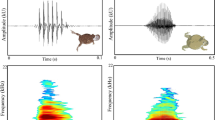
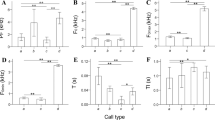
Abstract
Acoustic signals are one of the reproductive isolation mechanisms that can be a driving force for speciation. Evidence that geographic variation in mating calls can cause reproductive isolation is abundant for birds and frogs, but not for mammals. In the Callosciurus genus of tree squirrels distributed in Southeast Asia, males emit mating calls to attract females and the sound properties are known to be distinctly different among coexisting congeneric species. We investigated whether mating calls emitted by males differ between ten populations of the widely distributed C. erythraeus/C. finlaysonii complex. Based on the acoustic properties, the populations were divided into four groups (South Vietnam, Taiwan, eastern China, and western areas including Thailand and western China), which resulted in a high discrimination rate of 94.7% by a discriminant function analysis. A playback experiment was also conducted to determine whether the mating calls from different localities were as effective in attracting conspecific individuals as those in the same locality. In Kanagawa, Japan, where squirrels were introduced from Taiwan in the 1950s, squirrels responded more frequently to the playback sounds recorded in Taiwan compared to those recorded in Thailand. In addition, the response of squirrels decreased with an increase in the difference in acoustic characteristics compared to that from the original habitat. These results suggest that local differences in acoustic characteristics could be one of the mechanisms that promote reproductive isolation of geographically distant populations of Callosciurus.
Significance statement
Acoustic signals are one of the reproductive isolation mechanisms that can be a driving force for speciation. The tree squirrel genus Callosciurus (Sciuridae) is widely distributed in Southeast Asia and is one of the few suitable mammals for testing the premating isolation mechanism using acoustic signals. The mating calls of the C. erythraeus/C. finlaysonii complex were divided into four geographical groups (Taiwan, Vietnam, eastern China, and Thailand) by a discriminant function analysis. Playback experiments revealed that the squirrels responded more frequently to the sounds of their own group compared to those of other groups. In addition, the response of the squirrels decreased with an increase in the difference in acoustic characteristics compared to that from the original habitat. Local differences in acoustic characteristics could be one of the mechanisms that promote reproductive isolation of geographically distant populations of Callosciurus.





Similar content being viewed by others
Data availability
The data analyzed in this study are available as electronic supplementary material.
References
Abdullah SA, Yusoff-Rashid N, Idris AH (2001) Niche segregation among three sympatric species of squirrels inhabiting a lowland dipterocarp forest, Peninsular Malaysia. Mamm Study 26:133–144
Ahonen H, Harcourt RG, Stow AJ, Charrier I (2018) Geographical vocal variation and perceptual discrimination abilities in male Australian sea lions. Anim Cogn 21:235–243
Ahonen H, Stow AJ, Harcourt RG, Charrier I (2014) Adult male Australian sea lion barking calls reveal clear geographical variations. Anim Behav 97:229–239
Ayres JM, Clutton-Brock TH (1992) River boundaries and species range size in Amazonian primates. Am Nat 140:531–537
Baker MC (1982) Vocal dialect recognition and population genetic consequences. Am Zool 22:561–569
Baker MC, Cunningham MA (1985) The biology of bird-song dialects. Behav Brain Sci 8:85–100
Baker MC, Mewaldt LR (1978) Song dialects as barriers to dispersal in white-crowned sparrow, Zonotrichia leucophrys nuttalli. Evolution 32:712–722
Balakirev AE, Rozhnov VV (2019) Taxonomic revision of beautiful squirrels (Callosciurus, Rodentia: Sciuridae) from the Callosciurus erythraeus/finlaysonii complex and their distribution in eastern Indochina. Raffles Bull Zool 67:459–489
Blair WF (1974) Character displacement in frogs. Am Zool 14:1119–1125
Boonkhaw P, Prayoon U, Kanchanasaka B, Hayashi F, Tamura N (2017) Colour polymorphism and genetic relationships among twelve subspecies of Callosciurus finlaysonii in Thailand. Mamm Biol 85:6–13
Boughman JW (2002) How sensory drive can promote speciation. Trends Ecol Evol 17:571–577
Boul KE, Chris Funk W, Darst CR, Cannatella DC, Ryan MJ (2007) Sexual selection drives speciation in an Amazonian frog. Proc R Soc Lond B 274:399–406
Brockelman W, Ali R (1987) Methods for surveying and sampling forest primate populations. In: Marsh CW, Mittermeier RA (eds) Primate Conservation in the Tropical Rain Forest. Alan R Liss, New York, pp 23–62
Brown CH (2003) Ecological and physiological constraints for primate vocal communication. Primate audition Ethol Neurobiol 2003:127–149
Buainain N, de Assis CP, Raposo MA (2017) Geographic variation and taxonomy of the Arremon taciturnus (Hermann, 1783) species complex (Aves: Passerellidae). J Ornithol 158:631–650
Campbell P, Pasch B, Pino JL, Crimo OL, Phillips M, Phelps SM (2010) Geographic variation in the songs of neotropical singing mice: testing the relative importance of drift and local adaptation. Evolution 64:1955–1972
Charrier I, Mathevon N, Aubin T (2013) Bearded seal males perceive geographic variation in their trills. Behav Ecol Sociobiol 67:1679–1689
Corbet GB, Hill JE (1992) The mammals of the Indomalayan region. Natural History Museum Publication Oxford University Press, Oxford
Delgado RA (2006) Sexual selection in the loud calls of male primates: signal content and function. Int J Primatol 27:5–25
Dobzhansky T (1951) Genetics and the origin of species, 3rd edn. Columbia University Press, New York
Eiler KC, Banack SA (2004) Variability in the alarm call of golden-mantled ground squirrels (Spermophilus lateralis and S. saturatus). J Mamm 85:43–50
Farentinos RC (1972) Social dominance and mating activity in the tassel-eared squirrel (Sciurus aberti ferreus). Anim Behav 20:316–326
Fordham G, Shanee S, Peck M (2018) Biogeographic analysis of platyrrhines using updated taxonomic assessments to evaluate evidence for the Riverine Barrier Hypothesis. PeerJ 6:e26656v1
Freeman BG, Montgomery GA (2017) Using song playback experiments to measure species recognition between geographically isolated populations: a comparison with acoustic trait analyses. Auk 134:857–870
Funk WC, Cannatella DC, Ryan MJ (2009) Genetic divergence is more tightly related to call variation than landscape features in the Amazonian frogs Physalaemus petersi and P. freibergi. J Evol Biol 22:1839–1853
Gabrielli M, Cardoso YP, Benitez V, Gozzi AC, Guichón ML, Lizarralde MS (2014) Genetic characterization of Callosciurus (Rodentia: Sciuridae) Asiatic squirrels introduced in Argentina. Ital J Zool 81:328–343
Gerhardt HC (2013) Geographic variation in acoustic communication: reproductive character displacement and speciation. Evol Ecol Res 15:605–632
Graham BA, Sandoval L, Dabelsteen T, Mennill DJ (2016) A test of the acoustic adaptation hypothesis in three types of tropical forest: degradation of male and female rufous-and-white wren songs. Bioacoustics 26:37–61
Grant PR, Grant BR, Petren K (2000) The allopatric phase of speciation: the sharp-beaked ground finch (Geospiza difficilis) on the Galápagos islands. Biol J Linn Soc 69:287–317
Hamao S, Sugita N, Nishiumi I (2016) Geographic variation in bird songs: examination of the effects of sympatric related species on the acoustic structure of songs. Acta Ethol 19:81–90
Irwin DE, Price T (1999) Sexual imprinting, learning and speciation. Heredity 82:347–354
Irwin DE, Thimgan MP, Irwin JH (2008) Call divergence is correlated with geographic and genetic distance in greenish warblers (Phylloscopus trochiloides): a strong role for stochasticity in signal evolution? J Evol Biol 21:435–448
Janik VM, Slater PJB (1997) Vocal learning in mammals. Adv Stud Behav 26:59–99
Jones G (1997) Acoustic signals and speciation: the roles of natural and sexual selection in the evolution of cryptic species. Adv Stud Behav 26:317–354
Kanchanasaka B, Boonkhaw P, Hirankrilas K, Payoon U, Tamura N (2014) Color variation of Finlayson’s squirrel among populations and individuals in central Thailand. Mamm Study 39:237–244
Kirschel AN, Blumstein DT, Smith TB (2009) Character displacement of song and morphology in African tinkerbirds. P Natl Acad Sci USA 106:8256–8261
Koford RR (1982) Mating system of a territorial tree squirrel (Tamiasciurus douglasii) in California. J Mamm 63:274–283
Koprowski JL (1993) Behavioral tactics, dominance, and copulatory success among male fox squirrels. Ethol Ecol Evol 5:169–176
Lachlan RF, Servedio MR (2004) Song learning accelerates allopatric speciation. Evolution 58:2049–2063
Lin A, Liu H, Chang Y, Lu G, Feng J (2016) Behavioural response of the greater horseshoe bat to geographical variation in echolocation calls. Behav Ecol Sociobiol 70:1765–1776
Lishak RS (1982) Gray squirrel mating calls: a spectrographic and ontogenic analysis. J Mammal 63:661–663
Lurz PW, Hayssen V, Geissler K, Bertolino S (2013) Callosciurus erythraeus (Rodentia: Sciuridae). Mamm Spec 45:60–74
MacKinnon KS (1978) Stratification and feeding differences among Malayan squirrels. Malay Nat J 30:593–608
Marten K, Marler P (1977) Sound transmission and its significance for animal vocalization. 1 Temperate habitats. BehavEcolSociobiol 2:271–290
Marten K, Quine D, Marler P (1977) Sound transmission and its significance for animal vocalization. 2 Tropical forest habitats. Behav Ecol Sociobiol 2:291–302
Mayr E (1947) Ecological factors in speciation. Evolution 1:263–288
Mazzamuto MV, Galimberti A, Cremonesi G et al (2016) Preventing species invasion: a role for integrative taxonomy? Integr Zool 11:214–228
McComb K (1991) Female choice for high roaring rates in red deer, Cervus elaphus. Anim Behav 41:79–88
Miyamoto A, Tamura N, Sugimura K, Yamada F (2004) Predicting habitat distribution of the alien Formosan squirrel using logistic regression model. Glob Environ Res 8:13–22
Miyazaki M, Waas JR (2003) Acoustic properties of male advertisement and their impact on female responsiveness in little penguins Eudyptula minor. J Avian Biol 34:229–232
Moore JC, Tate GHH (1965) A study of the diurnal squirrels, Sciuridae, Sciurinae, of the Indian and Indochinese subregions. Fieldiana Zoology 48, Chicago Natural History Museum Press, Chicago
Morton ES (1975) Ecological sources of selection on avian sounds. Am Nat 109:17–34
Nelson DA, Soha JA (2004) Perception of geographical variation in song by male Puget Sound white-crowned sparrows, Zonotrichia leucophrys pugetensis. Anim Behav 68:395–405
Nicholls JA, Goldizen AW (2006) Habitat type and density influence vocal signal design in satin bowerbirds. J Anim Ecol 75:549–558
Oshida T, Dang CN, Nguyen ST, Nguyen NX, Endo H, Kimura J, Hayashi Y (2011) Phylogenetic relationship between Callosciurus caniceps and C. inornatus (Rodentia, Sciuridae): Implications for zoogeographical isolation by the Mekong River. Ital J Zool 78:328–335
Oshida T, Dang CN, Nguyen ST et al (2013) Phylogenetic position of Callosciurus erythraeus griseimanus from Vietnam in the genus Callosciurus. Mamm Stud 38(1):41–17
Oshida T, Torii H, Lin LK, Lee JK, Chen YJ, Endo H, Sasaki M (2007) A preliminary study on origin of Callosciurus squirrels introduced into Japan. Mamm Study 32:75–82
Oshida T, Yasuda M, Sasaki M (2016) Preliminary study on phylogeography of Callosciurus prevostii in Southeast Asia: mitochondrial DNA evidence supports riverine barrier hypothesis. Mamm Study 41:149–154
Otter KA, Mckenna A, LaZerte SE, Ramsay SM (2020) Continent-wide shifts in song dialects of white-throated sparrows. Curr Biol 30:3231–3235
Panhuis TM, Butlin R, Zuk M, Tregenza T (2001) Sexual selection and speciation. Trends Ecol Evol 16:364–371
Patten MA, Rotenberry JT, Zuk M (2004) Habitat selection, acoustic adaptation, and the evolution of reproductive isolation. Evolution 58:2144–2155
Podos J (2001) Correlated evolution of morphology and vocal signal structure in Darwin’s finches. Nature 409:185–188
Podos J, Warren PS (2007) The evolution of geographic variation in birdsong. Adv Stud Behav 37:403–458
Ptacek MB (2000) The role of mating preferences in shaping interspecific divergence in mating signals in vertebrates. Behav Process 51:111–134
Puechmaille SJ, Gouilh MA, Piyapan P, Yokubol M, Mie KM, Bates PJ, Petit EJ (2011) The evolution of sensory divergence in the context of limited gene flow in the bumblebee bat. Nat Commun 2:573
Reichert MS, Gerhardt HC (2013) The role of body size on the outcome, escalation and duration of contests in the grey treefrog, Hyla versicolor. Anim Behav 82:1357–1366
Ritchie MG (2007) Sexual selection and speciation. Annu Rev Ecol Evol S 38:79–102
Russo D, Mucedda M, Bello M, Biscardi S, Pidinchedda E, Jones G (2007) Divergent echolocation call frequencies in insular rhinolophids (Chiroptera): a case of character displacement? J Biogeogr 34:2129–2138
Ryan MJ (1990) Signals, species, and sexual selection. Am Sci 78:46–52
Ryan MJ, Brenowitz EA (1985) The role of body size, phylogeny and ambient noise in the evolution of bird song. Am Nat 126:87–100
Ryan MJ, Cocroft RB, Wilczynski W (1990) The role of environmental selection in intraspecific divergence of mate recognition signals in the cricket frog, Acris crepitans. Evolution 44:1869–1872
Ryan MJ, Guerra MA (2014) The mechanism of sound production in túngara frogs and its role in sexual selection and speciation. Curr Opin Neurol 28:54–59
Ryan MJ, Kime NM (2003) Selection on long-distance acoustic signals. In: Simmons A, Fay R, Popper A (eds) Acoustic communication. Springer, New York, pp 225–274
Ryan MJ, Perrill SA, Wilczynski W (1992) Auditory tuning and call frequency predict population-based mating preferences in the cricket frog, Acris crepitans. Am Nat 139:1370–1383
Ryan MJ, Rand AS (1990) The sensory basis of sexual selection for complex calls in the tungara frog, Physalaemus pustulosus (sexual selection for sensory exploitation). Evolution 44:305–314
Seddon N (2005) Ecological adaptation and species recognition drives vocal evolution in neotropical suboscine birds. Evolution 59:200–215
Slabbekoorn H, Smith TB (2002) Bird song, ecology and speciation. Phil Trans R Soc B 357:493–503
Tamura N (1993) Role of sound communication in mating of Malaysian Callosciurus (Sciuridae). J Mamm 74:468–476
Tamura N (1995) Postcopulatory mate guarding by vocalization in the Formosan squirrel. Behav Ecol Sociobiol 36:377–386
Tamura N, Boonkhaw P, Prayoon U, Kanchanasaka B, Hayashi F (2018) Mating calls are a sensitive indicator of phylogenetic relationships in tropical tree squirrels (Callosciurus spp.). Mamm Biol 93:198–206
Tamura N, Hayashi F, Miyashita K (1988) Dominance hierarchy and mating behavior of the Formosan squirrel, Callosciurus erythraeus thaiwanensis. J Mamm 69:320–331
Tamura N, Hayashi F, Miyashita K (1989) Spacing and kinship in the Formosan squirrel living in different habitats. Oecologia 79:344–352
Tamura N, Kasahi T, Kaneda M, Mitarai N, Shigeta M, ShigetaY HN (2013) Sound playback surveys to reveal the distribution of invasive alien Pallas’s squirrels, Callosciurus erythraeus. Mamm Study 38:97–103
Tamura N, Yong HS (1993) Vocalizations in response to predators in three species of Malaysian Callosciurus (Sciuridae). J Mammal 74:703–714
Thompson DC (1977) Reproductive behavior of the grey squirrel. Can J Zool 55:1176–1184
Timmins RJ, Duckworth JW (2008) Diurnal squirrels (Mammalia Rodentia Sciuridae) in Lao PDR: distribution, status and conservation. Trop Zool 21:11–56
Tobias JA, Aben J, Brumfield RT, Derryberry EP, Halfwerk W, Slabbekoorn H, Seddon N (2010) Song divergence by sensory drive in Amazonian birds. Evolution 64:2820–2839
Trivers R (1972) Parental investment and sexual selection. In: Campbell BG (ed) Sexual Selection and the Descent of Man, 1871–1971. Aldine, Chicago, pp 52–95
Uy JAC, Borgia G (2000) Sexual selection drives rapid divergence in bowerbird display traits. Evolution 54:273–278
Van Cise AM, Roch MA, Baird RW, Aran MT, Barlow J (2017) Acoustic differentiation of Shiho-and Naisa-type short-finned pilot whales in the Pacific Ocean. J Acoust Soc Am 141:737–748
Viljoen S (1983) Communicatory behaviour of southern African tree squirrels, Paraxerus palliatus ornatus, P. p. tongensis, P. c. cepapi and Funisciurus congicus. Mammalia 47:441–462
West-Eberhard MJ (1983) Sexual selection, social competition, and speciation. Q Rev Biol 58:155–183
Wilczynski W, Ryan MJ (1999) Geographic variation in animal communication systems. In: Foster SA, Endler JA (eds) Geographic variation in behavior. Oxford University Press, New York, pp 234–261
Wiley RH, Richards DG (1978) Physical constraints on acoustic communication in the atmosphere: implications for the evolution of animal vocalizations. Behav Ecol Sociobiol 3:69–94
Wilkins MR, Seddon N, Safran RJ (2013) Evolutionary divergence in acoustic signals: causes and consequences. Trends Ecol Evol 28:156–166
Wyman MT, Mooring MS, McCowan B, Penedo MCT, Reby D, Hart LA (2012) Acoustic cues to size and quality in the vocalizations of male North American bison, Bison bison. Anim Behav 84:1381–1391
Yasuda M, Ishii M, Okuda T, Hussein NA (2003) Small mammal community: habitat preference and effects after selective logging. In: Okuda T, Manokaran N, Matsumoto Y, Niiyama K, Thomas SC, Ashton PS (eds) Pasoh, ecology of a lowland rain forest in Southeast Asia. Springer, Tokyo, pp 533–546
Yu X, Peng Y, Aowphol A, Ding L, Brauth SE, Tang YZ (2011) Geographic variation in the advertisement calls of Gekko gecko in relation to variations in morphological features: implications for regional population differentiation. Ethol Ecol Evol 23:211–228
Acknowledgments
We are grateful H.S. Yong (University of Malaya), B. Kanchanasaka (Department of National Park Wildlife and Plant Conservation), P. Pipat (Chulalongkorn University), Y. Peng (Xishuangbanna Tropical Botanical Garden), and R. Tabuchi (Forest Products Research Institute) for making our study possible and E. Ohya (Forest Products Research Institute) for providing valuable advice regarding the acoustic analyses. We also thank the assistance provided by the staff of the following station: Ulu Gombak Field Station, Khao Ang Runai Wildlife Conservation, Khao Kheow Wildlife Sanctuary, Doi Suthep National Park, and Yokohama Nature Sanctuary. This study was financially supported by the Bureau of Industrial and Labor Affairs, Tokyo Metropolitan Government, and Forestry and Forest Products Research Institute, Japan. Comments from the editor and anonymous reviewers improved an earlier version of this manuscript.
Funding
This study was financially supported by the Bureau of Industrial and Labor Affairs, Tokyo Metropolitan Government, JSPS KAKENHI Grant Number 18H02247, and Forestry and Forest Products Research Institute, Japan.
Author information
Authors and Affiliations
Contributions
NT conceived and planned the study, conducted the experiment, collected data in the field, performed statistical analyses, interpreted results, and wrote the first draft of the manuscript; PB, UP, QTP, PY, XL, and FH collected data in the field and contributed to the interpretation of the results. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All applicable international, national, and/or institutional guidelines for conducting research were followed. According to ‘Act on Welfare and Management of Animals’ by the Ministries of Environment, Government of Japan, playback experiments at the present study site do not fall within the authority of the Local Ethical Commission in Japan and its approval was not required (www.env.go.jp/en/laws/nature/index.html). Playback trials were kept brief to minimize disturbance to our subjects. The sound power was adjusted to the level which was the same as the natural sounds.
Consent for publication
All authors consent to the publication of this article.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by E. Korpimäki
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tamura, N., Boonkhaw, P., Prayoon, U. et al. Geographical variation in squirrel mating calls and their recognition limits in the widely distributed species complex. Behav Ecol Sociobiol 75, 97 (2021). https://doi.org/10.1007/s00265-021-03022-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-021-03022-3