analyses. However, this is equally true for foundress-queen and worker-queen nests so should not impact comparisons of reproductive skew. No queen was recovered in five of the foundress-queen nests, but all offspring could be attributed to a single individual which was also the mother of the collected B1 adults. These nests were therefore treated as foundress-queen nests and retained in analyses.
Body size
The fecundity of a queen might be determined by her body size, as might her ability to control worker foraging. Both of these could lead to a relationship between body size and total number of offspring. Hence both the absolute size of the queen and the relative size difference between the queen and her average worker, calculated as (queen size − worker size)/queen size, were used as covariates in statistical models. The forewing of each collected adult was removed and placed between two microscope slides. It was then photographed and measured (× 1.6 magnification on a Leica M165 C). The body size of the five missing queens could not be determined and so they were removed from any analyses related to body size.
Weight of B2 reproductives
To better understand the differential investment in male and female B2 offspring, L. malachurum reproductives were collected and weighed. Following the end of worker foraging in August 2020, 55 male and 50 female reproductives were collected in flight using a net, stored individually on ice in a cool box for the rest of the day then placed in a fridge overnight. The following day, a CP64 Analytical Balance (Sartorius) was used to obtain each bee’s wet weight.
Statistical analyses
All data were analysed in R (ver 4.0.1) (R Core Team 2020). Means are reported ± one standard error, and statistical significance was assessed at the p = 0.05 level. With all generalised linear models (GLMs) and generalised linear mixed models (GLMMs), the significance of fixed effects was assessed using the ‘drop1’ function, which employs a log-likelihood ratio to tests to identify terms which can be removed from the model, followed by stepwise backwards elimination. Interaction terms were included in maximal models. Weights of male and female bees were not normally distributed, so were compared using a Mann–Whitney test, using the wilcox.test function. In general, we report only significant effects in the ‘Results’ section.
Reproductive success
To test whether the removal of queens had an effect on the total number of offspring produced, a quasipoisson GLM (‘stats’ package) was used. Total offspring produced was the response variable, and explanatory variables in the maximal model were treatment, maximum number of foragers, total days of foraging, whether foraging had finished when the nest was excavated (Y/N) and absolute queen size. Some explanatory variables could potentially be effects of treatment, e.g. group size and number of days foraging both might be influenced by worker-queens’ ability to control activity within the nest. As a check, a separate model was run using treatment as the only fixed effect. To do this, it was first determined using Wilcoxon tests that all uncontrolled sources of variation (group size, average worker size and total days foraging) were not significantly different between treatment groups. The model containing only treatment produced the same qualitative results as the models separating variance among other factors and so only the models accounting for other variables will be reported.
Foraging effort
All foraging effort modelling was carried out with GLMMs using the lme4 R package (Bates et al. 2014), with nest fitted as a random effect to account for the 2 days of filming modelled for each nest. A normal error structure was assumed with a log link function to account for normally distributed non-integer data that was bounded by zero. Treatment, number of foragers on the day of filming, and the relative size difference between the queen and her average worker were the explanatory variables tested in each model.
We first tested whether treatment affected the total time spent foraging by the group as a whole. We then tested whether treatment had an effect on the effort of an individual worker (number of trips undertaken per worker per day), modelled using a log-normal GLMM. Foraging trips per worker were calculated as total foraging trips per day divided by the number of foraging workers. Finally, we tested whether treatment had an effect on the foraging investment per offspring. Foraging trips per offspring were modelled as the response variable, calculated as foraging trips per day divided by offspring produced per day of foraging by the time nests were excavated.
Least square means were calculated for significant terms using the LSmeans package (Lenth 2016) and reported with 95% confidence intervals. Normality was checked using plotNormalHistogram in the rcompanion package (Mangiafico 2016). Dispersion and residuals were checked in all models using the DHARMa package (Hartig 2021).
Results
Worker-queen nests had 3.80 ± 0.23 foraging workers, and control nests had 3.91 ± 0.30. In nests with worker-queens, all B2 offspring were males — the absence of males in the B1 generation meant that B1 females are unable to mate. In comparison, foundress-queen nests were proportionally 0.62 ± 0.04 female. Adult female reproductives (gynes) weighed 20.12 ± 0.83 mg and males weighed 11.24 ± 0.42 mg (W = 2503.5, p < 0.001, n = 105). B1 females were 84.5 ± 0.7% the size of their queens.
Genetic relatedness
The expected relatedness between full sisters is 0.75 or 0.375 for half-sisters. Relatedness between workers was r = 0.49 [95% CI = 0.40, 0.57] and r = 0.57 [95% CI = 0.52, 0.63] in worker-queen and foundress-queen nests respectively. Relatedness between worker-queens and their workers was r = 0.47 [95% CI = 0.38, 0.56]. For a focal female altruist, the son of a sister would have an expected relatedness of 0.375 (worker-queen nests) whereas a brother would have an expected relatedness of 0.25 (foundress-queen nests). Relatedness between workers and male offspring was r = 0.30 [95% CI = 0.27, 0.32] and r = 0.24 [95% CI = 0.20, 0.27] in worker-queen and foundress-queen nests respectively. In foundress-queen nests, relatedness between workers and female offspring was r = 0.59 [95% CI = 0.54, 0.64].
Reproductive success
Worker-queens were the only possible mothers of 78% ± 7.0% of the offspring in their respective nests. The remaining offspring had more than one adult as a possible mother, but always including the worker-queen. No offspring in any worker-queen nest could be solely attributed to any individual other than the worker-queen. Relatedness between B2 offspring in worker-queen nests was close to the 0.5 expected for brothers (within-nests mean r = 0.49 [95% CI = 0.45, 0.53]). Together, these results indicate that worker-queens were monopolising offspring production in their respective nests.
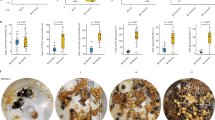
Nests in the two treatments did not differ in offspring number when excavated. Worker queens produced 14.65 offspring (95% CI: 11.67, 18.39). Foundress-queens produced 16.14 offpsring (95% CI: 13.94, 19.13). The removal of foundresses had no significant impact on the total number of offspring produced by the group (GLM: \({\chi }_{1}^{3}\) = 0.03, p = 0.86). Both treatment groups exhibited significant increases in total offspring produced with each additional worker (GLM: \({\chi }_{1}^{3}\) = 12.97, p < 0.001) (Fig. 1), with an additional 2.08 (95% CI: 1.48, 2.90) per worker. A quadratic term for group size was also tested and found to be non-significant (GLM: \({\chi }_{1}^{4}\) = 2.07, p = 0.15). There was an increase of 0.89 (95% CI: 0.77, 1.02) offspring produced per additional day of foraging (GLM: \({\chi }_{1}^{3}\) = 12.96, p < 0.001).
Relationship between the number of foraging workers in a group and the total number of offspring produced in nests with worker-queens and foundress-queens. 95% confidence intervals are indicated by dashed lines and fitted relationships by solid lines. Note that the x-axis scale is different for the two graphs
Foraging investment per offspring
The number of foraging trips taken per offspring increased with each additional worker and was smaller in worker-queen nests than foundress queen nests. Each additional worker increased the number of trips undertaken per offspring produced by 5.23 (95% CI: 3.92, 6.94) (GLMM: \({\chi }_{1}^{2}\) = 59.13, p < 0.001) (Fig. 2). Worker-queen nests had 20.86 (95% CI: 16.94, 25.68) trips per offspring while foundress-queen nests had 30.17 (95% CI: 25.87, 35.18) (GLMM: \({\chi }_{1}^{2}\) = 7.89, p = 0.005).
Relationship between the number of trips per brood and the number of foraging workers for each treatment. Trips per brood were calculated as foraging trips per day divided by offspring per day of foraging. 95% confidence intervals are indicated by dashed lines and fitted relationships by solid lines. Note that the x-axis scale is different for the two graphs
Individual and group level effort
The total time spent foraging by the group increased by 365.25 min per additional worker (95% CI: 329.87, 404.40) (GLMM: \({\chi }_{1}^{2}\) = 800.95, p < 0.001) and there was no difference between treatments (Fig. 3). Individual worker effort as a function of treatment and group size was considered as the number of trips taken by the average worker in a day. Each additional worker decreased the number of trips undertaken per worker per day by 0.36 (95% CI: 0.27, 0.41) (GLMM: \({\chi }_{1}^{2}\) = 3.94, p = 0.047) (Fig. 4). There was also a significant difference between worker queen nests and foundress-queen nests (GLMM: \({\chi }_{1}^{2}\) = 20.61, p < 0.001). Worker-queen nests had 5.36 (95% CI: 4.41, 6.51) trips per worker per day while foundress-queen nests had 9.52 (95% CI: 8.75, 10.36). This suggests that there is a difference in the length of trips between nest types, since total foraging time did not differ.
Relationship between the number of trips per worker and the number of workers for each treatment. Trips per worker were calculated as total days of foraging multiplied by foraging trips per day divided by number of workers. 95% confidence intervals are indicated by dashed lines and fitted relationships by solid lines. Note that the x-axis scale is different for the two graphs
Discussion
Nest inheritance by co-foundresses (Hart and Monnin 2006; Leadbeater et al. 2011; Hoffmann et al. 2012; Bang and Gadagkar 2012; Field and Leadbeater 2016) and workers (Franks et al. 1990; Bourke 1994; Heinze et al. 1997; Friend and Bourke 2014) is common in social insects, especially primitively eusocial taxa (Field & Toyoizumi 2020). The extent to which workers specialise for the worker role, and hence their role plasticity, should coevolve with their chance of inheritance (Bourke 1999). The higher the chance of inheritance, the greater the selection favouring plasticity, and the less specialised workers are expected to become. Hence, colony size will play an important role in the extent of plasticity seen in workers. Here, we quantified this plasticity in terms of several fitness components following experimentally induced inheritance of the queen position by workers in the primitively eusocial sweat bee Lasioglossum malachurum. Although worker-queens were 15% smaller than foundresses in our study population, they produced no fewer offspring than the foundresses that they replaced. At the same time, worker-queens maintained reproductive control in their nests by monopolising reproduction. However, worker-queens produced entirely male offspring, and social control may not have been complete: the number of foraging trips per worker per day in worker-queen nests was significantly fewer than in control nests.
Offspring production
Our finding that worker-queens produced the same number of offspring as foundress-queens suggests extensive worker plasticity (see also Rehan et al. 2010 in a carpenter bee). However, given the difference in offspring sex-ratios produced by the two queen types, it is important to account for any differences in the investment required to produce male and female offspring. In the present study, adult male weight was 54% that of adult female weight. There is evidence that hymenopteran females have higher metabolic conversion rates than males during development (Boomsma and Isaaks 1985; Nielsen et al. 1985; MacKay 1985), which could lead to larger body size from the same mass of pollen provided. However, it has also previously been shown that in sweat bees, male-producing pollen masses are lighter and of lower nutritional value than female pollen masses (Boomsma and Eickwort 1993; Richards and Packer 1994). If worker-queens are producing an equivalent number of offspring which are individually cheaper to produce than the average offspring in foundress-queen nests, a lower total investment in offspring must be occurring in worker-queen nests. This is reflected in the smaller number of foraging trips per offspring that we observed in worker-queen nests.
Reduced investment could partly reflect body size differences between worker-queens and foundresses. As worker-queens are producing individually cheaper offspring, we might expect them to produce a correspondingly larger number of offspring. This was not seen, and one possibility is that the smaller size of worker-queens imposes an insurmountable difference in reproductive potential. Fecundity is well known to depend on body size in insects (Stearns 1977; Head 1995). In experiments on worker reproduction in Bombus spp. a positive relationship was found between body size, fat body size, and productivity (Ayasse et al. 1995; Blacher et al. 2017), where fat body size was suggested to be a factor governing the ability of workers to be productive. Although body size per se did not influence offspring production in our study, the fat bodies of L. malachurum workers are considerably smaller than those of queens (Paxton et al. 2002). Lower reproductive investment by worker-queens could therefore reflect smaller energy reserves compared with foundress-queens. If worker-queen fecundity is limited, then in years or populations where larger numbers of workers are produced, there might be selection to favour greater sharing of egg-laying by worker-queens. An alternative explanation for our findings, however, is that egg-laying by all queens is constrained by the speed of cell construction and not by their ability to lay eggs. Each new offspring requires a new cell to be constructed before provisions can be provided. It is known that nest excavation is costly in Hymenoptera (Field et al. 2007; Ostwald et al. 2021) and observations of within nest behaviour in closely related taxa suggest foraging and cell construction are temporally separated behaviours which may limit a dynamic response to current conditions (Batra 1968). However these observations were on a small number of nests and there is limited knowledge of the interplay between cell construction and foraging in burrowing Halictidae (although see Leonard and Harmon-Threatt 2019). As the workers of L. malachurum likely carry out foraging, excavation, and guarding behaviours, a possible avenue of future research would be to determine how these tasks are divided among nest-mates, and whether there are correlated differences in body size and fat body size which might govern who inherits the nest upon queen death.
Our data do suggest that fecundity may be limited for all queens. The number of foraging trips per offspring was positively correlated with number of foragers across both nest types, consistent with the positive linear relationship between pollen ball weight and group size in another sweat bee species (Boomsma and Eickwort 1993). These patterns could reflect a combination of factors: limited queen fecundity (see Frank and Crespi 1989), a strategic decision to produce higher quality offspring when resources are abundant, or constraints imposed by costly cell construction. Larval provisioning is one of the most important determinants of body size in insects (Strohm 2000). Although larger size was not a determinant of reproductive success amongst female workers in our study, larger males often have an increased probability of mating success in Hymenoptera (Villalobos and Shelly 1991; Couvillon et al. 2010). In our study, it is possible that differences in fecundity were partially obscured because some nests were excavated before foraging was complete. However, this seems unlikely, since whether foraging was complete did not influence the effect of treatment.
Social control by worker-queens
Worker-queens should prefer to produce their own sons (r = 0.5) over the sons of their nest-mates (expected r = ca. 0.25). Our findings suggest that worker-queens do indeed successfully monopolize reproduction: relatedness between male offspring in their nests was close to 0.5, the value expected for brothers, and no offspring could be solely attributed to any individual other than the worker-queen. In another study of this same population, 3% of male offspring in foundress-queen nests were found to be worker laid (Pennell and Field 2021). Together this suggests that worker-queens are exhibiting the same level of reproductive control as the foundress-queens they have replaced. In highly eusocial species such as Apis mellifera, queens may signal their presence, and limit worker reproduction, using pheromones (Holman 2010; Richard and Hunt 2013; Oi et al. 2015; Grüter and Keller 2016). In primitively eusocial species, these pheromones can be indicative of fertility or dominance (Dapporto et al. 2007; Bhadra et al. 2010; Gadagkar 2016), but are not enough in isolation to prevent worker reproduction (Oi et al. 2019). Oophagy has been documented in sweat bees, and could be used to prevent worker reproduction (Batra 1968; Packer and Knerer 1986).
Aggressive dominance behaviour is also an important factor in primitively eusocial species (Reeve 1991; Roseler 1991; Tibbetts and Dale 2004; Thompson et al. 2014). In Bombus impatiens, the increase in group size over a queen’s lifetime leads to a change from aggressive behaviours to chemical signalling as the dominant method of maintaining reproductive dominance (Orlova et al. 2020). Small group sizes in L. malachurum suggest that aggressive behaviour is likely an important factor in the monopolisation of reproduction, and worker-queens lack the inherent size advantage that foundress-queens have over their nest-mates. In our population of L. malachurum and in Lasioglossum (Dialictus) zephyrum, the relative size of the foundress is a predictor of the level of worker reproduction in foundress-queen nests (Kukuk and May 1991; Pennell and Field 2021), suggesting that the smaller size of worker-queens could reduce their control over egg-laying, and over the foraging effort of their nest-mates. Although worker-queens in fact successfully monopolized reproduction, we found that helpers carried out significantly fewer foraging trips per day in worker-queen nests than in foundress-queen nests.
As well as size, another possible explanation for the difference between nest types in the number of foraging trips is the lower relatedness between workers and offspring in worker-queen nests. Worker-queens can monopolize reproduction, but foragers might still reduce costly effort when relatedness is lower, increasing their chance of surviving to potentially inherit the reproductive position. This relies on foragers having reliable cues indicating relatedness, hydrocarbon cues may carry insufficient information in L. malachurum (Soro et al. 2011), but the smaller size difference between helpers and the reproductive in worker-queen nests is a potentially reliable cue. Arguing against this, however, at least within the time frame of our study, is that we found no evidence of inheritance in worker-queen nests, perhaps because worker queens are relatively young bees: offspring relatedness was high, and all offspring genotypes were consistent with the single worker queen. One other possible explanation for the absence of worker reproduction in worker-queen nests is variation in individuals’ capacity to reproduce (Blacher et al. 2017), if the workers most capable of reproduction take on the replacement queen role. Indeed, the significant failure rate of nests where queens were removed might indicate that although plasticity into the reproductive role is extensive in successful nests, it may not be universally available to all workers. It will require more work to elucidate this further.
Conclusion
Overall, our results suggest that although L. malachurum workers are smaller than foundress queens, they retain a remarkably high level of plasticity, reflecting the relatively high chance that they will inherit the queen position when their mother dies. This high chance of nest inheritance leads to selection pressure to maintain plasticity. Experimentally induced worker-queens were able to produce the same number of offspring as foundress-queens. However, worker-queen broods likely involved a lower total energy investment by the group, as they comprised males only, which are the cheaper sex to produce. This suggests that worker plasticity might effectively be constrained following inheritance of the queen position, either by limited fecundity or because their smaller body size reduces their control over foraging by nest mates. The larger number of foraging trips per offspring in larger groups suggests a limit to queen egg laying across all queen types, and could lead to higher quality offspring at larger group sizes. Our work suggests that plasticity in other primitively eusocial species will repay further study.
Data availability
The datasets generated and analysed during the current study will be made available in a public repository following acceptance, and have been made available to the reviewers.
References
Almond EJ, Huggins TJ, Crowther LP et al (2019) Queen longevity and fecundity affect conflict with workers over resource inheritance in a social insect. Am Nat 193:256–266. https://doi.org/10.1086/701299
Arévalo E, Strassmann JE, Queller DC (1998) Conflicts of interest in social insects: male production in two species of Polistes. Evolution 52:797–805. https://doi.org/10.2307/2411273
Ayasse M, Marlovits T, Tengö J et al (1995) Are there pheromonal dominance signals in the bumblebee Bombus hypnorum L (Hymenoptera, Apidae)? Apidologie 26:163–180. https://doi.org/10.1051/apido:19950301
Bang A, Gadagkar R (2012) Reproductive queue without overt conflict in the primitively eusocial wasp Ropalidia marginata. Proc Natl Acad Sci U S A 109:14494–14499. https://doi.org/10.1073/pnas.1212698109
Bates D, Mächler M, Bolker B, Walker S (2014) Fitting linear mixed-effects models using lme4. submitted to J Stat Softw 67:51. https://doi.org/10.18637/jss.v067.i01
Batra SWT (1968) Behavior of some social and solitary halictine bees within their nests: a comparative study. J Kans Entomol Soc 41:120–133. http://www.jstor.org/stable/25083687
Bhadra A, Mitra A, Deshpande SA et al (2010) Regulation of reproduction in the primitively eusocial wasp Ropalidia marginata: on the trail of the queen pheromone. J Chem Ecol 36:424–431. https://doi.org/10.1007/s10886-010-9770-x
Blacher P, Huggins TJ, Bourke AFG (2017) Evolution of ageing, costs of reproduction and the fecundity–longevity trade-off in eusocial insects. Proc R Soc B 284:20170380. https://doi.org/10.1098/rspb.2017.0380
Boomsma JJ (1991) Adaptive colony sex ratios in primitively eusocial bees. Trends Ecol Evol 6:92–95. https://doi.org/10.1016/0169-5347(91)90182-W
Boomsma J, Eickwort GC (1993) Colony structure, provisioning and sex allocation in the sweat bee Halictus ligatus (Hymenoptera: Halictidae). Biol J Linn Soc 48:355–377. https://doi.org/10.1016/0024-4066(93)90006-A
Boomsma JJ, Isaaks JA (1985) Energy investment and respiration in queens and males of Lasius niger (Hymenoptera: Formicidae). Behav Ecol Sociobiol 18:19–27. https://doi.org/10.1007/BF00299234
Bourke AFG (1994) Worker matricide in social bees and wasps. J Theor Biol 167:283–292. https://doi.org/10.1006/jtbi.1994.1070
Bourke (1999) Colony size, social complexity and reproductive conflict in social insects. J Evol Biol 12:245–257. https://doi.org/10.1046/j.1420-9101.1999.00028.x
Bourke AFG, Franks NR (1995) Social evolution in ants. Princeton University Press, Princeton
Brand N, Chapuisat M (2012) Born to be bee, fed to be worker? The caste system of a primitively eusocial insect. Front Zool 9:35. https://doi.org/10.1186/1742-9994-9-35
Bridge C, Field J (2007) Queuing for dominance: gerontocracy and queue-jumping in the hover wasp Liostenogaster flavolineata. Behav Ecol Sociobiol 61:1253–1259. https://doi.org/10.1007/s00265-007-0355-9
Cant MA (2000) Social control of reproduction in banded mongooses. Anim Behav 59:147–158. https://doi.org/10.1006/anbe.1999.1279
Cant MA, Field J (2001) Helping effort and future fitness in cooperation animal societies. Proc R Soc B 268:1959–1964. https://doi.org/10.1098/rspb.2001.1754
Cant MA, Field J (2005) Helping effort in a dominance hierarchy. Behav Ecol 16:708–715. https://doi.org/10.1093/beheco/ari051
Cant MA, English S, Reeve HK, Field J (2006) Escalated conflict in a social hierarchy. Proc R Soc B 273:2977–2984. https://doi.org/10.1098/rspb.2006.3669
Clarke JM, Schluter D (2011) Colour plasticity and background matching in a threespine stickleback species pair. Biol J Linn Soc 102:902–914. https://doi.org/10.1111/j.1095-8312.2011.01623.x
Clarke FM, Miethe GH, Bennett NC (2001) Reproductive suppression in female Damaraland mole-rats Cryptomys damarensis: dominant control or self-restraint? Proc R Soc B 268:899–909. https://doi.org/10.1098/rspb.2000.1426
Couchoux C, Field J (2019) Parental manipulation of offspring size in social groups: a test using paper wasps. Behav Ecol Sociobiol 73:36. https://doi.org/10.1007/s00265-019-2646-3
Couvillon MJ, Hughes WOH, Perez-Sato JA et al (2010) Sexual selection in honey bees: colony variation and the importance of size in male mating success. Behav Ecol 21:520–525. https://doi.org/10.1093/beheco/arq016
Dapporto L, Romana Dani F, Turillazzi S (2007) Social dominance molds cuticular and egg chemical blends in a paper wasp. Curr Biol 17:504–505. https://doi.org/10.1016/j.cub.2007.05.002
De Souza AR, Rodrigues IL, Rocha JVA et al (2008) Foraging behavior and dominance hierarchy in colonies of the neotropical social wasp Polistes ferreri (Hymenoptera, Vespidae) in different stages of development. Sociobiology 52:293–303
DeWitt TJ, Sih A, Wilson DS (1998) Costs and limits of phenotypic plasticity. Trends Ecol Evol 13:77–81. https://doi.org/10.1016/S0169-5347(97)01274-3
Field J, Foster W (1999) Helping behaviour in facultatively eusocial hover wasps: an experimental test of the subfertility hypothesis. Anim Behav 57:633–636. https://doi.org/10.1006/anbe.1999.0995
Field J, Leadbeater E (2016) Cooperation between non-relatives in a primitively eusocial paper wasp, Polistes dominula. Philos Trans R Soc Lond B Biol Sci 371:20150093. https://doi.org/10.1098/rstb.2015.0093
Field J, Toyoizumi H (2020) The evolution of eusociality: no risk-return tradeoff but the ecology matters. Ecol Lett 23:518–526. https://doi.org/10.1111/ele.13452
Field J, Turner E, Fayle T, Foster W (2007) Costs of egg-laying and offspring provisioning: multifacted parental investment in a digger waps. Proc R Soc B 274:445–451. https://doi.org/10.1098/rspb.2006.3745
Field J, Paxton RJ, Soro A, Bridge C (2010) Cryptic plasticity underlies a major evolutionary transition. Curr Biol 20:2028–2031. https://doi.org/10.1016/j.cub.2010.10.020
Frank SA, Crespi BJ (1989) Synergism between sib-rearing and sex ratio in Hymenoptera. Behav Ecol Sociobiol 24:155–162. https://doi.org/10.1007/BF00292098
Franks NR, Ireland B, Bourke AFG (1990) Conflicts, social economics and life history strategies in ants. Behav Ecol Sociobiol 27:175–181. https://doi.org/10.1007/BF00180301
Friend LA, Bourke AFG (2014) Workers respond to unequal likelihood of future reproductive opportunities in an ant. Anim Behav 97:165–176. https://doi.org/10.1016/j.anbehav.2014.09.013
Gadagkar R (2016) Evolution of social behaviour in the primitively eusocial wasp Ropalidia marginata: do we need to look beyond kin selection? Proc R Soc B:20150094. https://doi.org/10.1098/rstb.2015.0094
Goodnight KF, Queller DC (1999) Computer software for performing likelihood tests of pedigree relationship using genetic markers. Mol Ecol 8:1231–1234. https://doi.org/10.1046/j.1365-294x.1999.00664.x
Grüter C, Keller L (2016) Inter-caste communication in social insects. Curr Opin Neurobiol 38:6–11. https://doi.org/10.1016/j.conb.2016.01.002
Hart AG, Monnin T (2006) Conflict over the timing of breeder replacement in vertebrate and invertebrate societies. Insectes Soc 53:375–389. https://doi.org/10.1007/s00040-005-0895-4
Hartig F (2021) DHARMa: Residual Diagnostics for Hierarchical (Multi-Level / Mixed) Regression Models. R package version 0.4.4. http://florianhartig.github.io/DHARMa/ Accessed 26 Feburary 2021
Head G (1995) Selection on fecundity and variation in the degree of sexual size dimorphism among spider species (Class Araneae). Evolution 49:776–781. https://doi.org/10.2307/2410330
Heinze J, Puchinger W, Hölldobler B (1997) Worker reproduction and social hierarchies in Leptothoraxants. Anim Behav 54:849–864. https://doi.org/10.1006/anbe.1996.0511
Hoffmann K, Foster KR, Korb J (2012) Nest value mediates reproductive decision making within termite societies. Behav Ecol 23:1203–1208. https://doi.org/10.1093/beheco/ars103
Holman L (2010) Queen pheromones. Commun Integr Biol 3:558–560. https://doi.org/10.4161/cib.3.6.12976
Kapheim KM, Nonacs P, Smith AR et al (2015) Kinship, parental manipulation and evolutionary origins of eusociality. Proc R Soc B 282:20142886. https://doi.org/10.1098/rspb.2014.2886
Kapheim KM, Smith AR, Ihle KE et al (2012) Physiological variation as a mechanism for developmental caste-biasing in a facultatively eusocial sweat bee. Proc R Soc B 279:1437–1446. https://doi.org/10.1098/rspb.2011.1652
Kukuk PF, May B (1991) Colony dynamics in a primitively eusocial halictine bee Lasioglossum (Dialictus) zephyrum (Hymenoptera: Halictidae). Insectes Soc 38:171–188. https://doi.org/10.1007/BF01240967
Leadbeater E, Carruthers JM, Green JP et al (2011) Nest inheritance is the missing source of direct fitness in a primitively eusocial insect. Science 333:874–876. https://doi.org/10.1126/science.1205140
Lenth RV (2016) Least-Squares Means: The R Package lsmeans. J Stat Softw 69:1–33. https://doi.org/10.18637/jss.v069.i01
Leonard RJ, Harmon-Threatt AN (2019) Methods for rearing ground-nesting bees under laboratory conditions. Apidologie 50:689–703. https://doi.org/10.1007/s13592-019-00679-8
Lopez-Vaamonde C, Raine NE, Koning JW et al (2009) Lifetime reproductive success and longevity of queens in an annual social insect. J Evol Biol 22:983–996. https://doi.org/10.1111/j.1420-9101.2009.01706.x
Lourenço AP, Martins JR, Torres FAS et al (2019) Immunosenescence in honey bees (Apis mellifera L.) is caused by intrinsic senescence and behavioral physiology. Exp Gerontol 119:174–183. https://doi.org/10.1016/j.exger.2019.02.005
MacKay WP (1985) A comparison of the energy budgets of three species of Pogonomyrmex harvester ants (Hymenoptera: Formicidae). Oecologia 66:484–494. https://doi.org/10.1007/BF00379338
Mangiafico SS (2016) Summary and analysis of extension program evaluation in R. Cooperative Extension (Rutgers NJAES), New Brunswick
Maruska KP (2014) General and comparative endocrinology social regulation of reproduction in male cichlid fishes. Gen Comp Endocrinol 207:2–12. https://doi.org/10.1016/j.ygcen.2014.04.038
Moore D, Liebig J (2013) Reproductive restraint without policing in early stages of a social insect colony. Anim Behav 85:1323–1328. https://doi.org/10.1016/j.anbehav.2013.03.022
Mueller UG (1991) Haplodiploidy and the evolution of facultative sex ratios in a primitively eusocial bee. Science 254:442–444. https://doi.org/10.1126/science.254.5030.442
Nicholls JA, Double MC, Rowell DM, Magrath RD (2000) The evolution of cooperative and pair breeding in thornbills Acanthiza (Pardalotidae). J Avian Biol 31:165–176. https://doi.org/10.1034/j.1600-048X.2000.310208.x
Nielsen MG, Skyberg N, Peakin G (1985) Respiration in the sexuals of the ant Lasius flavus. Physiol Entomol 10:199–204. https://doi.org/10.1111/j.1365-3032.1985.tb00035.x
Oi CA, Oliveira RC, van Zweden JS et al (2019) Do primitively eusocial wasps use queen pheromones to regulate reproduction? A case study of the paper wasp Polistes satan. Front Ecol Evol 7. https://doi.org/10.3389/fevo.2019.00199
Oi CA, van Zweden JS, Oliveira RC et al (2015) The origin and evolution of social insect queen pheromones: novel hypotheses and outstanding problems. BioEssays 37:808–821. https://doi.org/10.1002/bies.201400180
Orlova M, Treanore E, Amsalem E (2020) Built to change: dominance strategy changes with life stage in a primitively eusocial bee. Behav Ecol 31:1361–1368. https://doi.org/10.1093/beheco/araa093
Ostwald MM, Fox TP, Harrison JF et al (2021) Social consequences of energetically costly nest construction in a facultatively social bee. Proc R Soc B 288:20210033–20210033. https://doi.org/10.1098/rspb.2021.0033
Packer L, Knerer G (1985) Social evolution and its correlates in bees of the subgenus Evylaeus (Hymenoptera; Halictidae). Behav Ecol Sociobiol 17:143–149. https://doi.org/10.1007/BF00299246
Packer L, Knerer G (1986) The biology of a subtropical population of Halictus ligatus Say (Hymenoptera: Halictidae). Behav Ecol Sociobiol 18:363–375. https://doi.org/10.1007/BF00299667
Parsons PJ, Couchoux C, Horsburgh GJ et al (2017) Identification of 24 new microsatellite loci in the sweat bee Lasioglossum malachurum (Hymenoptera: Halictidae). BMC Res Notes 10:753. https://doi.org/10.1186/s13104-017-3089-4
Paxton RJ, Ayasse M, Field J, Soro A (2002) Complex sociogenetic organisation and reproductive skew in a primatively eusocial sweat bee, Lagioglossum malachurum, as revealed by microsatellites. Mol Ecol 11:2405–2416. https://doi.org/10.1046/j.1365-294X.2002.01620.x
Paxton RJ, Arevalo E, Field J (2003) Microsatellite loci for the eusocial Lasioglossum malachurum and other sweat bees (Hymenoptera, Halictidae). Mol Ecol Notes 3:82–84. https://doi.org/10.1046/j.1471-8286.2003.00357.x
Peeters C, Molet M (2010) Colonial reproduction and life histories. In: Lach L, Parr CL, Abbott KL (eds) Ant Ecology. Oxford University Press, Oxford, pp 159–176
Pennell TM, Field J (2021) Split sex ratios and genetic relatedness in a primitively eusocial sweat bee. Behav Ecol Sociobiol 75:5. https://doi.org/10.1007/s00265-020-02944-8
Queller DC, Goodnight KF (1989) Estimating relatedness using genetic markers. Evol 43(2):258–275. https://doi.org/10.1111/j.1558-5646.1989.tb04226.x
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online at: https://www.R-project.org. Accessed 26 May 2020
Reeve H (1991) Polistes. In: Ross K, Matthews R (eds) The social biology of wasps. Cornell University Press, Ithaca, pp 99–148
Rehan SM, Richards MH, Adams M, Schwarz MP (2014) The costs and benefits of sociality in a facultatively social bee. Anim Behav 97:77–85. https://doi.org/10.1016/j.anbehav.2014.08.021
Rehan SM, Richards MH, Schwarz MP (2010) Social polymorphism in the Australian small carpenter bee, Ceratina (Neoceratina) australensis. Insectes Soc 57:403–412. https://doi.org/10.1007/s00040-010-0097-y
Richards MH, Packer L (1994) Trophic aspects of caste determination in Halictus ligatus, a primitively eusocial sweat bee. Behav Ecol Sociobiol 34:385–391. https://doi.org/10.1007/BF00167329
Richard F-J, Hunt JH (2013) Intracolony chemical communication in social insects. Insectes Soc 60:275–291. https://doi.org/10.1007/s00040-013-0306-6
Richards CL, Bossdorf O, Muth NZ et al (2006) Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol Lett 9:981–993. https://doi.org/10.1111/j.1461-0248.2006.00950.x
Richards MH, French D, Paxton RJ (2005) It’s good to be queen: classically eusocial colony structure and low worker fitness in an obligately social sweat bee. Mol Ecol 14:4123–4133. https://doi.org/10.1111/j.1365-294X.2005.02724.x
Richardson DS, Jury FL, Blaakmeer K et al (2001) Parentage assignment and extra-group paternity in a cooperative breeder: the Seychelles warbler (Acrocephalus sechellensis). Mol Ecol 10:2263–2273. https://doi.org/10.1046/j.0962-1083.2001.01355.x
Roseler P-F (1991) 9. Reproductive competition during colony establishment. In: Ross KG, Matthews RW (eds) The social biology of wasps. Cornell University Press, Ithaca, pp 309–335
Saigo T, Tsuchida K (2004) Queen and worker policing in monogynous and monandrous colonies of a primitively eusocial wasp. Proc R Soc Lond B:271S509–S512. https://doi.org/10.1098/rsbl.2004.0238
Schwarz MP, Richards MH, Danforth BN (2007) Changing paradigms in insect social evolution: insights from halictine and allodapine bees. Annu Rev Entomol 52:127–150. https://doi.org/10.1146/annurev.ento.51.110104.150950
Shreeves G, Field J (2002) Group size and direct fitness in social queues. Am Nat 159:81–95. https://doi.org/10.1086/324125
Smith, AR (2010) Developmental Plasticity. In: Breed, MD and Moore, J (ed) Encyclopedia of Animal Behavior. Academic Press, Oxford, pp. 507–512
Soro A, Ayasse M, Zobel MU et al (2011) Kin discriminators in the eusocial sweat bee Lasioglossum malachurum: the reliability of cuticular and Dufour’s gland odours. Behav Ecol Sociobiol 65:641–653. https://doi.org/10.1007/s00265-010-1066-1
Stearns SC (1977) The evolution of life history traits: a critique of the theory and a review of the data. Annu Rev Ecol Evol Syst 8:145–171. https://doi.org/10.1146/annurev.es.08.110177.001045
Stevens M, Rong CP, Todd PA (2013) Colour change and camouflage in the horned ghost crab Ocypode ceratophthalmus. Biol J Linn Soc 109:257–270. https://doi.org/10.1111/bij.12039
Strohm E (2000) Factors affecting body size and fat content in a digger wasp. Oecologia 123:184–191. https://doi.org/10.1007/s004420051004
Strohm E, Bordon-Hauser A (2003) Advantages and disadvantages of large colony size in a halictid bee: The queen’s perspective. Behav Ecol 14:546–553. https://doi.org/10.1093/beheco/arg039
Taylor BA, Cini A, Cervo R et al (2020) Queen succession conflict in the paper wasp Polistes dominula is mitigated by age-based convention. Behav Ecol 31:992–1002. https://doi.org/10.1093/beheco/araa045
Thompson FJ, Donaldson L, Johnstone RA et al (2014) Dominant aggression as a deterrent signal in paper wasps. Behav Ecol 25:706–715. https://doi.org/10.1093/beheco/aru063
Tibbetts EA, Dale J (2004) A socially enforced signal of quality in a paper wasp. Nature 432:218–222. https://doi.org/10.1038/nature02949
Toth AL, Robinson GE (2005) Worker nutrition and division of labour in honeybees. Anim Behav 69:427–435. https://doi.org/10.1016/j.anbehav.2004.03.017
Van Der Westhuizen L, Jarvis J, Bennett N, Van Wyk J (2013) A case of natural queen succession in a captive colony of naked mole-rats, Heterocephalus glaber. Afr Zool 48:56–63. https://doi.org/10.3377/004.048.0119
Villalobos EM, Shelly TE (1991) Correlates of male mating success in two species of Anthidium bees (Hymenoptera : Megachilidae). Behav Ecol Sociobiol 29:47–53. https://doi.org/10.1007/BF00164294
Wenseleers T, Helanterä H, Hart A, Ratnieks FLW (2004) Worker reproduction and policing in insect societies: An ESS analysis. J Evol Biol 17:1035–1047. https://doi.org/10.1111/j.1420-9101.2004.00751.x
West-Eberhard MJ (2005) Developmental plasticity and the origin of species differences. Proc Natl Acad Sci U S A 102:6543–6549. https://doi.org/10.1073/pnas.0501844102
Wyman LM, Richards MH (2003) Colony social organization of Lasioglossum malachurum Kirby (Hymenoptera, Halictidae) in southern Greece. Insectes Soc 50:201–211. https://doi.org/10.1007/s00040-003-0647-7
Yanega D (1989) Caste determination and differential diapause within the first brood of Halictus rubicundus in New York (Hymenoptera: Halictidae). Behav Ecol Sociobiol 24:97–107. https://doi.org/10.1007/BF00299641
Zanette L, Field J (2009) Cues, concessions, and inheritance: dominance hierarchies in the paper wasp Polistes dominulus. Behav Ecol 20:773–780. https://doi.org/10.1093/beheco/arp060
Acknowledgements
We would like to thank Tanya Pennell, Lucy Neame, and Antoine Melet for valuable assistance in the field. Many thanks also to Tom Carter, Emily Costello, and Jess Stokes for assisting with video data collection. We are also grateful to Charles Burrell, Penny Green, and the team at Knepp Estate for their support and permission to conduct research on the site.
Funding
This work is part of a project that has received funding from the European Research Council (ERC) under the European Horizon’s 2020 research and innovation programme (grant agreement no. 695744 awarded to JF).
Author information
Authors and Affiliations
Contributions
Project conceptualisation and design: TNP and JF. Data collection and analysis: TNP. Supervision: JF. The first draft of the manuscript was written by TNP; then, both authors edited it.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by A. Toth
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Price, T.N., Field, J. Sisters doing it for themselves: extensive reproductive plasticity in workers of a primitively eusocial bee. Behav Ecol Sociobiol 76, 85 (2022). https://doi.org/10.1007/s00265-022-03196-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-022-03196-4