Abstract
Pancreatic cancer remains a challenging disease with limited treatment options, resulting in high mortality rates. The predominant approach to managing pancreatic cancer patients continues to be systemic cytotoxic chemotherapy. Despite substantial advancements in immunotherapy strategies for various cancers, their clinical utility in pancreatic cancer has proven less effective and durable. Whether administered as monotherapy, employing immune checkpoint inhibitors, tumor vaccines, chimeric antigen receptors T cells, or in combination with conventional chemoradiotherapy, the clinical outcomes remain underwhelming. Extensive preclinical experiments and clinical trials in the realm of pancreatic cancer have provided valuable insights into the complexities of immunotherapy. Chief among the hurdles are the immunosuppressive tumor microenvironment, limited immunogenicity, and the inherent heterogeneity of pancreatic cancer. In this comprehensive review, we provide an overview and critical analysis of current clinical immunotherapy strategies for pancreatic cancer, emphasizing their endeavors to overcome immunotherapy resistance. Particular focus is placed on strategies aimed at reshaping the immunosuppressive microenvironment and enhancing T cell-mediated tumor cell killing. Ultimately, through deeper elucidation of the underlying pathogenic mechanisms of pancreatic cancer and the refinement of therapeutic approaches, we anticipate breakthroughs that will pave the way for more effective treatments in this challenging disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer, ranking as the seventh leading cause of cancer-related fatalities, remains a formidable malignancy characterized by a grim prognosis and a mortality-to-incidence ratio of 94% [1]. Although there has been a modest improvement in its 5-year survival rate in recent years, a lack of breakthrough treatment options persists. Several factors contribute to the dismal outlook for pancreatic cancer patients. Firstly, the absence of early diagnostic markers or discernible clinical symptoms suitable for pancreatic tumor screening often results in the diagnosis of locally advanced or metastatic disease at the time of presentation. Secondly, treatment options for pancreatic cancer are exceedingly limited, further compounding the poor prognosis. Conventional treatments, such as FOLFIRINOX (comprising fluorouracil, leucovorin, irinotecan, and oxaliplatin) or a combination of nab-paclitaxel plus gemcitabine, afford a median overall survival (OS) of merely 11.1 and 8.5 months, respectively, for patients in good health who receive these standard systemic chemotherapy regimens [2,3,4]. Surgery represents the sole potential cure for this disease; however, a mere 20% of patients diagnosed with localized pancreatic cancer are eligible candidates for surgical intervention. Furthermore, recurrence of pancreatic cancer remains commonplace, even among patients who receive standard adjuvant therapy following surgery [5]. Consequently, there exists an urgent imperative for novel therapeutic strategies in the battle against pancreatic cancer.
Immunotherapy, in essence, entails the augmentation of the human immune system's capacity to recognize and eliminate tumor cells through various mechanisms. Its overarching objective is the complete eradication of malignant cells while sparing normal cell function. Immunotherapy has spawned numerous drug regimens grounded in diverse mechanisms and has exhibited remarkable success in the realm of malignancies such as metastatic melanoma and hematological tumors. Nevertheless, its clinical utility in solid tumors, notably pancreatic cancer, has been less auspicious, though this does not preclude its promising potential [6].
In this comprehensive review, we provide an overview of current clinical immunotherapies for pancreatic cancer, elucidate their underlying mechanisms, and assess their efficacy. Furthermore, we spotlight technological advancements, encompassing improvements in antibody technology, the manipulation and amplification of cancer-killing cells, and cancer vaccines, all of which have injected innovation into the field of immunotherapy and hold the promise of a brighter future in the battle against pancreatic cancer.
Immune checkpoint-based treatment options
T cells represent indispensable components of the human immune system, regulated by costimulatory and inhibitory signals as they engage in the recognition and elimination of tumor cells in vivo [7, 8]. Immune checkpoints constitute a category of immunosuppressive molecules that operate under physiological conditions to maintain self-tolerance and prevent the body from inadvertently harming its healthy cells during infection or excessive inflammation [9,10,11]. Research has revealed that tumor cells can elude immune cell recognition and destruction by binding to immune checkpoints on the surface of immune cells, thereby effecting immune evasion [12,13,14,15]. Therapeutic strategies centered on disrupting the interaction between tumor cells and immune cell checkpoints hold the potential to reverse immune evasion. Notably, PD-1 (Programmed cell death protein 1) and PD-L1 (Programmed death-ligand 1), as well as CTLA-4 (cytotoxic T-lymphocyte-associated protein 4), were the first immune checkpoints to be discovered, garnering significant attention as therapeutic targets [15] (Fig. 1). Corresponding monoclonal antibodies can prevent PD-1 from binding with PD-L1 and PD-L2, as well as inhibit the binding of PD-L1 to CD80 protein, thereby reinstating the immune system's ability, particularly that of T cells, to target and eliminate tumor cells, while preserving normal immune function [16, 17]. Similarly, by disrupting the ligand-receptor interactions of B7-1/B7-2 and CTLA-4, these interventions enable effector cells to sustain their recognition and killing functions.
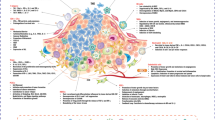
Current clinical immunotherapy strategies for pancreatic cancer. This schematic shows the monotherapies and combined therapies for pancreatic cancer. The clinical immunotherapy strategies for pancreatic cancer are shown as monotherapy, including administrated with tumors or angiogenesis-targeted antibodies, immune checkpoint inhibitors, tumor vaccines, chimeric antigen receptor (CAR) T cells, and also as in combination with chemotherapy or radiotherapy. Upper left: Monoclonal antibodies that target tumors or angiogenesis, inhibit tumor development, and promote apoptosis of tumor cells. They are usually used in combination with chemotherapy or radiotherapy. Upper right: Treatment options based on ICB. These treatments are usually used in combination with drugs that inhibit tumor growth, improve the tumor microenvironment, and also in combination with conventional chemotherapy or radiotherapy. Bottom left: Whole cell vaccines, peptide vaccines, dendritic cell vaccines, and nucleic acid vaccines are prepared differently. Tumor vaccines are often used in combination with chemotherapy, ICB, etc. Bottom right: T cells are extracted from the patient's body and genetically modified to express chimeric antigen receptors (CARs), enabling them to mount a more potent and sustained response. These engineered T cells circulate within the body, delivering precise and efficient anti-tumor capabilities
In addition to immune checkpoint inhibitors, another class of drugs has garnered attention due to its distinct mechanism of action. CD40, a member of the TNF (tumor necrosis factor) receptor superfamily, exhibits wide distribution among antigen-presenting cells and plays a pivotal role in activating antigen-presenting cells and enhancing T cell-mediated anti-tumor immunity. Consequently, CD40 represents a compelling antagonistic target [18,19,20] (Fig. 1). Activation of CD40 sets in motion tumor suppression through various pathways in diverse CD40 agonist-based combination therapy regimens. For instance, CD40-activated macrophages enhance the intratumoral concentration of chemotherapeutic drugs by disrupting the extracellular matrix. Moreover, CD40 agonists heighten the efficacy of the adaptive immune system against tumor cells by augmenting antigen presentation, bolstering CD8 + T cell activity, and fostering immune cell infiltration at tumor sites, effectively transforming "cold" tumors into immunologically active environments [19].
In summary, the discovery of immune checkpoints and the evolution of clinical applications involving immune checkpoint inhibitors and agonists have broadened the landscape of anti-tumor treatment options, charting novel directions for anti-tumor research and bring about a resurgence in the exploration of anti-tumor immunity (Clinical trials of drugs targeting immune checkpoints are listed in Table 1).
Monotherapy using single immune checkpoint drugs or combinations
Blocking the PD-1 and PD-L1 axis or preventing CTLA-4 binding to its ligands with monoclonal antibodies has demonstrated definitive efficacy in many patients with solid tumors [21,22,23,24]. However, the clinical effectiveness of this monotherapy approaching pancreatic cancer patients has been disappointing. Currently, the following immune checkpoint drugs are commonly employed in clinical trials and therapies for pancreatic cancer: PD-1 inhibitors such as pembrolizumab, nivolumab, camrelizumab, and toripalimab; PD-L1 inhibitors like atezolizumab and durvalumab; and CTLA-4 inhibitors including ipilimumab and tremelimumab.
Pembrolizumab (MK-3475), a potent PD-1-specific IgG4-κ humanized monoclonal antibody, obstructs the binding of PD-1 to its ligands, PD-L1 and PD-L2. In a phase I study, pembrolizumab exhibited variable efficacy among different advanced solid tumor patient populations, with both pancreatic cancer patients experiencing disease progression during treatment [25].
Similarly, clinical trials of anti-PD-L1 monotherapy have yielded discouraging results. An early phase I trial involving seven pancreatic cancer patients treated with BMS-936559, a high-affinity, IgG4 human-derived anti-PD-L1 monoclonal antibody, failed to elicit any partial responses (PR) [26]. In another phase I study, one pancreatic cancer patient achieved progression-free survival (PFS) for 12.2 months following treatment with atezolizumab, an engineered immunoglobulin monoclonal antibody targeting PD-L1, showing promise but lacking sufficient evidence to draw definitive conclusions [27].
Conversely, clinical trials of anti-CTLA-4 monotherapy have produced even more disheartening outcomes, with no evidence of efficacy and the emergence of unmanageable side effects. In a phase II study of 27 patients with locally advanced or metastatic pancreatic cancer treated with ipilimumab, a fully humanized CTLA-4 antibody, only 40% (eight of 20) completed the treatment course due to disease progression and side effects. Notably, three immunological adverse events of grade ≥ 3 were observed, one of which resulted in the patient's death. According to the response evaluation criteria in solid tumors, it was concluded that single-agent ipilimumab, at a dose of 3.0 mg/kg, was an effective therapy for pancreas adenocarcinoma [28].
Furthermore, combination therapy involving anti-PD-1/L1 and anti-CTLA-4 demonstrated limited efficacy in pancreatic ductal adenocarcinoma (PDAC), in contrast to its success in other solid tumors [29, 30]. O'Reilly EM et al. conducted a phase II randomized clinical trial in patients with metastatic PDAC, administering a combination of durvalumab, a human anti-PD-L1 monoclonal antibody, and tremelimumab, a human anti-CTLA-4 monoclonal antibody. The objective response rate (ORR) was 3.1% for patients receiving combination therapy and 0% for those receiving monotherapy [31].
Interestingly, the combination therapy of anti-PD-L1 and anti-CTLA-4 demonstrated some efficacy in pancreatic neuroendocrine tumors (pNETs). In a study involving a combination of ipilimumab and nivolumab, an anti-PD-1 monoclonal antibody, three out of seven (43%) pNET patients achieved objective responses in CA209-538, particularly those with high-grade tumors, including one previously refractory to single-agent anti-PD-1 therapy [32]. This suggests that patients with high-grade pNETs can significantly benefit from dual checkpoint blockade.
In summary, immune checkpoint inhibitor monotherapy, with limited data, has demonstrated little to no impact on overall prognosis in pancreatic cancer patients. However, the clinical benefit observed in individual patients suggests the therapeutic potential of this approach, particularly in vaccinated patients who exhibit stronger responses to immune checkpoint drugs [28]. Additionally, the combination strategies of anti-CTLA-4 plus anti-PD-1 or anti-PD-L1 have shown anti-tumor activity, especially in pNETs, although further clinical trials are required to establish their potential value. These findings underscore the need for combination therapy strategies based on immune checkpoint drugs to combat pancreatic cancer effectively.
Immune checkpoint drugs combined with chemotherapy
anti-PD-1 combined with (m) FOLFIRINOX or gemcitabine-based chemotherapy
Both the combination of anti-PD-1 with (modified) FOLFIRINOX and gemcitabine-based chemotherapy have demonstrated superior efficacy compared to chemotherapy alone.
A phase II study involving a combination of modified FOLFIRINOX, a standard adjuvant therapy for patients with resectable and advanced disease [3, 33,34,35,36], and nivolumab as a first-line treatment for 31 metastatic pancreatic cancer patients yielded promising results. FOLFIRINOX was modified by having a lower dose of fluorouracil compared to standard FOLFIRINOX. This regimen resulted in a median OS of 13.40 months (90% CI 10.87–15.24) and a median PFS of 7.39 months (90% CI 3.88–7.59), with a 1-year survival rate of 54.8% (90% CI 39.1–68.1%). Notably, the most frequently reported grade 3–4 drug-related adverse event was neutrophil count decrease (38.7%) [37]. Overall, the addition of a PD-1 antibody to (m) FOLFIRINOX has shown slight improvements over the known efficacy of (m) FOLFIRINOX alone [38, 39].
Furthermore, the CISPD-4 study, a randomized phase II trial investigating the combination of PD-1 antibodies with modified FOLFIRINOX for borderline resectable and locally advanced pancreatic cancer, is currently underway [40], which may provide additional positive data for this combination therapy regimen.
The combination therapy of anti-PD-1 with gemcitabine and nab-paclitaxel (AG) chemotherapy as a first-line treatment has also shown promising efficacy. In a previous study, this combination achieved a remarkable 100% disease control rate (DCR) in patients with advanced pancreatic cancer as a first-line therapy [41]. Patients in this study achieved a median OS of 15 months and a median PFS of 9.1 months. Similar encouraging data regarding DCR were observed in other clinical trials. In a single-arm, single-center exploratory study that enrolled 20 metastatic PDAC patients, camrelizumab, an anti-PD-1 antibody, combined with AG as a first-line therapy resulted in a 60% ORR and an 85% DCR [42]. Similarly, in a phase II trial, 80% of patients with advanced pancreatic cancer achieved an ORR when treated with NAPPCG (N/nivolumab plus AP/albumin-bound paclitaxel plus P/paricalcitol and plus C/cisplatin + G/gemcitabine) as a first-line therapy, with a median PFS of 8.2 months [43].
The trial data mentioned above suggest that the combination therapy of anti-PD-1 with gemcitabine-based chemotherapy elicits a stronger initial response in pancreatic cancer patients. However, investigating methods to sustain this anti-tumor response and prolong patients' survival remains a key area for further research. Notably, a case report documented a patient with metastatic PDAC who achieved durable responses and tolerated well a triple combination therapy of toripalimab (a humanized IgG4K monoclonal antibody specific for human PD-1), gemcitabine, and nab-paclitaxel, even though pseudoprogression was observed in this patient, suggesting that some patients may derive substantial benefit from this protocol [44].
anti-CTLA-4 combined with gemcitabine
The safety of tremelimumab, a human monoclonal IgG2 antibody targeting CTLA-4, in combination with gemcitabine has been established in patients with metastatic pancreatic cancer [45]. However, compared to PD-1, the results of the combination of CTLA-4 and chemotherapy have been less optimistic. In a phase I study conducted in 2020, patients with advanced pancreatic cancer treated with ipilimumab plus gemcitabine achieved a median PFS of 2.5 months (95% CI 0.8–4.8) and a median OS of 8.5 months (95% CI 2.2–10.3), which did not surpass the efficacy of gemcitabine monotherapy. Common grade 3 or 4 adverse events included anemia (48%), leukopenia (48%), and neutropenia (43%) [46].
The combination of anti-CTLA-4 and chemotherapy does not appear to demonstrate superiority over the combination of PD-1 with chemotherapy, which may be attributed to differences in chemotherapy regimens and the varying therapeutic effects of anti-CTLA-4 and anti-PD-1 [47].
anti-PD-1 plus anti-CTLA4 plus chemotherapy
In contrast to the combination of anti-CTLA-4 with gemcitabine, the addition of PD-1 and CTLA-4 antibodies to chemotherapy has shown better survival benefits, albeit with increased side effects. The combination of gemcitabine and nab-paclitaxel (Nab-P) serves as the standard first-line treatment for advanced PDAC. In the recent Canadian Cancer Trials Group PA.7 trial (NCT02879318), a randomized phase II study, the combination of gemcitabine plus nab-paclitaxel with durvalumab and tremelimumab improved patients' OS (9.8 months versus 8.8 months; HR 0.94; P: 0.72) and ORR (30.3% versus 23.0%; OR 1.49; P 0.28) compared to the control group. However, there was no significant difference in PFS (5.5 months versus 5.4 months), and the overall incidence of adverse events in the experimental group was higher (68.91% versus 44.3%) [48].
Based on the results of clinical trials involving the combination of anti-PD-1 or anti-CTLA-4 with chemotherapy, it appears that the three-drug regimen combining anti-PD-1 and anti-CTLA-4 with chemotherapy may not warrant further research for the treatment of pancreatic cancer. This is due to the fact that, in addition to the limited improvement in clinical benefits compared to the two-drug combination regimen, the tolerability of self-toxicity and side effects in advanced pancreatic cancer patients remains a significant concern.
CD40 agonist combined with gemcitabine-based chemotherapy
As early as 2011, the combination of CD40 agonistic monoclonal antibodies (mAbs), such as selicrelumab, with gemcitabine was observed to induce tumor regression in advanced pancreatic cancer patients. Among 21 patients, four developed a partial response, 11 had stable disease (SD), and four experienced disease progression (PD). The median PFS for these patients was 5.6 months (95% confidence interval, 4.0 months to not estimable), and the median OS was 7.4 months (95% CI, 5.5 to 12.8 months). This combination demonstrated better clinical benefit than historical gemcitabine monotherapy, highlighting the potential of CD40 agonistic mAbs when combined with chemotherapy [20, 49].
More recently, the safety and tolerability of combination therapy with CD40 agonists and AG (gemcitabine and nab-paclitaxel) have been demonstrated. In an open-label, multicenter, phase Ib study involving APX005M (sotigalimab), another CD40 agonistic monoclonal antibody, combined with chemotherapy, 14 out of 24 patients with metastatic pancreatic adenocarcinoma responded positively, with eight of them receiving additional nivolumab. Common grade 3–4 treatment-related adverse events included decreased lymphocyte counts (20; 67%) and decreased neutrophil counts (9; 30%). The most common serious adverse event was pyrexia (6 [20%] of 30) [50]. Overall, based on the observed tolerability and clinical activity, this study suggests a viable combination therapy option for advanced pancreatic cancer patients.
However, in the recently published phase II results of this study, the experimental group receiving AG plus sotigalimab with or without nivolumab did not exhibit improved efficiency compared to the control group. The 1-year OS rates for the two groups were 48.1% (sotigalimab plus chemo, p = 0.062, n = 36) and 41.3% (sotigalimab, nivo plus chemo, p = 0.223, n = 35), respectively. There was no significant improvement observed in ORR or PFS in either arm [51, 52]. This lack of improvement may be attributed to functional exhaustion following T cell hyperactivation and potential efficacy antagonism introduced by the combination therapy. While the results of this study are negative, they provide crucial insights for future research into combination therapy strategies and the underlying immune mechanisms of pancreatic cancer.
Furthermore, the addition of CD40 agonists to neoadjuvant chemotherapy has demonstrated immunological changes and clinical benefits. In one study, selicrelumab, an agonistic CD40 mAb, was added to the standard chemotherapy regimen of gemcitabine and nab-paclitaxel before surgery for 16 patients with resectable pancreatic cancer. This combination proved to have tolerable toxicity, resulting in an OS of 23.4 months (95% CI, 18.0–28.8 months). Additionally, immunological changes, such as increased proliferation and activation of T cells in the tumor microenvironment (TME) and a decrease in M2-like tumor-associated macrophages, were observed in postoperative patients compared to preoperative untreated patients [53]. Focusing on these immunological changes within the TME under these treatments may pave the way for future studies aimed at overcoming the current efficacy challenges faced by regimens combining CD40 agonists with chemotherapeutics.
For patients with pancreatic cancer, the combined treatment strategy of immune checkpoint drugs with chemotherapy offers certain advantages over chemotherapy alone. However, the contradiction between chemotherapy drugs' immune system-suppressing effects and the immune system's dependence on immune checkpoint drugs may limit the efficacy of this combination strategy. Additionally, the rapid progression of pancreatic cancer and the immune cell exhaustion in its late stages weakens the immune system's anti-tumor capabilities, thereby limiting the clinical impact of immune checkpoint drugs. Furthermore, the potential autoimmune toxicity resulting from ICIs or CD40 agonists, as well as the tolerability of combination therapy, is important considerations before initiating treatment, particularly for patients with abnormal autoimmune function or those who have undergone multiple rounds of chemotherapy.
Combination of immune checkpoint drugs with other drugs
In pancreatic cancer, the immune microenvironment characterized by immunosuppression presents a significant challenge to the efficacy of immunotherapy. Simultaneously, targeting different nodes involved in anti-tumor immunosuppression holds the theoretical promise of producing a synergistic effect.
Anti-PD-1 combined with anti-colony-stimulating factor-1
Colony-stimulating factor-1 (CSF-1) is produced by various tumors and recruits cells like myeloid-derived suppressor cells (MDSCs) to promote immunosuppression. Preclinical data in a pancreatic cancer mouse model suggest that blocking CSF-1/CSF-1R signaling can lead to anti-tumor T cell responses and tumor regression [54, 55]. However, the effectiveness of anti-PD-1 combined with anti-CSF1R therapy in pancreatic cancer remains inconclusive. A study involving solid tumor patients, including those with pancreatic cancer, treated with 200 mg pembrolizumab plus 1100 mg AMG 820, an anti-CSF1R monoclonal antibody, did not yield sufficiently robust anti-tumor activity to warrant further investigation [56].
Anti-PD-1/L1 combined with transforming growth factor β receptor inhibitor
Transforming growth factor β (TGF-β) is a multifunctional cytokine in the transforming growth factor superfamily that promotes immunosuppression, angiogenesis, tumor cell epithelial-to-mesenchymal transition (EMT), and other tumor-promoting processes. Blocking the TGF-β pathway may sensitize tumors to immune checkpoint inhibitors by affecting immune cell function [57,58,59]. Preclinical studies have shown that TGF-β receptor inhibitors (TGFβ-RI) can efficiently block the TGF-β canonical pathway [60,61,62], and dual inhibition of TGFβ-RI and PD-L1 may synergistically enhance anti-tumor efficacy by downregulating PD-1 expression while relieving TGF-β's inhibitory effects on CD8 + T cells [63, 64]. However, a two-part, single-arm, multinational, phase Ib study combining galunisertib, a TGF-β type I receptor kinase inhibitor, with durvalumab in recurrent/refractory metastatic pancreatic cancer patients reported inconsistent results. The study showed a median OS of 5.72 months (95% CI: 4.01 to 8.38) and PFS of 1.87 months (95% CI: 1.58 to 3.09), with a DCR of 25.0% and a confirmed ORR of 3.1% [65].
Similarly, clinical trials targeting both PD-L1 and TGF-β pathways have yielded pessimistic results. M7824 (MSB0011359C), a novel bifunctional fusion protein consisting of a human IgG1 monoclonal antibody against PD-L1 fused to the extracellular domain of TGF-β receptor II (TGF-βRII), was tested in a phase I study with patients suffering from solid tumors. While durable and confirmed partial responses were observed in one of five patients (20%) with pancreatic cancer [66], the overall results were not promising.
Anti-PD-L1 combined with vascular endothelial growth factor (VEGF) inhibitor
VEGF and its receptors are essential regulators of angiogenesis under normal physiological conditions. VEGF is expressed in various tumors, promoting tumor angiogenesis and metastasis. In the case of pancreatic cancer, most tumors are VEGF-positive, making it a potential target [67]. The combination of anti-PD-L1 with a VEGF inhibitor has shown effectiveness in pNET patients. Halperin DM et al. treated 20 pNET patients with atezolizumab in combination with the VEGF inhibitor bevacizumab. The study reported a PFS of 14.9 months (95% CI, 4.4–32.0) and an ORR of 0%; (95% CI, 5.7%-43.7%) [68].
Anti-PD-L1/CTLA-4 combined with anti-CC chemokine receptor 4 mAb
CC chemokine receptor 4 (CCR4), expressed in various tumor and Treg cells, has been shown to increase the number of CD56 + NK cells and induce potent ADCC (antibody-dependent cellular cytotoxicity)-mediated anti-tumor effects as well as decrease the number of Foxp3 + Treg cells in peripheral in a tumor-bearing mouse model when targeted by its monoclonal antibody [69,70,71,72]. However, clinical trials investigating the combination of anti-CCR4 and anti-PD-L1 at tolerated levels have not shown significant efficacy. In a phase I clinical trial, Zamarin D et al. enrolled 64 patients with solid tumors, including 27 with pancreatic cancer, who were treated with mogamulizumab, a humanized, defucosylated immunoglobulin G1 kappa mAb, in combination with durvalumab or tremelimumab. Despite tolerability, the ORR was only 5.3% (95% CI, 0.1%–26.0%), with one patient achieving a partial response [73]. The lackluster results may be attributed to inadequate drug concentration within the local tumor to trigger pharmacological effects or the absence of specific immune cell types in the pancreatic cancer tumor microenvironment, limiting the anti-tumor efficacy of CCR4 mAb. Additionally, the associated depletion of effector T cell populations may counteract the positive therapeutic effects of Treg depletion.
Anti-PD-1 combined with inhibitor of CXCR4-CXCL12 axis
The COMBAT trial has shown promising signs of efficacy in combining anti-PD-1 therapy with a CXCR4 inhibitor and chemotherapy for pancreatic cancer patients. This trial enrolled patients with metastatic PDAC who had progressed after first-line therapy. They were subsequently treated with BL-8040 (a CXCR4 antagonist) and pembrolizumab as second-line regimens. The results indicated a median OS of 7.5 months. In the expansion cohort, where the NAPOLI-1 regimen (nanosome irinotecan, fluorouracil, and leucovorin) was added, the ORR, DCR, and median persistence were 32%, 77%, and 7.8 months, respectively, for the experimental group consisting of 22 patients [74]. However, subsequent data for 43 patients treated with this regimen showed a decrease in ORR (21%), DCR (63.02%), and median duration of clinical benefit (5.7 months) compared to previous data. In the intention-to-treat population, median PFS was 3.8 months, and median OS was 6.6 months. Importantly, the triple combination was safe and well-tolerated, with a low incidence of grade 3 or higher neutropenia and infection (7%) [75]. These alterations in efficacy may be attributed to the modulation of tumor immunosuppression in pancreatic cancer.
Conversely, a clinical study involving anti-CXCL12 (NOX-A12) showed positive results. In patients with microsatellite-stable disease, unresponsive to anti-PD-1 therapy, cotreatment with a 2-week start-up period of NOX-A12 followed by pembrolizumab resulted in disease stabilization observed in 2 (22%) of nine patients. Clinical activity was also observed in other patients, characterized by significantly longer treatment duration than before [76]. Given that chemotherapeutic drugs can increase the exposure of tumor neoantigens to the immune environment through direct cell destruction and that inhibition of the CXCR4-CXCL12 pathway can reprogram the tumor microenvironment [77,78,79], the combination of chemotherapy and CXCR4-CXCL12 pathway blockade holds significant promise.
Immune checkpoint drugs combined with kinase inhibitor
Bruton's tyrosine kinase (BTK), a non-receptor kinase, has been implicated in the immunosuppressive function of myeloid-derived cells in the tumor microenvironment [80, 81]. While BTK inhibitors have shown the ability to inhibit pancreatic cancer progression in mouse models [82], the clinical combination therapy of a PD-1 inhibitor with a BTK inhibitor has demonstrated poor efficacy. A randomized phase II study evaluating pembrolizumab and acalabrutinib in comparison with acalabrutinib alone in patients with advanced pancreatic cancer failed to reveal a significant improvement in the ORR (7.9%, 95% CI: 1.7% to 21.4% in the combination therapy arm, versus 0%, 95% CI: 0% to 10%, in the monotherapy arm) and median PFS (1.4 months, 95% CI: 1.3 to 1.4 months, and 1.4 months, 95% CI: 1.3 to 1.5 months) [83]. Furthermore, the study did not assess changes in MDSCs and T cell subsets in the tumor microenvironment.
Similarly, anlotinib, a tyrosine kinase inhibitor targeting VEGF receptors 2, has shown the ability to reduce vascular density in tumor tissue and inhibit tumor growth [84, 85]. However, combining a tyrosine kinase inhibitor (TKI) with PD-1 therapy in pancreatic cancer has not improved patient survival. In an observational, prospective study involving patients with advanced pancreatic cancer, the combination regimen of anlotinib plus anti-PD-1 antibodies resulted in the worst PFS compared to other solid tumors, with a PFS of 1.61 months versus 8.37 months (95% CI: 6.5–10.0 months) [86].
Likewise, the phase Ia/Ib PACT study demonstrated the stable safety and durable clinical activity of LY3300054, a new PD-L1 inhibitor with a modified Fc domain that prevents PD-L1–expressing T cell depletion. However, combinations of this drug with tyrosine-protein kinase (MET) inhibitors have been less effective in patients with pancreatic cancer. After treatment with LY3300054 and merestinib (a type II MET kinase inhibitor), most pancreatic cancer patients experienced disease progression [87]. Moreover, in a recent phase Ib clinical trial, the combination of avelumab (a PD-L1 blocking human IgG1 monoclonal antibody) and binimetinib (a small-molecule MEK1/2 inhibitor) also showed limited clinical activity in metastatic pancreatic ductal adenocarcinoma (mPDAC) patients [88].
Immune checkpoint drugs combined with kinase inhibitor and chemotherapy
The incorporation of chemotherapy agents alongside ICIs and kinase inhibitors appears to ameliorate the unfavorable outcome associated with the two-drug combination regimen. Focal adhesion kinase (FAK), a tyrosine kinase overactivated in the majority of PDAC and linked to a poor prognosis, was targeted in a recent multicenter, open-label, phase 1 study. The three-drug combination of defactinib, a small-molecule FAK inhibitor, pembrolizumab, and gemcitabine, demonstrated good tolerability and safety in patients with advanced refractory pancreatic cancer. Notably, among the 10 evaluable patients, one achieved a PR, seven showed SD, and two exhibited PD [89].
Immune checkpoint drugs combined with PARP inhibitors and platinum-based therapy
Niraparib, a poly (ADP ribose) polymerase (PARP) inhibitor inhibiting DNA repair and inducing tumor cell death, significantly enhanced progression-free survival in patients with advanced ovarian cancer. In a recent randomized phase Ib/II study of niraparib combined with nabuliumab or ipilimumab in patients with platinum-sensitive advanced pancreatic cancer [90], despite 50% of patients experiencing grade 3–4 treatment-related AEs, the nira/ipi group was deemed superior due to a 59.6% progression-free survival rate at 6 months (PFS6) and a 17.3-month mOS as opposed to the nira/nivo group [91]. This precision targeting strategy may offer personalized immune drug selection for patients with distinct characteristics.
Immune checkpoint drugs combined with activator of the Toll-like receptor 9 pathway
Pixatimod, a compound augmenting innate immunity by activating the Toll-like receptor 9 pathway, has been reported to potentially enhance the efficacy of ICIs. Unfortunately, a phase Ib open-label multicenter study of solid tumors treated with nivolumab revealed no responders among 18 mPDAC patients [92]. This implies that augmenting the anti-tumor capability of innate immunity may not eliminate T cell rejection and restriction in advanced PDAC; yet, we believe that enhancing the innate immune system may exert a more potent effect on early-stage pancreatic cancer treatment.
It is apparent that drugs targeting immune system suppression have clinical efficacy limitations. Contributing factors may include:
-
a.
Recruitment of patients with a history of multiple failed treatments in trials, potentially leading to unknown changes in tumor-localized microenvironments.
-
b.
Heterogeneity in local tumor cellular composition and an insufficient number of recruited patients in studies.
-
c.
Persistence of immunosuppressive components in the tumor microenvironment and the emergence of adaptive resistance mechanisms.
-
d.
Challenges in achieving adequate drug penetration into local tumor sites and maintaining sustained effects within the tumor microenvironment.
Nevertheless, research in this area warrants attention and ongoing exploration to enhance our comprehension of the local environment and molecular mechanisms of pancreatic cancer.
Therapeutic regimen based on immune checkpoint drugs and radiotherapy
Radiation therapy is believed to induce a distal anti-tumor immune response through the abscopal effect, enhancing tumor cell sensitivity to immune cell killing. The combination with ICIs may potentially reverse the cold tumor characteristics of pancreatic cancer. In a single-arm, non-randomized, phase 2 trial combining radiation, ipilimumab, and nivolumab in patients with metastatic microsatellite-stable (MSS) PDAC, a response was observed in patients receiving radiation therapy, with a DCR of 29% (5/17; 95%CI: 10–56%), and an ORR of 18% (3/17; 95%CI: 4–43%). This study confirms the ability of radiation therapy to improve the response rate to immunotherapy [93].
The safety of ICIs combined with stereotactic body radiotherapy (SBRT) was demonstrated in a phase I clinical trial [94]. In another randomized phase II study of nivolumab with or without ipilimumab combined with SBRT, there were also positive results for refractory metastatic pancreatic cancer. Compared to patients treated with SBRT/nivolumab, those in the SBRT nivolumab/ipilimumab group achieved a higher clinical benefit rate (CBR) (37.2% vs. 17.1%) and a higher percentage of patients who achieved a PR (14.0% vs. 2.4%). In conclusion, the regimen exhibited clinically significant anti-tumor activity and a good safety profile [95].
Similarly, the addition of anti-PD-1 therapy plus KRAS inhibitor to chemoradiotherapy regimens has demonstrated both safety and improved efficacy when compared to control groups. In a phase II study involving 198 patients with postoperative local recurrence of pancreatic cancer characterized by mutant KRAS and positive immunohistochemical staining of PD-L1, eligible participants received chemotherapy (mFOLFIRINOX or 5-fluorouracil). Following this, they were administered SBRT (35–40 Gy in five fractions), intravenous pembrolizumab (200 mg/3 weeks), and trametinib (2 mg/day) or SBRT (with the same regimen) and intravenous gemcitabine (1000 mg/m(2)) on day 1 and 8 of a 21-day cycle for eight cycles. The results indicated that the group receiving SBRT plus pembrolizumab and trametinib achieved a median OS of 24.9 months (95% CI 23.3–26.5), whereas the control group (SBRT + gemcitabine) had a median OS of 22.4 months (95% CI 21.2–23 6). This demonstrated a notable improvement with a hazard ratio (HR) of 0.60 (95% CI 0.44–0.82; p = 0.0012). The most common grade 3 or 4 adverse effects observed were increased alanine aminotransferase or aspartate aminotransferase (ten [12%] of 85 in SBRT plus pembrolizumab and trametinib group vs. six [7%] of 85 in SBRT plus gemcitabine group) [96].
However, the outcomes of a phase II study examining the effectiveness of a multi-drug combination involving ICIs and radiotherapy for refractory pancreatic cancer were not as promising as anticipated. At the data cutoff, no response was observed in 26 patients treated with ipilimumab, nivolumab, tocilizumab (an anti-IL-6 receptor monoclonal antibody), and SBRT. Five patients (19%; 95%CI, 7–39) demonstrated stability. The median overall survival was 5.3 months (95%CI 2.3–8.0). Furthermore, 19 patients (73%) experienced treatment-related adverse events. This outcome may be associated with the intricate role of IL-6 in the specific tumor microenvironment of pancreatic cancer [97].
In general, the efficacy of ICIs in combination with radiotherapy or chemoradiotherapy met expectations and indeed enhanced the adaptive anti-tumor immune response. However, the effectiveness of incorporating other types of drugs into this combination strategy varies. The amalgamation of precise radiotherapy with more personalized and diversified immunotherapy regimens may represent one of the avenues to unlock the therapeutic potential of ICIs in the future.
Immune checkpoint drugs plus other treatments
Oncolytic viruses, capable of entering tumor cells and causing persistent killing without harming normal tissues, hold promise for selective anti-tumor effects and promoting anti-tumor immune responses, particularly when utilizing wild-type or gene-edited viruses. Pelareorep, an intravenously delivered oncolytic reovirus, has shown safety and efficacy in combination with chemotherapy for various malignant tumors [98, 99]. However, its application in pancreatic cancer patients has yet to yield positive clinical results. In a phase Ib study, pelareorep and pembrolizumab were added to chemotherapy. This combination led to encouraging efficacy, with three out of 10 patients achieving disease control, one patient experiencing a partial response, and two patients achieving stable disease, which persisted for 9 and 4 months, respectively [100].
In a noncontrolled, single-arm, open-label, phase 2 clinical trial, seven patients with first-line treatment-resistant mPDAC were given oncolytic parvovirus (H-1PV, ParvOryx). Two out of seven achieved PR and lived up to 326 and 555 days, respectively. In addition, ParvOryx was well-tolerated by the patients [101].
Evofosfamide, an investigational hypoxia-activated prodrug, holds promise in the treatment of pancreatic cancer. In a recent phase I dose-escalation study combining ipilimumab with evofosfamide in advanced pancreatic cancer patients, while no patients achieved a confirmed partial response, five out of seven patients exhibited stable disease [102]. The safety of this combination was demonstrated, and responders displayed increased proliferation of peripheral T cells and greater infiltration of intratumoral T cells into the hypoxic tumor microenvironment, providing a basis for further investigation of this combination therapy.
Moreover, immune checkpoint drugs combined with topical therapy, specifically electroporation, have been explored in the context of pancreatic cancer. Depletion of tumor stroma has the potential to enhance the killing of tumor cells by cytotoxic T lymphocytes, thus synergizing with immunotherapy [103, 104]. Irreversible electroporation (IRE), a surgical therapy that directly damages cancer tissue, has also been employed in patients with PDAC [105, 106]. The combination of IRE and anti-PD-1 therapy has demonstrated impressive safety and efficacy. In a phase Ib clinical trial involving 10 patients with stage 4, unresectable pancreatic cancer, two patients did not receive planned treatment and relapsed at 3 months. The remaining eight patients achieved a median PFS of 6.8 months (95% CI 3.5–10.0). The median OS reached 18 months (95% CI 9.2–26.8), with only one patient experiencing a nivolumab-related adverse event. The most common adverse reactions included pain, fatigue, diarrhea, nausea, and hypertension. Encouraged by these positive results, a phase II trial is currently underway [107].
While the combination of ICIs with oncolytic viruses, adjuvants, or electroporation has demonstrated varying clinical effects, it suggests that non-traditional treatment methods may enhance anti-tumor effects within the context of immunotherapy. Research into these effective non-traditional treatments represents a critical aspect of the battle against pancreatic cancer, and further clinical trials are essential to fully realize their potential.
In summary, mounting evidence suggests that monotherapy with anti-PD-1/L1, anti-CTLA-4, and CD40 agonists has limited clinical efficacy in pancreatic cancer patients. Combination strategies involving immune checkpoint blockades and therapies or drugs targeting various mechanisms, particularly radiotherapy and oncolytic viruses, have shown some improvements in outcomes, although the variability and magnitude of improvement remain limited. This may be attributed to the challenging-to-reverse tumor microenvironment of pancreatic cancer, which hinders the sustained action of drugs and anti-tumor immune cells. As a result, efforts are focused on identifying ways to modify the tumor microenvironment of pancreatic cancer, thereby enhancing anti-tumor activity. This includes investigating new mechanisms for inhibiting tumor development, discovering novel immune checkpoints to optimize immune cell anti-tumor functions, and developing additional combination treatment strategies.
Treatment options targeting tumors and blood vessels
Since the pioneering use of hybridoma technology to create highly specific monoclonal antibodies in 1975 [108], the monoclonal antibody industry has continued to expand and plays an indispensable role in immunology and tumor therapy. Despite their high specificity and pharmacological advantages, monoclonal antibodies face several challenges in their application, such as off-target effects, limitations related to antibody size, and the risk of immune responses. To address these issues, various approaches have been explored, including humanization technology, Fc region modification, the design of drug delivery platforms targeting tumor cells, and the development of bispecific antibodies, all of which show promise [109].
Tumor-associated antigens, typically overexpressed on the surface of tumor cells and under-expressed or absent in normal cells, play crucial roles in tumor development, migration, and intercellular communication [110, 111]. Targeting these antigens can directly inhibit or kill tumors through various mechanisms. The following sections describe four types of monoclonal antibodies targeting tumors and blood vessels currently under investigation in pancreatic cancer clinical research (Fig. 1). Relevant drug information and clinical trials are also listed in Table 1.
Blocking cell growth factor signaling
Epidermal growth factor receptor (EGFR) inhibitor plus gemcitabine
The combination of erlotinib (an oral EGFR inhibitor) and panitumumab (a fully human monoclonal antibody inhibiting EGFR-expressing tumors) with gemcitabine in patients with advanced pancreatic cancer demonstrated improved median OS (8.3 months versus 4.2 months; HR, 0.817; 95% CI, 0.530–1.260; p = 0.1792) and PFS (3.6 months versus 2.0 months; HR, 0.843; 95% CI, 0.555–1.280; p = 0.4190). However, this improved efficacy was accompanied by increased toxicity, with patients in the experimental group experiencing a higher frequency of grade 3 and higher non-hematologic toxicities (82.6% vs. 52.2%; p = 0.0018) [112]. These findings do not support this regimen as a superior treatment option.
EGFR inhibitor plus gemcitabine plus radiotherapy
In a prospective phase II study, patients with locally advanced pancreatic cancer (LAPC) received maintenance therapy with gemcitabine or gemcitabine plus cetuximab, a monoclonal antibody targeting EGFR, following a combination therapy consisting of cetuximab, gemcitabine, and intensity-modulated radiation therapy (IMRT). The results indicated that the addition of cetuximab did not improve the survival benefit of patients after chemoradiotherapy, with a 13-month median OS showing no clear advantage compared to historical data [113].
EGFR plus HER2 inhibitor plus gemcitabine
In a phase II multicenter study, first-line therapy in advanced pancreatic cancer patients involved gemcitabine, trastuzumab (a monoclonal antibody targeting the HER2 receptor), and erlotinib. The study observed partial tumor responses in 19% of patients, disease stabilization in 56%, and a DCR of 74.6% (95% CI: 61.8–85.0; 44/59 patients). The median PFS was 3.5 months (95% CI: 2.4–3.8), and the median OS was 7.9 months (95% CI: 5.1–10.2) [114]. However, this combination did not demonstrate clear superiority over standard therapy.
Inducing apoptosis
DR5 agonist plus gemcitabine
Death receptor 5 (DR5), a cell surface receptor of the TNF-receptor superfamily, mediates apoptosis. Conatumumab, which binds to and activates DR5, can induce tumor cell apoptosis. In a randomized, placebo-controlled phase II study in patients with metastatic pancreatic cancer, the combination of conatumumab and gemcitabine resulted in a 6-month survival rate of 59% (42–73) compared to 50% (33–64) in the gemcitabine arm, with neutropenia being the most common grade ≥ 3 adverse event [115]. Additionally, a combination of tigatuzumab (a humanized monoclonal antibody activating DR5) and gemcitabine demonstrated clinical activity in a phase II trial involving patients with advanced unresectable pancreatic cancer. The study reported a PFS of 52.5% (95% CI, 39.3–64.1%) at 4 months, with the most common adverse events being nausea (35.5%) and fatigue (32.3%) [116]. Both studies support further research into drugs with apoptosis-inducing mechanism.
Anti-mesothelin
Mesothelin, an antigen differentially expressed on the surface of tumors and normal cells, is a potentially effective target for pancreatic cancer. LMB-100, a conjugate of a mesothelin antibody and pseudomonas toxin A, demonstrated limited effectiveness in a phase I study involving patients with advanced solid tumors [117]. Notably, its effectiveness in pancreatic cancer patients was suboptimal.
Another multicenter study involving anetumab ravtansine, another antibody conjugate targeting mesothelin, enrolled 148 patients in phase II with various types of solid tumors. None of the patients with pancreatic cancer achieved stable disease, partial response, or complete response [118]. These results may further underscore that antibody–drug conjugates (ADCs) targeting mesothelin are unlikely to revolutionize advanced pancreatic cancer therapy.
Targeting angiogenesis-VEGF inhibitor plus chemotherapy regimens
Bevacizumab, a VEGF inhibitor, showed tolerability and clinical activity when combined with gemcitabine in patients with pancreatic cancer [119]. Additionally, heavily pretreated patients with advanced solid tumors demonstrated good tolerability and safety when treated with AG (albumin-bound paclitaxel and gemcitabine) plus bevacizumab. Among the 15 patients with pancreatic cancer, one achieved partial response with a 57% reduction in tumor size, and 10 (67%) had stable disease [120].
A phase I/II multicenter single-arm study of FABLOx (Metronomic 5-Fluorouracil Plus nab-Paclitaxel, Bevacizumab, Leucovorin, and Oxaliplatin) in patients with metastatic pancreatic cancer revealed the regimen's tolerability. The most common grade ≥ 3 adverse events were abdominal pain and fatigue. The ORR was 33%, with median PFS and OS of 5.6 (95% CI, 1.7–11.3) and 9.9 (95% CI, 4.4–13.2) months, respectively [121]. However, the phase II data alone are insufficient to draw positive conclusions, but the confirmation of safety in these trials has paved the way for subsequent clinical investigations.
In summary, monoclonal antibodies targeting cell growth signals have not shown superior efficacy in pancreatic cancer, even when combined with systemic chemotherapy. The potential of apoptosis-inducing strategies in combination with chemotherapy warrants further investigation, but the outlook may be uncertain at present. The effectiveness of targeting stroma and blood vessels in combination with chemotherapy remains unclear.
Tumor vaccine
Tumor vaccines constitute a vital component of contemporary tumor immunotherapy, leveraging tumor cells or tumor antigen components to stimulate specific immune and humoral responses within the patient's immune system. This process enhances the body's ability to detect and combat tumors. Tumor vaccines encompass whole-cell vaccines, dendritic cell (DC) vaccines, peptide-based vaccines, and nucleic acid (DNA and mRNA) vaccines (Fig. 1). These vaccines differ in their mechanisms, and their combination therapies yield varying outcomes in clinical trials (Table 1).
Whole-cell vaccines
Whole-cell vaccines involve the use of irradiated or otherwise treated self or allogeneic tumor cells, rendering them incapable of proliferation while retaining their immunogenicity. These vaccines, as a whole, can elicit anti-tumor immune responses within the body. Whole-cell vaccines offer multiple potential antigens, reducing the likelihood of antigen loss and enhancing immune responses to varying degrees.
Combination of whole-cell vaccine and chemotherapy: CRS-207 plus cyclophosphamide (Cy) plus GVAX
GVAX is a granulocyte–macrophage colony-stimulating factor (GM-CSF) gene-transfected tumor cell vaccine. It relies on two irradiated, GM-CSF-secreting allogeneic PDA cell lines. In the context of the GVAX pancreatic vaccine, low-dose cyclophosphamide was added as a neoadjuvant chemotherapy regimen, offering superior benefits over GVAX alone. Notably, the regimen induced tertiary lymphoid structures and anti-tumor immunological changes, such as Treg depletion, in most subsequent specimens. This suggests that vaccination can alter the "non-immunogenic" characteristics of pancreatic cancer by enhancing immune-infiltrating cells in the TME. However, the median survival of 4.3 months fell short of expectations [122, 123]. Nevertheless, given the observed immunological changes, the inclusion of CY when using GVAX appears to be a consensus.
Le DT and colleagues invested significant efforts in studying the combined therapeutic approach of GVAX and CRS-207, a tumor vaccine utilizing Listeria bacteria to activate the immune response to mesothelin. Their findings indicated that heterologous prime/boost with Cy/GVAX and CRS-207 as third-line therapy significantly improved OS in metastatic pancreatic cancer patients compared to Cy plus GVAX (6.1 months vs. 3.9 months; HR, 0.59; p = 0.02) in a phase II study [124]. Subsequently, after demonstrating the regimen's tolerability, the authors conducted a controlled experiment comparing this regimen to conventional chemotherapy. The results suggested that, in comparison with conventional chemotherapy, treatment with Cy/GVAX plus CRS-207 or CRS-207 monotherapy did not significantly improve survival. The data showed median OS values of 3.7 (95% CI 2.9–5.3), 5.4 (95% CI 4.2–6.4), and 4.6 (95% CI 4.2–5.7) months and PFS of approximately 2.2 months for all arms, including the group receiving chemotherapy [125].
Additionally, based on these two studies, the authors performed immunological analyses of patients with prolonged survival using single-cell mass cytometry and identified two cell subsets associated with improved OS: CD8( +)CD45RO(-)CCR7(-)CD57( +) cells and CD14( +)CD33( +)CD85j( +) cells. The former subset was more abundant, and the latter less so [126].
Combination of whole-cell vaccine and immune checkpoint drugs
The combination of tumor vaccines and ICIs has shown promise in preclinical models. This combination, along with low-dose cyclophosphamide and PD-1 blockade, can inhibit the immunosuppressive function of Tregs and the PD-1/L1 axis, enhancing the CD8 + T cell responses induced by tumor vaccines [127, 128].
However, the role of immune checkpoint antibodies in combination with whole-cell vaccines remains controversial. In another study by Tsuji Kawa T et al., Cy/GVAX and CRS-207 were combined with nivolumab. Although the experiment maintained a good safety profile, there was no significant improvement in OS, DCR, and median PFS in the experimental group compared to the control group without nivolumab (median OS: 5.9 [95% CI, 4.7–8.6] vs. 6.1 [95% CI, 3.5–7.0] months, HR: 0.86 [95% CI, 0.55–1.34]; DCR: 13.7% vs. 9.5%; median PFS: approximately 2.2 months for both arms). Moreover, the 12-month and 18-month OS rates trended less impressively in the experimental group compared to the control group [129].
In contrast, the combination of the pancreatic cancer GVAX vaccine (with low-dose cyclophosphamide), nivolumab, and urelumab (an anti-CD137 agonist antibody that enhances T-cell immunity) as adjuvant or neoadjuvant therapy in patients with resectable pancreatic cancer has demonstrated potential efficacy. In comparison with the group of patients receiving only the GVAX vaccine, those administered the three-drug combination exhibited a significant increase in disease-free survival (33.51 months vs. 13.90 months, HR = 0.55, p = 0.242) and overall survival (35.55 months vs. 23.59 months, HR = 0.59, p = 0.377). Additionally, an augmentation in activated cytotoxic T cells within the tumor was observed. However, this promising trial had certain limitations, including a smaller enrollment in the three-drug combination group and a higher proportion receiving adjuvant (m) FOLFIRINOX [130].
It is worth noting that patients treated with GVAX and the CTLA-4 antagonist ipilimumab experienced significant changes in the TCR repertoire [129]. In addition, ipilimumab treatment led to the expansion of clones associated with longer survival. However, when a phase II study of GVAX combined with ipilimumab was conducted, this regimen failed to improve OS compared to the addition of ICIs (HR: 1.85, 95% CI: 1.03–3.33, p = 0.036) and was prematurely closed. Nonetheless, improvements in immune cell infiltration following treatment were observed in both studies. Therefore, the combination therapy of tumor vaccination and ICIs warrants further investigation [131].
In conclusion, whole-cell vaccines, whether as monotherapy or in combination with immunotherapies, have not demonstrated superior therapeutic outcomes compared to traditional regimens. However, given the consistently low toxicity of immunotherapy and the advanced stage of disease progression in the primary study population, future developments in therapeutic strategies based on whole-cell vaccines hold promise.
DC vaccine
DCs, known for their potent antigen-presenting abilities, serve as the crucial link between antigens and immune responses. DC-based anticancer vaccines employ native or patient-derived dendritic cells loaded with various forms of antigens in vitro to generate antigen-specific cytotoxic T cells. This approach results in the targeted destruction of tumor cells.
DC vaccine monotherapy
pMUC1-peptide pulsed dendritic cells (DCs):
Mucin 1 (MUC1), an extensively studied tumor-associated antigen (TAA), is aberrantly expressed in various malignancies [132,133,134]. In a phase I trial, autologous DCs pulsed with MUC-1 and subcutaneously administered to induce an immune response demonstrated safety in seven patients with advanced pancreatic cancer. Additionally, the vaccine induced anti-tumor immune responses against MUC-1[135].
Allogeneic lysate-dendritic cells (DCs):
In a phase I study involving a DC vaccine loaded with an allogeneic tumor cell lysate derived from multiple myeloma cell lines as adjuvant therapy, 10 pancreatic cancer patients who underwent surgical resection and received standard-of-care with no radiographic progression observed achieved an 80% 1-year disease-free survival rate. While the expected median OS and median PFS were not met, 70% of patients experienced no disease progression or recurrence during a median follow-up of 25 months [136].
DC vaccine plus chemotherapy plus immune cell therapy
Zoledronate-pulsed DCs (Zol-DCs) vaccine plus gemcitabine plus T cell therapy:
In addition to combination with chemotherapy, integrating other agents with distinct mechanisms enriches the therapeutic strategy for DC vaccines. Dendritic cells loaded with TAA were treated with zoledronate, a commonly used bisphosphonate in the clinic. This DC vaccine is known as the Zol-DCs vaccine and can promote the activation of Vγ9γδ T cells. A phase I/II study investigated a combination regimen of Zol-DCs with chemotherapy and intravenous infusion of αβT cells in 15 patients with locally advanced pancreatic cancer. Approximately 50% of patients achieved stable disease, with median PFS and median OS of 5.5 months and 12.0 months, respectively. Although these results were comparable to OS inpatients receiving standard gemcitabine-based chemoradiotherapy, the regimen demonstrated a 30.8% 2-year survival rate, outperforming patients who received chemoradiotherapy alone. Notably, improved survival correlated with a pre-treatment neutrophil/lymphocyte ratio (NLR) less than 5.0 and increased CD8 + /Treg ratio in post-treatment SD patients, suggesting their potential as biomarkers for Zol-DC immune combination therapy [137].
DC vaccine alone or DC vaccine plus lymphokine-activated killer [LAK] cell plus chemotherapy (gemcitabine with or without S-1):
In a retrospective study involving 49 patients with inoperable pancreatic carcinoma refractory to standard treatment, a combination therapy of DC vaccine alone or DC vaccine plus LAK cell and chemotherapy (gemcitabine + /S-1) resulted in 2 patients achieving complete response, five achieving partial response, and 10 achieving stable disease. The cohort exhibited a median survival of 360 days, suggesting potential for improving the prognosis of advanced pancreatic cancer patients. The addition of LAK cells enhanced treatment efficacy. Immunological analysis revealed that the reduction of regulatory T cells was closely associated with improved prognosis rather than an increase in tumor antigen-specific T cells [138]. These findings support the potential of combining cell therapy and chemotherapy with DC vaccines as a treatment option for pancreatic cancer patients.
DC vaccine plus adjuvant
Toll-like receptor (TLR)-3 agonist poly-ICLC plus DC vaccine (three distinct A2-restricted peptides):
Capitalizing on enhanced T cell responses observed at the cellular level when the TLR3 agonist poly-ICLC was combined with DCs, Mehrotra S et al. developed poly-ICLC as an adjuvant in combination with DC vaccine for 12 patients with metastatic (nine) or locally advanced unresectable (three) pancreatic cancer. Eight patients were assessed as having stable disease and progressive disease at day 56. The experimental median OS was 7.7 months; a notable improvement compared to the 4.2 to 4.9 months median OS achieved with second-line chemotherapy regimens for metastatic pancreatic cancer patients. This regimen was well-tolerated, with fatigue and self-limiting flu-like symptoms as the most common side effects. Although the experiment lacked data on immune changes in the tumor microenvironment post-treatment, the regimen exhibited superiority over existing standard treatment regimens [139].
Personalized neoantigen peptides (PEP-DC) plus nivolumab plus SOC chemotherapy plus aspirin
In a phase Ib trial, a novel proteo-genomic antigen discovery pipeline was designed for neoantigen prediction and selection. The trial combined nivolumab with chemotherapy in a treatment approach based on tumor vaccines. Aspirin was added to suppress immunosuppressive cells. CD4 + T cell responses against PEP candidates were detected in all three donors, although CD8 + T cell responses were not observed. This protocol highlights the feasibility of neoantigen vaccine production [140], offering innovative prospects for future vaccine treatment strategies.
While DC vaccine monotherapy has exhibited safety in earlier studies [135], its clinical benefits for pancreatic cancer have been modest. Conversely, combining DC vaccines with chemotherapy shows promise, and the inclusion of toll-like receptor (TLR)-3 agonists demonstrates favorable efficacy. The exploration of neoantigens in follow-up studies holds great potential for future vaccine treatment strategies.
Peptide vaccines
Peptide vaccines, compared to other vaccine types, offer the advantage of lower toxicity and simplified synthesis. However, this ease of use comes with the potential for reduced immunogenicity. Hence, the active use of adjuvants or immunomodulators becomes crucial to elicit robust responses. Despite extensive clinical trials, peptide vaccines derived from sources such as telomerase have not demonstrated a significant clinical efficacy when compared to standard chemotherapy. Therefore, the potential of neoantigen vaccines holds promise for future developments.
Peptide vaccines monotherapy
Personalized peptide vaccination (PPV):
A retrospective study conducted in 2021 explored iNeo-Vac-P01, a personalized neoantigen-based peptide vaccine, in seven advanced pancreatic cancer patients with low tumor mutation burden (TMB). Concurrent administration of other treatments, including ICIs, was permitted. The vaccine was well-tolerated, with no significant vaccine-related adverse immune reactions reported. Patients exhibited a mean OS of 24.1 months, vaccine-related OS of 8.3 months, and PFS of 3.1 months. The DCR reached 85.71%, and the 1-year survival rate approached 50%. A substantial increase in antigen-specific TCR clones was observed in one patient who achieved long-term survival, suggesting iNeo-Vac-P01's potential to activate specific T cell subsets against tumor cells [141]. In summary, personalized peptide vaccines offer considerable clinical benefits, with immunological changes providing valuable insight for future research in this innovative strategy.
Peptide vaccines plus chemotherapy
Peptides WT1-pulsed dendritic cell vaccine combined with chemotherapy.
An early phase I study investigated the use of DCs pulsed with Wilms' tumor 1 (WT1)-specific peptides (DC/WT1-I, II, or I/II) in combination with gemcitabine for seven PDA patients. One patient achieved partial response, and the remaining six demonstrated stable disease. Notably, delayed-type hypersensitivity (DTH) responses specific to WT1 peptides were detected in four of the seven patients. Patients with a robust DTH response to WT1 peptide exhibited improved survival. The regimen also maintained a favorable safety profile, with grade 1 skin reactions at the vaccination site, mirroring those reported in previous gemcitabine trials. Additionally, vaccination promoted the long-term maintenance of WT1-specific memory CD8 + T cells [142, 143].
Yanagisawa R et al. conducted a phase I trial involving patients with surgically resected pancreatic cancer. The patient was administered a three-drug combination of antigen-pulsed DCs loaded with WT1 peptides (highly expressed in pancreatic cancer), S-1, and OK-432 (an adjuvant for the WT1-DC vaccine). No significant adverse effects were reported, and seven out of eight patients with surgically resected pancreatic cancer exhibited durable WT1-specific CTL immune responses. A 2-year OS rate of approximately 62.5% suggests its potential as a treatment option [144]. The regimen's superiority, stemming from these results, indicates its therapeutic potential, especially when combined with chemotherapy.
SVN-2B plus gemcitabine and/or tegafur/gimeracil/oteracil plus IFN beta(ADJUVANT).
A study involving 29 patients with metastatic pancreatic cancer, who had previously failed gemcitabine-based therapy, investigated KIF20A-66, an HLA-A24-restricted peptide vaccine derived from KIF20A. Among the patients who completed at least one course of treatment, 21 achieved stable disease, while eight experienced progressive disease [145]. This outcome underscores the potential activity of the vaccine.
Similarly, SVN-2B, another HLA-A24-restricted peptide vaccine with IFN-β as an adjuvant, was evaluated in 83 HLA-A24-positive pancreatic cancer patients. These patients were divided into three groups: SVN-2B plus IFN-β, SVN-2B alone, and a placebo group. Although differences in PFS and DCR were evident among the three groups, the variance in OS was less pronounced (102 days, 96.5 days, and 111 days, respectively; p = 0.4565). However, an increase in surviving 2B-specific CTLs was observed in the SVN‐2B plus IFN-β group, suggesting potential for long-term survival improvement [146].
OCV-C01 plus gemcitabine:
OCV-C01, comprising epitope peptides from KIF20A, VEGFR1, and VEGFR2, combined with gemcitabine resulted in a higher median DFS of 15.8 months in 30 resected pancreatic cancer patients (95% CI, 11.1–20.6), compared to gemcitabine alone (DFS 12.0 months). Furthermore, the DFS rate at 18 months reached 34.6% (95% CI, 18.3–51.6), with median OS not reached. The OS rate at 18 months was 69.0% (95% CI, 48.8–82.5). The combination was well-tolerated, with no significant differences in adverse effects between gemcitabine plus OCV-C01 and gemcitabine alone (p = 0.504). Nonetheless, this non-randomized trial and the low expression rate of KIF20A suggest the need for a larger sample size for more conclusive results [147].
Different combination regimens of peptide vaccines and chemotherapy exhibit varying efficacy among groups of pancreatic cancer patients. Overall, they demonstrate reliable safety profiles but fall short of significantly improving OS. These findings underscore the need to address performance limitations despite their favorable safety profiles.
nucleic acid vaccine
DNA vaccine plus chemotherapy
Algenpantucel-L plus neoadjuvant SOC chemotherapy (FOLFIRINOX or gemcitabine/nab-paclitaxel):
Algenpantucel-L (AL) is an allogeneic pancreatic cancer vaccine designed based on the concept of hyperacute rejection. It consists of two human pancreatic ductal adenocarcinoma cell lines genetically engineered to express αGal through retroviral transfer using the murine αGT gene [148]. A recent phase III study aimed to assess the potential of AL as a neoadjuvant chemotherapy regimen to enhance patient outcomes. In this study, 303 patients with borderline resectable or locally advanced PDAC were divided into two groups. Group A received neoadjuvant standard-of-care chemotherapy (FOLFIRINOX or gemcitabine/nab-paclitaxel), while group B received the same standard neoadjuvant regimen with the addition of HAPa immunotherapy. The results indicated that there was no significant difference in median OS (14.9 months vs. 14.3 months; HR: 1.02, 95% CI 0.66–1.58; p = 0.98) and median PFS (13.4 months vs. 12.4 months; HR 1.33, 95% CI 0.72–1.78; p = 0.59) between the two groups [149]. It is evident that the incorporation of AL did not result in improvement.
DNA vaccine plus IL-12
Telomerase plays a pivotal role in tumor cell proliferation. The telomerase complex is essential for maintaining telomere length at chromosome ends during DNA replication. Human telomerase reverse transcriptase (hTERT), a catalyst within the telomerase complex, is highly expressed in tumors and promotes tumor growth by facilitating cell proliferation, epithelial-to-mesenchymal transition, and other pathways. Consequently, hTERT becomes a valuable anti-tumor target.
In a phase I trial evaluating the safety of DNA vaccines targeting hTERT in solid tumor patients, 34 pancreatic cancer patients who exhibited no evidence of disease (NED) after front-line therapy were enrolled in two groups. These groups received modified plasmid DNA encoding hTERT variants (INO-1400/INO-1401) alone or in combination with an interleukin 12 plasmid (INO-9012). Both groups displayed excellent tolerance. In terms of clinical activity, the median DFS was 9 months, with 41.4% of patients remaining disease-free at 18 months. Additionally, the production of hTERT-specific CD4 + and CD8 + T cells was observed in the remaining patients, which correlated with survival benefits [150].
RNA vaccine plus anti-PD-1 plus chemotherapy
RNA vaccines have garnered significant anticipation in recent years due to their relatively simple production process, high efficiency, and accuracy. A recent phase I clinical trial illuminated the potential application of neoantigen RNA vaccines in treating patients with surgically resected pancreatic cancer. In this clinical trial, Luis A. Rojas et al. sequentially administered atezolizumab, autologous neoantigen-specific RNA vaccine, and mFOLFIRINOX to patients, observing favorable tolerability with only one out of 16 patients experiencing grade 3 AEs. Additionally, the vaccine demonstrated the ability to induce high-intensity polyclonal neoantigen-specific T cell responses in 50% of patients, resulting in a longer median recurrence-free survival compared to non-responders (non-responders: 13.4 months vs. responders: not reached) at an 18-month median follow-up. This exciting outcome is evidently contingent on the presence of tumors [151]. The safety and effectiveness of this vaccine in pancreatic cancer patients await validation through larger-scale clinical trials. Nevertheless, this clinical trial underscores the feasibility of an mRNA-based neoantigen vaccine for treating pancreatic cancer [152].
Most of the vaccines evaluated in current clinical experiments have demonstrated good safety and tolerance. Relevant clinical data have shown certain immunological changes and clinical activity, resulting in varying degrees of disease remission. These effects are primarily reflected in the proportion of patients achieving stable disease and improvements in PFS. However, the impact on OS remains limited. To provide more reliable treatment options, it may be necessary to explore more diverse and precise directions.
Adoptive cell transfer therapy
Adoptive cell therapy harnesses the body's own cells to enhance cellular resistance and eliminate rejection by re-engineering them. T cells, the cornerstone of immunotherapy, are extracted from the patient's body and genetically modified to express chimeric antigen receptors (CARs), enabling them to mount a more potent and sustained response. These engineered T cells circulate within the body, delivering precise and efficient anti-tumor capabilities [153] (Fig. 1). While adoptive cell therapy has demonstrated remarkable clinical benefits in hematological malignancies [154], its effectiveness in solid tumors, with their complex tumor microenvironments, presents a challenge in predicting outcomes.
CD133, a transmembrane protein highly expressed in various solid tumors, including pancreatic cancer, emerges as a potential immunotherapy target for patients with advanced CD133-positive tumors [155]. Wang Y et al. investigated the efficacy of CD133-directed CAR-T cells in patients with advanced metastatic malignancies. Results from 23 patients, including 14 with hepatocellular carcinomas and seven with pancreatic carcinomas, showed that three achieved partial remission, and 14 achieved stable disease. The median PFS was 5 months, with the main observed toxicity being a decrease in hemoglobin/platelet counts [156]. Given the immunosuppressive nature of intrahepatic and pancreatic cancers, this regimen holds promise for pancreatic cancer patients.
Mesothelin, highly expressed in pancreatic cancer cells, represents a target for multiple immunotherapy approaches. In one phase I trial, engineered T cells were designed to transiently express a CAR specific for mesothelin. These cells were administered to six patients with chemotherapy-refractory metastatic PDAC. Of these patients, two achieved stable disease, with PFS ranging from 3.8 to 5.4 months. Monitoring of metabolic active volume (MAV) in individual tumor lesions revealed stability in three patients and a 69.2% decrease in MAV in one patient with confirmed mesothelin expression [157].
The authors also employed a lentiviral CAR-expression system to stably express CART-meso in T cells. This phase I trial included patients with malignant pleural mesothelioma (MPM), ovarian adenocarcinoma (OVCA), and PDAC. Results indicated that 11 of 15 patients achieved stable disease, with peak CART-meso cell numbers detected in peripheral blood at 6–14 days, although they persisted only transiently. Pre-treatment with cyclophosphamide enhanced CART-meso expansion but did not prolong persistence beyond 28 days. Additionally, CART-meso DNA was detected in 70% (seven of 10) tumor biopsies, and human anti-chimeric antibodies (HACA) were detected in the blood of 57.1% (eight of 14) patients. The limited clinical activity may be attributed to low mesothelin expression in the patient population. Overall, CART-meso cells demonstrated good tolerability and expansion in the blood of all patients but yielded limited clinical activity [158]. Furthermore, clinical trials evaluating mesothelin-specific CARs containing fully human scFv (NCT03054298 and NCT03323944) are ongoing to address concerns about murine scFv-induced immune clearance of CAR-T cells.
Human epidermal growth factor receptor 2 (HER2), a cell surface receptor highly expressed by tumor cells, has become a target for CAR-T cell therapy. In a phase I trial conducted by Feng KC et al., HER2-targeting CAR-T cells were administered to patients with advanced unresectable pancreatic cancer following pre-treatment with nab-paclitaxel and cyclophosphamide. The results demonstrated that two pancreatic cancer patients achieved stable disease, with PFS durations of 5.3 and 8.3 months, respectively. Notably, this data compare favorably to an overall median PFS of 4.8 months (range, 1.5–8.3 months) in the same group, suggesting that HER2-targeted CAR-T therapy may benefit specific pancreatic cancer patient populations [159].
Similarly, in another phase I trial [160], 16 patients with EGFR-positive metastatic pancreatic cancer underwent cyclophosphamide preconditioning followed by CART-EGFR cell therapy. Fourteen evaluable patients achieved partial responses lasting 2–4 months, and eight patients experienced stable disease. The median OS for all 14 evaluable patients was 4.9 months, with a median PFS of 3 months from the start of treatment. Notably, the occurrence of grade ≥ 3 adverse events, such as fever/fatigue, nausea/vomiting, and mucous membrane/skin toxicity, significantly improved after treatment. Overall, while the results were not ideal, they were encouraging.
Similarly encouraging efficacy was observed in seven locally advanced and metastatic pancreatic cancer patients with adoptive anti-CD3 x anti-EGFR bispecific antibody armed activated T cells (BATs). In this phase I/II clinical trial, seven patients with PC survived more than a year, including one who was still alive at 54 months. The median OS was 31 months, and two patients notably achieved CR after restarting chemotherapy [161]. In conclusion, BATs infusion is considered safe and may improve survival in patients with pancreatic cancer by inducing an adaptive anti-tumor immune response.
Despite the promise of adoptive cell transfer therapy, limited clinical trials exist due to the high cost and time-consuming nature of this approach (Table 1). Industrial production of off-the-shelf, universal CAR-T cells for patient treatment holds the potential to make this therapy more accessible. Additionally, improving the efficacy of this therapy in solid tumors will require further exploration. For pancreatic cancer, the challenges of antigen selection and the immunosuppressive microenvironment persist. However, monotherapy regimens continue to demonstrate clinical activity. Furthermore, cell therapies utilizing other immune effector cells such as NK cells and macrophages as carriers for CARs have shown promise in preclinical and clinical experiments [162, 163].
Conclusion and discussion
It is evident that regardless of the chosen treatment modality, single-mechanism-based immunotherapy strategies face significant challenges in altering the grim prognosis of pancreatic cancer patients. Combining various immunotherapy approaches with conventional radiotherapy, chemotherapy, molecular targeted therapy, and other diverse treatment modalities has exhibited a more expansive realm of development and heightened therapeutic potential in clinical trials. This inclination is closely tied to the inherent attributes of PC itself.
The complex immunosuppressive microenvironment intrinsic to PC, compounded by the physical barriers erected by fibroproliferative stroma, and the distinctive immunological traits characterized by low TMB collectively exert a profound influence on pancreatic cancer's onset and progression. These factors impact multiple facets of the body's immune response against tumor cells. Strategies for future pancreatic cancer treatment must focus on mitigating or reversing these adversarial effects through comprehensive systemic treatment regimens.
Specifically, this involves deploying technical innovations such as constructing carrier systems to enhance drug delivery to tumor sites, breaching physical barriers, and conducting in-depth research into the complex pancreatic cancer tumor microenvironment to modify its immunosuppressive attributes. By doing so, we can unleash the full potential of anti-tumor immune cells like effector T cells, boosting their capacity to recognize and eliminate tumor cells.
Pancreatic cancer's low tumor mutational burden sets it apart from other solid tumors, resulting in fewer neoantigens, limited immune activation, and restricted options for targeting tumor cells. Additionally, tumor cell heterogeneity and the rarity of identical neoantigens across different individuals make it challenging to mount effective immunotherapies that target a single or a small number of neoantigens. These unique features and constraints make pancreatic cancer appear daunting to treat. However, a judicious combination of therapeutic strategies will undoubtedly enhance patient prognoses, paving the way for precise and personalized immunotherapy programs within the context of comprehensive treatment strategies.
The status and abundance of immune cells in the TME vary among different cancer types [164]. Considering the influence of the complex and unique tumor microenvironment of PDAC on immunotherapy and the diverse responses of different populations to tumor cells, further research on neoantigen vaccines is crucial to achieve personalized precision treatment strategies. Deepening our understanding of the correlation between different components in the TME of pancreatic cancer is essential. Adopting appropriate treatment plans tailored to patients with distinct TME characteristics or immune profiles is integral to personalized immunotherapy against pancreatic cancer.
Data from single-cell RNA sequencing revealed that, despite heterogeneity, immunosuppressive myeloid and macrophage populations predominate in the PDAC TME, with prevalent expression of immune checkpoints on dysfunctional T cells and NK cells [165]. Within the TME, the interactions of immune cells with other components such as the cellular matrix are complex. H. Sadozai et al. analyzed the composition of pancreatic cancer stroma and infiltrating immune cells, finding that immune cells may influence the PFS of patients with surgical resection of pancreatic cancer by altering the composition of pancreatic stroma. Levels of CD3 and CD206 can serve as prognostic indicators of PFS [166]. Another study also identified that long-term survivors (LTS) of PDAC exhibited higher infiltration levels of immune cells, particularly T cells, and positivity for TLS in the TME. Stromal iNOS cells and CD68 cells were considered to have greater prognostic value [167].
M. Wartenberg et al. integrated data on immune cell background and histological characteristics of patients with pancreatic cancer, identifying three subtypes with different immunological characteristics and prognosis: "immune escape," “immune enrichment,” and "immune exhausted." Approximately 35% of patients belong to the "immune enrichment” subtype, characterized by T cell enrichment, lower levels of FOXP3 + Tregs, and mutations in CDKN2A and PIK3CA, indicating a poorer prognosis [168].
Similarly, an analysis based on transcriptomic signatures of immune infiltration categorized PDAC into adaptive, innate, and immune-exclusion subtypes. Innate immune subtypes associated with poorer survival exhibited an enrichment of NK cells and neutrophils and an exclusion of other tumor-infiltrated lymphocytes. This suggests that therapies targeting NK cells and neutrophils may yield better therapeutic outcomes in these patients. The “T cell dominant” subtype, being the most immunogenic, demonstrated enrichment of adaptive immune-associated subsets, making it more suitable for immune checkpoint-related therapies. The “tumor dominant" subtype, characterized by low immune cell infiltration in the TME, indicates prominent metabolic adaptation [169].
The crosstalk between different components of the TME and immune cells is intricate. Identifying immunosensitive or drug-resistant patients through the analysis of tumor interstitial and immune backgrounds is crucial for predicting treatment responses [170]. Simultaneously, for patients who are not candidates for surgical removal, this approach is challenging but equally indispensable. In any case, promoting the infiltration of local anti-tumor immune cells and reversing the adverse effects of the tumor microenvironment on these cells are imperative to overcome the heterogeneous TME of pancreatic cancer.
Presently, several studies have identified immunosuppressive factors in pancreatic cancer that originate with gene mutations and intensify as tumors progress, ultimately culminating in the establishment of advanced local immune privilege. Preventing and detecting tumors early have always been paramount, particularly for diseases like pancreatic cancer that lack early symptoms and effective screening methods. Therefore, devising effective early screening strategies and corresponding interventions is of paramount importance. Furthermore, stratifying pancreatic cancer patients based on distinct genetic, pathological, and other attributes to tailor personalized treatments is another avenue worthy of exploration. Researchers have categorized patients into immune escape phenotypes, enrichment phenotypes, and exhaustion phenotypes based on molecular characteristics and prognosis, underscoring the inaccuracy of a one-size-fits-all treatment approach. Swift patient stratification via reliable early stage biomarkers and the selection of suitable individualized combination therapies may constitute a reliable future treatment option, albeit a challenging one.
In conjunction with several preclinical studies, we have observed consistent efficacy of oncolytic viruses against pancreatic cancer [171]. Additionally, targeting immune activation markers such as OX40 [172], CD40 [173], and 4-1BB [174], in combination with immunotherapy, consistently demonstrates potential therapeutic value. Moreover, an anticipated research direction involves enhancing the impact of immunotherapy by altering the pancreatic cancer TME with low immune invasion through matrix destruction or targeted interventions [175,176,177]. TLS, identified not only as a prognostic indicator of immunotherapy but also as inducible by immunotherapy under specific conditions, becomes an immune element in the anti-tumor immune response within tumors [178]. Given that the generation of TLS is invariably associated with the presence of high endothelial venules, the continual generation and maintenance of TLS at the tumor site can overcome the solid stromal barriers of pancreatic cancer, facilitating extensive immune cell infiltration and sustained anti-tumor immune responses.
Recent studies have illuminated the influence of the gut microbiome and its metabolites on the immune system in patients with pancreatic cancer [179]. Specifically, sachharopolyspora, pseudoxanthomonas, and streptomyces are significantly enriched in patients with long-term survival (LTS), promoting the recruitment and activation of T cells at the tumor site [180]. Beyond influencing the adaptive immune response, the gut microbiota can contribute to the progression of PDAC by regulating the innate immune system, particularly by inhibiting the infiltration and activation of NK cells [181]. A more profound understanding of the crosstalk between distinct intestinal environments and PDAC immune microenvironments may constitute a means to achieve personalized immunotherapy.
Simultaneously, the limitations of mouse models in pancreatic cancer research have become increasingly apparent. This underscores the imperative for more refined pancreatic cancer research models, emphasizing the disparities in experimental outcomes stemming from differences between human and mouse models. Exploring these distinctions holds the potential to deepen our comprehension of tumor immune mechanisms in pancreatic cancer.
Current clinical treatment and scientific investigation of pancreatic cancer face substantial hurdles. Overcoming these obstacles necessitates numerous endeavors and collaborations between researchers and clinicians. The joint dedication and cooperation of these stakeholders are indispensable in this endeavor.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2020) GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-a Cancer J Clin 71(2021):209–249. https://doi.org/10.3322/caac.21660
Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Chone L, Francois E, Artru P, Biagi JJ, Lecomte T, Assenat E, Faroux R, Ychou M, Volet J, Sauvanet A, Breysacher G, Di Fiore F, Cripps C, Kavan P, Texereau P, Bouhier-Leporrier K, Khemissa-Akouz F, Legoux JL, Juzyna B, Gourgou S, O’Callaghan CJ, Jouffroy-Zeller C, Rat P, Malka D, Castan F, Bachet JB, G. Canadian Canc Trials, G.I.P.G. Unicanc (2018) FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. New Engl J Med 379:2395–2406. https://doi.org/10.1056/NEJMoa1809775
Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, Bennouna J, Bachet JB, Khemissa-Akouz F, Pere-Verge D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M, U. Grp Tumeurs Digestives, P. Intergrp (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. New Engl J Med 364:1817–1825. https://doi.org/10.1056/NEJMoa1011923
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369:1691–1703.
Sinn M, Bahra M, Liersch T, Gellert K, Messmann H, Bechstein W, Waldschmidt D, Jacobasch L, Wilhelm M, Rau BM, Grützmann R, Weinmann A, Maschmeyer G, Pelzer U, Stieler JM, Striefler JK, Ghadimi M, Bischoff S, Dörken B, Oettle H, Riess H (2017) CONKO-005: Adjuvant chemotherapy with gemcitabine plus erlotinib versus gemcitabine alone in patients after r0 resection of pancreatic cancer: a multicenter randomized phase III trial. J Clin Oncol Official J Am Soc Clin Oncol 35:3330–3337.
Carreau NA, Pavlick AC (2019) Nivolumab and ipilimumab: immunotherapy for treatment of malignant melanoma. Future Oncol (London, England) 15:349–358.
Zhang Q, Vignali DAA (2016) Co-stimulatory and co-inhibitory pathways in autoimmunity. Immunity 44:1034–1051.
Chen L, Flies DB (2013) Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol 13:227–242.
Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH (2006) Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med 203:883–895
Keir ME, Butte MJ, Freeman GJ, Sharpe AH (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26:677–704.
Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, Azuma M, Krummel MF, Bluestone JA (2009) Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol 10:1185–1192.
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–264.
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L (2002) Tumor-associated B7–H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8:793–800
Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N (2002) Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 99:12293–12297
Wei SC, Duffy CR, Allison JP (2018) Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 8:1069–1086.
Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng S-L, Shen X, Boyd Z, Hegde PS, Chen DS, Vogelzang NJ (2014) MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515:558–562. https://doi.org/10.1038/nature13904
Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ (2001) PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2:261–268
Djureinovic D, Wang M, Kluger HM (2021) Agonistic CD40 antibodies in cancer treatment Cancers 13. https://doi.org/10.3390/cancers13061302
Vonderheide RH (2020) CD40 agonist antibodies in cancer immunotherapy. In: Klotman ME (ed) Annual review of medicine 71:47–58
Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun WJ, Huhn RD, Song WR, Li DG, Sharp LL, Torigian DA, O’Dwyer PJ, Vonderheide RH (2011) CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331:1612–1616. https://doi.org/10.1126/science.1198443
Herbst RS, Arkenau H-T, Santana-Davila R, Calvo E, Paz-Ares L, Cassier PA, Bendell J, Penel N, Krebs MG, Martin-Liberal J, Isambert N, Soriano A, Wermke M, Cultrera J, Gao L, Widau RC, Mi G, Jin J, Ferry D, Fuchs CS, Petrylak DP, Chau I (2019) Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): a multicohort, non-randomised, open-label, phase 1a/b trial. Lancet Oncol 20:1109–1123. https://doi.org/10.1016/S1470-2045(19)30458-9
Topalian SL, Drake CG, Pardoll DM (2015) Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27:450–461. https://doi.org/10.1016/j.ccell.2015.03.001
Sharpe AH, Pauken KE (2018) The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol 18:153–167. https://doi.org/10.1038/nri.2017.108
Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, Weber JS, Joshua AM, Hwu W-J, Gangadhar TC, Patnaik A, Dronca R, Zarour H, Joseph RW, Boasberg P, Chmielowski B, Mateus C, Postow MA, Gergich K, Elassaiss-Schaap J, Li XN, Iannone R, Ebbinghaus SW, Kang SP, Daud A (2014) Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet (London, England) 384:1109–1117. https://doi.org/10.1016/S0140-6736(14)60958-2
Patnaik A, Kang SP, Rasco D, Papadopoulos KP, Elassaiss-Schaap J, Beeram M, Drengler R, Chen C, Smith L, Espino G, Gergich K, Delgado L, Daud A, Lindia JA, Li XN, Pierce RH, Yearley JH, Wu D, Laterza O, Lehnert M, Iannone R, Tolcher AW (2015) Phase I study of pembrolizumab (MK-3475; Anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res 21:4286–4293. https://doi.org/10.1158/1078-0432.CCR-14-2607
Brahmer JR, Tykodi SS, Chow LQM, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen SM, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366:2455–2465. https://doi.org/10.1056/NEJMoa1200694
Mizugaki H, Yamamoto N, Murakami H, Kenmotsu H, Fujiwara Y, Ishida Y, Kawakami T, Takahashi T (2016) Phase I dose-finding study of monotherapy with atezolizumab, an engineered immunoglobulin monoclonal antibody targeting PD-L1, Japanese patients with advanced solid tumors. Invest New Drugs 34:596–603. https://doi.org/10.1007/s10637-016-0371-6
Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, Rosenberg SA (2010) Phase 2 trial of single agent ipilimumab (Anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother 33:828–833. https://doi.org/10.1097/CJI.0b013e3181eec14c
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hogg D, Hill A, Marquez-Rodas I, Haanen J, Guidoboni M, Maio M, Schoffski P, Carlino MS, Lebbe C, McArthur G, Ascierto PA, Daniels GA, Long GV, Bastholt L, Rizzo JI, Balogh A, Moshyk A, Hodi FS, Wolchok JD (2019) Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 381:1535–1546. https://doi.org/10.1056/NEJMoa1910836
Motzer R, Rini BI, McDermott DF, Frontera OA, Hammers HJ, Carducci MA, Salman P, Escudier B, Beuselinck B, Amin A, Porta C, George S, Neiman V, Bracarda S, Tykodi SS, Barthelemy P, Leibowitz-Amit R, Plimack ER, Oosting SF, Redman B, Melichar B, Powles T, Nathan P, Oudard S, Pook D, Choueiri TK, Donskov F, Grimm MO, Gurney H, Heng DYC, Kollmannsberger CK, Harrison MR, Tamita Y, Duran I, Grunwold V, McHenry MB, Mekan S, Tannir NM, CheckMate I (2019) Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol 20:1370–1385. https://doi.org/10.1016/s1470-2045(19)30413-9
O’Reilly EM, Oh DY, Dhani N, Renouf DJ, Lee MA, Sun WJ, Fisher G, Hezel A, Chang SC, Vlahovic G, Takahashi O, Yang Y, Fitts D, Philip PA (2019) Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: a phase 2 randomized clinical trial. Jama Oncol 5:1431–1438. https://doi.org/10.1001/jamaoncol.2019.1588
Klein O, Kee D, Markman B, Michael M, Underhill C, Carlino MS, Jackett L, Lum C, Scott C, Nagrial A, Behren A, So JY, Palmer J, Cebon J (2020) Immunotherapy of ipilimumab and nivolumab in patients with advanced neuroendocrine tumors: a subgroup analysis of the CA209-538 clinical trial for rare cancers. Clin Cancer Res 26:4454–4459. https://doi.org/10.1158/1078-0432.Ccr-20-0621
Blazer M, Wu C, Goldberg RM, Phillips G, Schmidt C, Muscarella P, Wuthrick E, Williams TM, Reardon J, Ellison EC, Bloomston M, Bekaii-Saab T (2015) Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol 22:1153–1159. https://doi.org/10.1245/s10434-014-4225-1
Nevala-Plagemann C, Hidalgo M, Garrido-Laguna I (2020) From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nat Rev Clin Oncol 17:108–123. https://doi.org/10.1038/s41571-019-0281-6
Ramanathan RK, McDonough S, Philip PA, Hingorani SR, Lacy J, Kortmansky JS, Thumar J, Chiorean EG, Shields AF, Behl D, Mehan PT, Gaur R, Seery T, Guthrie K, Hochster HS (2019) Phase IB/II randomized study of FOLFIRINOX plus pegylated recombinant human hyaluronidase versus FOLFIRINOX alone in patients with metastatic pancreatic adenocarcinoma: SWOG S1313. J Clin Oncol 37:1062. https://doi.org/10.1200/jco.18.01295
Springfeld C, Jäger D, Büchler MW, Strobel O, Hackert T, Palmer DH, Neoptolemos JP (1983) Chemotherapy for pancreatic cancer, Presse Medicale (Paris. France 48(2019):e159–e174. https://doi.org/10.1016/j.lpm.2019.02.025
Ueno M, Morizane C, Ikeda M, Sudo K, Hirashima Y, Kuroda M, Fukuyama Y, Okusaka T, Furuse J (2022) A phase II study of nivolumab in combination with modified FOLFIRINOX for metastatic pancreatic cancer. J Clin Oncol 40:553. https://doi.org/10.1200/JCO.2022.40.4_suppl.553
Lambert A, Gavoille C, Conroy T (2017) Current status on the place of FOLFIRINOX in metastatic pancreatic cancer and future directions. Ther Adv Gastroenterol 10:631–645. https://doi.org/10.1177/1756283x17713879
Kang H, Jo JH, Lee HS, Chung MJ, Bang S, Park SW, Song SY, Park JY (2018) Comparison of efficacy and safety between standard-dose and modified-dose FOLFIRINOX as a first-line treatment of pancreatic cancer. World J Gastrointest Oncol 10:421–430. https://doi.org/10.4251/wjgo.v10.i11.421
Chen YW, Guo CX, Bai XL, Gao SL, Shen Y, Zhang M, Wu J, Que RS, Li X, Liang TB, Wu J (2022) Randomized phase II trial of neoadjuvant chemotherapy with modified FOLFIRINOX versus modified FOLFIRINOX and PD-1 antibody for borderline resectable and locally advanced pancreatic cancer (the CISPD-4 study). J Clin Oncol 40:562. https://doi.org/10.1200/JCO.2022.40.4_suppl.562
Weiss GJ, Blaydorn L, Beck J, Bornemann-Kolatzki K, Urnovitz H, Schutz E, Khemka V (2018) Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. Invest New Drugs 36:96–102. https://doi.org/10.1007/s10637-017-0525-1
Cui JJ, Yang HY, Hu J, Yao JY, Wang Y, Liang YY, Wang YC, Jiao F, Zhang XF, Zhang X, Han T, Mao TB, Xia Q, Xiao XY, Wang LW (2021) Anti-PD-1 antibody combined with albumin-bound paclitaxel and gemcitabine (AG) as first-line therapy and Anti-PD-1 monotherapy as maintenance in metastatic pancreatic ductal adenocarcinoma (PDAC). J Clin Oncol 39:e16218. https://doi.org/10.1200/JCO.2021.39.15_suppl.e16218
Borazanci EH, Jameson GS, Borad MJ, Ramanathan RK, Korn RL, Caldwell L, Ansaldo K, Hendrickson K, Marceau K, Von Hoff DD (2018) A phase II pilot trial of nivolumab (N) plus albumin bound paclitaxel (AP) plus paricalcitol (P) plus cisplatin (C) plus gemcitabine (G) (NAPPCG) in patients with previously untreated metastatic pancreatic ductal adenocarcinoma (PDAC). J Clin Oncol 36:358. https://doi.org/10.1200/JCO.2018.36.4_suppl.358
Shui L, Cheng K, Li XF, Shui PX, Li SS, Peng Y, Li J, Guo FZ, Yi C, Cao D (2020) Durable response and good tolerance to the triple combination of toripalimab, gemcitabine, and nab-paclitaxel in a patient with metastatic pancreatic ductal adenocarcinoma. Front Immunol 11:1127. https://doi.org/10.3389/fimmu.2020.01127
Aglietta M, Barone C, Sawyer MB, Moore MJ, Miller WH, Bagala C, Colombi F, Cagnazzo C, Gioeni L, Wang E, Huang B, Fly KD, Leone F (2014) A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol 25:1750–1755. https://doi.org/10.1093/annonc/mdu205
Kamath SD, Kalyan A, Kircher S, Nimeiri H, Fought AJ, Benson A, Mulcahy M (2020) Ipilimumab and gemcitabine for advanced pancreatic cancer: a phase Ib study. Oncologist 25:E808–E815. https://doi.org/10.1634/theoncologist.2019-0473
Zhang H, Dai Z, Wu W, Wang Z, Zhang N, Zhang L, Zeng W-J, Liu Z, Cheng Q (2021) Regulatory mechanisms of immune checkpoints PD-L1 and CTLA-4 in cancer. J Exp Clin Cancer Res CR 40:184. https://doi.org/10.1186/s13046-021-01987-7
Renouf DJ, Dhani NC, Kavan P, Jonker DJ, Wei ACC, Hsu T, Tang PA, Graham B, Gallinaro L, Hasan T, Li WW, Hart K, Tu DS, O’Callaghan CJ (2018) The Canadian Cancer Trials Group PA 7 trial: results from the safety run in of a randomized phase II study of gemcitabine (GEM) and nab-paclitaxel (Nab-P) versus GEM, nab-P, durvalumab (D), and tremelimumab (T) as first-line therapy in metastatic pancreatic ductal adenocarcinoma (mPDAC). J Clin Oncol 36:349. https://doi.org/10.1200/JCO.2018.36.4_suppl.349
Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol Official J Am Soc Clin Oncol 15:2403–2413
O’Hara MH, O’Reilly EM, Varadhachary G, Wolff RA, Wainberg ZA, Ko AH, Fisher G, Rahma O, Lyman JP, Cabanski CR, Mick R, Gherardini PF, Kitch LJ, Xu JY, Samuel T, Karakunnel J, Fairchild J, Bucktrout S, LaVallee TM, Selinsky C, Till JE, Carpenter EL, Alanio C, Byrne KT, Chen RO, Trifan OC, Dugan U, Horak C, Hubbard-Lucey VM, Wherry EJ, Ibrahim R, Vonderheide RH (2021) CD40 agonistic monoclonal antibody APX005M (sotigalimab) and chemotherapy, with or without nivolumab, for the treatment of metastatic pancreatic adenocarcinoma: an open-label, multicentre, phase 1b study. Lancet Oncol 22:118–131
O’Hara MH, O’Reilly EM, Wolff RA, Wainberg ZA, Ko AH, Rahma OE, Fisher GA, Lyman JP, Cabanski CR, Karakunnel JJ, Gherardini PF, Kitch LJ, Bucktrout S, Christopher E, Mick R, Chen R, Trifan OC, Salvador L, O’Donnell-Tormey J, Vonderheide RH (2021) Gemcitabine (Gem) and nab-paclitaxel (NP) ± nivolumab (nivo) ± CD40 agonistic monoclonal antibody APX005M (sotigalimab), in patients (Pts) with untreated metastatic pancreatic adenocarcinoma (mPDAC): Phase (Ph) 2 final results. J Clin Oncol 39:4019–4019. https://doi.org/10.1200/JCO.2021.39.15_suppl.4019
Padron LJ, Maurer DM, O’Hara MH, O’Reilly EM, Wolff RA, Wainberg ZA, Ko AH, Fisher G, Rahma O, Lyman JP, Cabanski CR, Yu JX, Pfeiffer SM, Spasic M, Xu JY, Gherardini PF, Karakunnel J, Mick R, Alanio C, Byrne KT, Hollmann TJ, Moore JS, Jones DD, Tognetti M, Chen RO, Yang XD, Salvador L, Wherry EJ, Dugan U, O’Donnell-Tormey J, Butterfield LH, Hubbard-Lucey VM, Ibrahim R, Fairchild J, Bucktrout S, LaVallee TM, Vonderheide RH (2022) Sotigalimab and/or nivolumab with chemotherapy in first-line metastatic pancreatic cancer: clinical and immunologic analyses from the randomized phase 2 PRINCE trial. Nat Med 9:1829. https://doi.org/10.1038/s41591-022-01829-9
Byrne KT, Betts CB, Mick R, Sivagnanam S, Bajor DL, Laheru DA, Chiorean EG, O’Hara MH, Liudahl SM, Newcomb C, Alanio C, Ferreira AP, Park BS, Ohtani T, Huffman AP, Vayrynen SA, Costa AD, Kaiser JC, Lacroix AM, Redlinger C, Stern M, Nowak JA, Wherry EJ, Cheever MA, Wolpin BM, Furth EE, Jaffee EM, Coussens LM, Vonderheide RH (2021) Neoadjuvant selicrelumab, an agonist CD40 antibody, induces changes in the tumor microenvironment in patients with resectable pancreatic cancer. Clin Cancer Res 27:4574–4586. https://doi.org/10.1158/1078-0432.Ccr-21-1047
Holmgaard RB, Brachfeld A, Gasmi B, Jones DR, Mattar M, Doman T, Murphy M, Schaer D, Wolchok JD, Merghoub T (2016) Timing of CSF-1/CSF-1R signaling blockade is critical to improving responses to CTLA-4 based immunotherapy. Oncoimmunology 5:1151595. https://doi.org/10.1080/2162402x.2016.1151595
Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo JQ, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG (2014) CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Can Res 74:5057–5069. https://doi.org/10.1158/0008-5472.Can-13-3723
Razak ARA, Cleary JM, Moreno V, Boyer M, Aller EC, Edenfield W, Tie J, Harvey RD, Rutten A, Shah MA, Olszanski AJ, Jager D, Lakhani N, Ryan DP, Rasmussen E, Juan G, Wong HS, Soman N, Smit MAD, Nagorsen DKP (2020) Papadopoulos, Safety and efficacy of AMG 820, an anti-colony-stimulating factor 1 receptor antibody, in combination with pembrolizumab in adults with advanced solid tumors. J Immunother Cancer 8:6. https://doi.org/10.1136/jitc-2020-001006
Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, Kadel EE, Koeppen H, Astarita JL, Cubas R, Jhunjhunwala S, Banchereau R, Yang Y, Guan Y, Chalouni C, Ziai J, Şenbabaoğlu Y, Santoro S, Sheinson D, Hung J, Giltnane JM, Pierce AA, Mesh K, Lianoglou S, Riegler J, Carano RAD, Eriksson P, Höglund M, Somarriba L, Halligan DL, van der Heijden MS, Loriot Y, Rosenberg JE, Fong L, Mellman I, Chen DS, Green M, Derleth C, Fine GD, Hegde PS, Bourgon R, Powles T (2018) TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554:544–548. https://doi.org/10.1038/nature25501
Derynck R, Zhang YE (2003) Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425:577–584
Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, Sevillano M, Ibiza S, Cañellas A, Hernando-Momblona X, Byrom D, Matarin JA, Calon A, Rivas EI, Nebreda AR, Riera A, Attolini CS-O, Batlle E (2018) TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554:538–543. https://doi.org/10.1038/nature25492
Herbertz S, Sawyer JS, Stauber AJ, Gueorguieva I, Driscoll KE, Estrem ST, Cleverly AL, Desaiah D, Guba SC, Benhadji KA, Slapak CA, Lahn MM (2015) Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des Dev Ther 9:4479–4499. https://doi.org/10.2147/dddt.S86621
Melisi D, Garcia-Carbonero R, Macarulla T, Pezet D, Deplanque G, Fuchs M, Trojan J, Oettle H, Kozloff M, Cleverly A, Smith C, Estrem ST, Gueorguieva I, Lahn MMF, Blunt A, Benhadji KA, Tabernero J (2018) Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br J Cancer 119:1208–1214. https://doi.org/10.1038/s41416-018-0246-z
Holmgaard RB, Schaer DA, Li YX, Castaneda SP, Murphy MY, Xu XH, Inigo I, Dobkin J, Manro JR, Iversen PW, Surguladze D, Hall GE, Novosiadly RD, Benhadji KA, Plowman GD, Kalos M, Driscoll KE (2018) Targeting the TGF beta pathway with galunisertib, a TGF beta RI small molecule inhibitor, promotes anti-tumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade. J Immunother Cancer 6:4. https://doi.org/10.1186/s40425-018-0356-4
Principe DR, Park A, Dorman MJ, Kumar S, Viswakarma N, Rubin J, Torres C, McKinney R, Munshi HG, Grippo PJ, Rana A (2019) TGF beta blockade augments PD-1 inhibition to promote T-cell-mediated regression of pancreatic cancer. Mol Cancer Ther 18:613–620. https://doi.org/10.1158/1535-7163.Mct-18-0850
Sow HS, Ren J, Camps M, Ossendorp F, ten Dijke P (2019) Combined inhibition of TGF—signaling and the PD-L1 immune checkpoint is differentially effective in tumor models. Cells 8:320. https://doi.org/10.3390/cells8040320
Melisi D, Oh D, Hollebecque A, Calvo E, Varghese A, Borazanci E, Macarulla T, Merz V, Zecchetto C, Zhao YM, Gueorguieva I, Man M, Gandhi L, Estrem ST, Benhadji KA, Lanasa MC, Avsar E, Guba SC, Garcia-Carbonero R (2021) Safety and activity of the TGF beta receptor I kinase inhibitor galunisertib plus the anti-PD-L1 antibody durvalumab in metastatic pancreatic cancer. J Immunother Cancer 9:2068. https://doi.org/10.1136/jitc-2020-002068
Strauss J, Heery CR, Schlom J, Madan RA, Cao L, Kang ZG, Lamping E, Marte JL, Donahue RN, Grenga I, Cordes L, Christensen O, Mahnke L, Helwig C, Gulley JL (2018) Phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGF beta. Adv Solid Tumors Clin Cancer Res 24:1287–1295. https://doi.org/10.1158/1078-0432.Ccr-17-2653
Seo Y, Baba H, Fukuda T, Takashima M, Sugimachi K (2000) High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer 88:2239–2245
Halperin DM, Liu SY, Dasari A, Fogelman D, Bhosale P, Mahvash A, Estrella JS, Rubin L, Morani AC, Knafl M, Overeem TA, Fu SC, Solis LM, Cuentas EP, Verma A, Chen HL, Gite S, Subashchandrabose P, Dervin S, Schulze K, Darbonne WC, Yun C, Wistuba PAF II, Woodman SE, Yao JC (2022) Assessment of clinical response following atezolizumab and bevacizumab treatment in patients with neuroendocrine tumors a nonrandomized clinical trial. Jama Oncol. https://doi.org/10.1001/jamaoncol.2022.0212
Ishida T, Ueda R (2006) CCR4 as a novel molecular target for immunotherapy of cancer. Cancer Sci 97:1139–1146. https://doi.org/10.1111/j.1349-7006.2006.00307.x
Kurose K, Ohue Y, Sato E, Yamauchi A, Eikawa S, Isobe M, Nishio Y, Uenaka A, Oka M, Nakayama E (2015) Increase in activated treg in TIL in lung cancer and in vitro depletion of treg by ADCC using an antihuman CCR4 mAb (KM2760). J Thorac Oncol 10:74–83. https://doi.org/10.1097/jto.0000000000000364
Kurose K, Ohue Y, Wada H, Iida S, Ishida T, Kojima T, Doi T, Suzuki S, Isobe M, Funakoshi T, Kakimi K, Nishikawa H, Udono H, Oka M, Ueda R, Nakayama E (2015) Phase Ia study of FoxP3(+) CD4 treg depletion by infusion of a humanized anti-CCR4 antibody, KW-0761. Cancer Patients Clin Cancer Res 21:4327–4336. https://doi.org/10.1158/1078-0432.Ccr-15-0357
Saito T, Kurose K, Kojima T, Funakoshi T, Sato E, Nishikawa H, Nakajima J, Seto Y, Kakimi K, Iida S, Doki Y, Oka M, Ueda R, Wada H (2021) Phase Ib study on the humanized anti-CCR4 antibody, KW-0761, in advanced solid tumors. Nagoya J Med Sci 83:827–840. https://doi.org/10.18999/nagjms.83.4.827
Zamarin D, Hamid O, Nayak-Kapoor A, Sahebjam S, Sznol M, Collaku A, Fox FE, Marshall MA, Hong DS (2020) y Mogamulizumab in combination with durvalumab or tremelimumab in patients with advanced solid tumors: a phase i study. Clin Cancer Res 26:4531–4541. https://doi.org/10.1158/1078-0432.Ccr-20-0328
Bockorny B, Semenisty V, Macarulla T, Borazanci E, Wolpin BM, Stemmer SM, Golan T, Geva R, Borad MJ, Pedersen KS, Park JO, Ramirez RA, Abad DG, Feliu J, Munoz A, Ponz-Sarvise M, Peled A, Lustig TM, Bohana-Kashtan O, Shaw SM, Sorani E, Chaney M, Kadosh S, Haras AV, Von Hoff DD, Hidalgo M (2020) BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the COMBAT trial. Nat Med 26:878. https://doi.org/10.1038/s41591-020-0880-x
Bockorny B, Macarulla T, Semenisty V, Borazanci E, Feliu J, Ponz-Sarvise M, Abad DG, Oberstein P, Alistar A, Munoz A, Geva R, Guillen-Ponce C, Fernandez MS, Peled A, Chaney M, Gliko-Kabir I, Shemesh-Darvish L, Ickowicz D, Sorani E, Kadosh S, Vainstein-Haras A, Hidalgo M (2021) Motixafortide and pembrolizumab combined to nanoliposomal irinotecan, fluorouracil, and folinic acid in metastatic pancreatic cancer: the COMBAT/KEYNOTE-202 trial. Clin Cancer Res 27:5020–5027. https://doi.org/10.1158/1078-0432.Ccr-21-0929
Suarez-Carmona M, Williams A, Schreiber J, Hohmann N, Pruefer U, Krauss J, Jager D, Fromming A, Beyer D, Eulberg D, Jungelius JU, Baumann M, Mangasarian A, Halama N (2021) Combined inhibition of CXCL12 and PD-1 in MSS colorectal and pancreatic cancer: modulation of the microenvironment and clinical effects. J Immunother Cancer 9:2505. https://doi.org/10.1136/jitc-2021-002505
Feig C, Jones JO, Kraman M, Wells RJB, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, Teichmann SA, Janowitz T, Jodrell DI, Tuveson DA, Fearon DT (2013) Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci USA 110:20212–20217. https://doi.org/10.1073/pnas.1320318110
Seo YD, Jiang XY, Sullivan KM, Jalikis FG, Smythe KS, Abbasi A, Vignali M, Park JO, Daniel SK, Pollack SM, Kim TS, Yeung R, Crispe IN, Pierce RH, Robins H, Pillarisetty VG (2019) Mobilization of CD8(+) T Cells via CXCR4 blockade facilitates PD-1 checkpoint therapy in human pancreatic cancer. Clin Cancer Res 25:3934–3945. https://doi.org/10.1158/1078-0432.Ccr-19-0081
Peled A (2019) Combination of BL-8040, anti PD-1 and chemotherapy significantly reduced pancreatic tumor growth and changed the balance between CD4+/FOXP3+cells and CD8+cells in the tumor. J Immunother Cancer 7
Pal Singh S, Dammeijer F, Hendriks RW (2018) Role of Bruton’s tyrosine kinase in B cells and malignancies. Mol Cancer 17:57. https://doi.org/10.1186/s12943-018-0779-z
Wang M, Rule S, Zinzani PL, Goy A, Casasnovas O, Smith SD, Damaj G, Doorduijn J, Lamy T, Morschhauser F, Panizo C, Shah B, Davies A, Eek R, Dupuis J, Jacobsen E, Kater AP, Le Gouill S, Oberic L, Robak T, Covey T, Dua R, Hamdy A, Huang X, Izumi R, Patel P, Rothbaum W, Slatter JG, Jurczak W (2018) Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet (London, England) 391:659–667. https://doi.org/10.1016/S0140-6736(17)33108-2
Gunderson AJ, Kaneda MM, Tsujikawa T, Nguyen AV, Affara NI, Ruffell B, Gorjestani S, Liudahl SM, Truitt M, Olson P, Kim G, Hanahan D, Tempero MA, Sheppard B, Irving B, Chang BY, Varner JA, Coussens LM (2016) Bruton tyrosine kinase-dependent immune cell cross-talk drives pancreas cancer. Cancer Discov 6:270–285. https://doi.org/10.1158/2159-8290.CD-15-0827
Overman M, Javle M, Davis RE, Vats P, Kumar-Sinha C, Xiao LC, Mettu NB, Parra ER, Benson A, Lopez CD, Munugalavadla V, Patel P, Tao L, Neelapu S, Maitra A (2020) Randomized phase II study of the Bruton tyrosine kinase inhibitor acalabrutinib, alone or with pembrolizumab in patients with advanced pancreatic cancer. J Immunother Cancer 8:587. https://doi.org/10.1136/jitc-2020-000587
Shen G, Zheng F, Ren D, Du F, Dong Q, Wang Z, Zhao F, Ahmad R, Zhao J (2018) Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol 11:120. https://doi.org/10.1186/s13045-018-0664-7
Xie C, Wan X, Quan H, Zheng M, Fu L, Li Y, Lou L (2018) Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci 109:1207–1219. https://doi.org/10.1111/cas.13536
Yuan M, Zhu ZZ, Mao W, Wang H, Qian H, Wu JG, Guo XL, Xu Q (2021) Anlotinib combined with anti-PD-1 antibodies therapy in patients with advanced refractory solid tumors: a single-center, observational, Prospective Study. Front Oncol 11:683502. https://doi.org/10.3389/fonc.2021.683502
Patnaik A, Yap TA, Chung HC, de Miguel MJ, Bang YJ, Lin CC, Su WC, Italiano A, Chow KH, Szpurka AM, Yu DN, Zhao YM, Carlsen M, Schmidt S, Vangerow B, Gandhi L, Xu XJ, Bendell J (2021) Safety and Clinical activity of a new anti-PD-L1 antibody as monotherapy or combined with targeted therapy in advanced solid tumors: the PACT phase Ia/Ib trial. Clin Cancer Res 27:1267–1277. https://doi.org/10.1158/1078-0432.Ccr-20-2821
Rodon Ahnert J, Tan DS, Garrido-Laguna I, Harb W, Bessudo A, Beck JT, Rottey S, Bahary N, Kotecki N, Zhu Z, Deng S, Kowalski K, Wei C, Pathan N, Laliberte RJ, Messersmith WA (2023) Avelumab or talazoparib in combination with binimetinib in metastatic pancreatic ductal adenocarcinoma: dose-finding results from phase Ib of the JAVELIN PARP MEKi trial. ESMO Open 8:101584. https://doi.org/10.1016/j.esmoop.2023.101584
Wang-Gillam A, Lim KH, McWilliams R, Suresh R, Lockhart AC, Brown A, Breden M, Belle JI, Herndon J, Bogner SJ, Pedersen K, Tan B, Boice N, Acharya A, Abdiannia M, Gao F, Yoon HH, Zhu M, Trikalinos NA, Ratner L, Aranha O, Hawkins WG, Herzog BH, DeNardo DG (2022) Defactinib, pembrolizumab, and gemcitabine in patients with advanced treatment refractory pancreatic cancer: a phase I dose escalation and expansion study. Clin Cancer Res 28:5254–5262. https://doi.org/10.1158/1078-0432.Ccr-22-0308
Wu XH, Zhu JQ, Yin RT, Yang JX, Liu JH, Wang J, Wu LY, Liu ZL, Gao YN, Wang DB, Lou G, Yang HY, Zhou Q, Kong BH, Huang Y, Chen LP, Li GL, An RF, Wang K, Zhang Y, Yan XJ, Lu X, Lu WG, Hao M, Wang L, Cui H, Chen QH, Abulizi G, Huang XH, Tian XF, Wen H, Zhang C, Hou JM, Mirza MR (2021) Niraparib maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer using an individualized starting dose (NORA): a randomized, double-blind, placebo-controlled phase III trial(☆). Ann Oncol 32:512–521. https://doi.org/10.1016/j.annonc.2020.12.018
Reiss KA, Mick R, Teitelbaum U, O’Hara M, Schneider C, Massa R, Karasic T, Tondon R, Onyiah C, Gosselin MK, Donze A, Domchek SM, Vonderheide RH (2022) Niraparib plus nivolumab or niraparib plus ipilimumab in patients with platinum-sensitive advanced pancreatic cancer: a randomised, phase 1b/2 trial. Lancet Oncol 23:1009–1020. https://doi.org/10.1016/s1470-2045(22)00369-2
Lemech C, Dredge K, Bampton D, Hammond E, Clouston A, Waterhouse NJ, Stanley AC, Leveque-El Mouttie L, Chojnowski GM, Haydon A, Pavlakis N, Burge M, Brown MP, Goldstein D (2023) Phase Ib open-label, multicenter study of pixatimod, an activator of TLR9, in combination with nivolumab in subjects with microsatellite-stable metastatic colorectal cancer, metastatic pancreatic ductal adenocarcinoma and other solid tumors. J Immunother Cancer 11:6136. https://doi.org/10.1136/jitc-2022-006136
Parikh AR, Szabolcs A, Allen JN, Clark JW, Wo JY, Raabe M, Thel H, Hoyos D, Mehta A, Arshad S, Lieb DJ, Drapek LC, Blaszkowsky LS, Giantonio BJ, Weekes CD, Zhu AX, Goyal L, Nipp RD, Dubois JS, Van Seventer EE, Foreman BE, Matlack LE, Ly L, Meurer JA, Hacohen N, Ryan DP, Yeap BY, Corcoran RB, Greenbaum BD, Ting DT, Hong TS (2021) Radiation therapy enhances immunotherapy response in microsatellite stable colorectal and pancreatic adenocarcinoma in a phase II trial. Nat Cancer 2:1124–1135. https://doi.org/10.1038/s43018-021-00269-7
Xie C, Duffy AG, Brar G, Fioravanti S, Mabry-Hrones D, Walker M, Bonilla CM, Wood BJ, Citrin DE, Gil Ramirez EM, Escorcia FE, Redd B, Hernandez JM, Davis JL, Gasmi B, Kleiner D, Steinberg SM, Jones JC, Greten TF (2020) Immune checkpoint blockade in combination with stereotactic body radiotherapy in patients with metastatic pancreatic ductal adenocarcinoma. Clin Cancer Res 26:2318–2326. https://doi.org/10.1158/1078-0432.Ccr-19-3624
Chen IM, Johansen JS, Theile S, Hjaltelin JX, Novitski SI, Brunak S, Hasselby JP, Willemoe GL, Lorentzen T, Madsen K, Jensen BV, Wilken EE, Geertsen P, Behrens C, Nolsoe C, Hermann KL, Svane IM, Nielsen D (2022) Randomized phase II study of nivolumab with or without ipilimumab combined with stereotactic body radiotherapy for refractory metastatic pancreatic cancer (CheckPAC). J Clin Oncol 40:3180–3189. https://doi.org/10.1200/jco.21.02511
Zhu XF, Cao YS, Liu WY, Ju XP, Zhao XZ, Jiang LG, Ye YS, Jin G, Zhang HJ (2021) Stereotactic body radiotherapy plus pembrolizumab and trametinib versus stereotactic body radiotherapy plus gemcitabine for locally recurrent pancreatic cancer after surgical resection: an open-label, randomised, controlled, phase 2 trial. Lancet Oncol 22:1093–1102. https://doi.org/10.1016/s1470-2045(21)00286-2
Chen IM, Donia M, Chamberlain CA, Jensen AWP, Draghi A, Theile S, Madsen K, Hasselby JP, Toxværd A, Høgdall E, Lorentzen T, Wilken EE, Geertsen P, Svane IM, Johansen JS, Nielsen D (2023) Phase 2 study of ipilimumab, nivolumab, and tocilizumab combined with stereotactic body radiotherapy in patients with refractory pancreatic cancer (TRIPLE-R). Eur J Cancer 180:125–133. https://doi.org/10.1016/j.ejca.2022.11.035
Fukuhara H, Ino Y, Todo T (2016) Oncolytic virus therapy: A new era of cancer treatment at dawn. Cancer Sci 107:1373–1379. https://doi.org/10.1111/cas.13027
Eissa IR, Bustos-Villalobos I, Ichinose T, Matsumura S, Naoe Y, Miyajima N, Morimoto D, Mukoyama N, Zhiwen W, Tanaka M, Hasegawa H, Sumigama S, Aleksic B, Kodera Y, Kasuya H (2018) The current status and future prospects of oncolytic viruses in clinical trials against melanoma, glioma, pancreatic, and breast cancers. Cancers 10:356. https://doi.org/10.3390/cancers10100356
Mahalingam D, Wilkinson GA, Eng KH, Fields P, Raber P, Moseley JL, Cheetham K, Coffey M, Nuovo G, Kalinski P, Zhang B, Arora SP, Fountzilas C (2020) Pembrolizumab in combination with the oncolytic virus pelareorep and chemotherapy in patients with advanced pancreatic adenocarcinoma: a phase Ib study. Clin Cancer Res 26:71–81. https://doi.org/10.1158/1078-0432.Ccr-19-2078
Hajda J, Leuchs B, Angelova AL, Frehtman V, Rommelaere J, Mertens M, Pilz M, Kieser M, Krebs O, Dahm M, Huber B, Engeland CE, Mavratzas A, Hohmann N, Schreiber J, Jäger D, Halama N, Sedlaczek O, Gaida MM, Daniel V, Springfeld C, Ungerechts G (2021) Phase 2 trial of oncolytic h-1 parvovirus therapy shows safety and signs of immune system activation in patients with metastatic pancreatic ductal adenocarcinoma. Clin Cancer Res 27:5546–5556. https://doi.org/10.1158/1078-0432.Ccr-21-1020
Hegde A, Jayaprakash P, Couillault CA, Piha-Paul S, Karp D, Rodon J, Pant S, Fu SQ, Dumbrava EE, Yap TA, Subbiah V, Bhosale P, Coarfa C, Higgins JP, Williams ET, Wilson TF, Lim J, Meric-Bernstam F, Sumner E, Zain H, Nguyen D, Nguyen LM, Rajapakshe K, Curran MA, Hong DS (2021) A phase I dose-escalation study to evaluate the safety and tolerability of evofosfamide in combination with ipilimumab in advanced solid malignancies. Clin Cancer Res 27:3050–3060. ttps://doi.org/10.1158/1078-0432.Ccr-20-4118
Khan SM, Nazzal M, Zelenock G, Qu W, Aplin B, Baldawi M, Arishi A, Stoller J (2015) A nationwide comparison of early outcomes after carotid endarterectomy and carotid artery stenting. J Vasc Surg 62:819–820. https://doi.org/10.1016/j.jvs.2015.06.147
Khan S, Ebeling MC, Chauhan N, Thompson PA, Gara RK, Ganju A, Yallapu MM, Behrman SW, Zhao H, Zafar N, Singh MM, Jaggi M, Chauhan SC (2015) Ormeloxifene suppresses desmoplasia and enhances sensitivity of gemcitabine in pancreatic cancer. Can Res 75:2292–2304. https://doi.org/10.1158/0008-5472.CAN-14-2397
Martin RCG, Kwon D, Chalikonda S, Sellers M, Kotz E, Scoggins C, McMasters KM, Watkins K (2015) Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: safety and efficacy. Ann Surg 262:1441. https://doi.org/10.1097/SLA.0000000000001441
Holland MM, Bhutiani N, Kruse EJ, Weiss MJ, Christein JD, White RR, Huang K-W, Martin RCG (2019) A prospective, multi-institution assessment of irreversible electroporation for treatment of locally advanced pancreatic adenocarcinoma: initial outcomes from the AHPBA pancreatic registry. HPB (Oxford) 21:1024–1031. https://doi.org/10.1016/j.hpb.2018.12.004
O’Neill C, Hayat T, Hamm J, Healey M, Zheng QQ, Li Y, Martin RC (2020) A phase 1b trial of concurrent immunotherapy and irreversible electroporation in the treatment of locally advanced pancreatic adenocarcinoma. Surgery 168:610–616. https://doi.org/10.1016/j.surg.2020.04.057
Kohler G, Milstein C (1975) Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495–497. https://doi.org/10.1038/256495a0
Boland AJ, O’Kane AA, Buick R, Longley DB, Scott CJ (2021) Antibody therapy in pancreatic cancer: mAb-ye we’re onto something? Biochim Biophys Acta Rev Cancer 1876:188557. https://doi.org/10.1016/j.bbcan.2021.188557
Zahavi D, Weiner L (2020) Monoclonal antibodies in cancer therapy. Antibodies 9:34. https://doi.org/10.3390/antib9030034
Kunjiappan S, Pavadai P, Vellaichamy S, Pandian SRK, Ravishankar V, Palanisamy P, Govindaraj S, Srinivasan G, Premanand A, Sankaranarayanan M, Theivendren P (2021) Surface receptor-mediated targeted drug delivery systems for enhanced cancer treatment: a state-of-the-art review. Drug Dev Res 82:309–340. https://doi.org/10.1002/ddr.21758
Halfdanarson TR, Foster NR, Kim GP, Meyers JP, Smyrk TC, McCullough AE, Ames MM, Jaffe JP, Alberts SR (2019) A phase II randomized trial of panitumumab, erlotinib, and gemcitabine versus erlotinib and gemcitabine in patients with untreated, metastatic pancreatic adenocarcinoma: North Central Cancer Treatment Group Trial N064B (Alliance). Oncologist 24:589. https://doi.org/10.1634/theoncologist.2018-0878
Liermann J, Munter M, Naumann P, Abdollahi A, Krempien R, Debus J (2022) Cetuximab, gemcitabine and radiotherapy in locally advanced pancreatic cancer: long-term results of the randomized controlled phase II PARC trial, Clinical and Translational. Radiat Oncol 34:15–22. https://doi.org/10.1016/j.ctro.2022.03.003
Assenat E, Mineur L, Mollevi C, Lopez-Crapez E, Lombard-Bohas C, Samalin E, Portales F, Walter T, de Forges H, Dupuy M, Boissiere-Michot F, Ho-Pun-Cheung A, Ychou M, Mazard T (2021) PhaseIIstudy evaluating the association of gemcitabine, trastuzumab and erlotinib as first-line treatment in patients with metastatic pancreatic adenocarcinoma (GATE1). Int J Cancer 148:682–691. https://doi.org/10.1002/ijc.33225
Kindler HL, Richards DA, Garbo LE, Garon EB, Stephenson JJ, Rocha-Lima CM, Safran H, Chan D, Kocs DM, Galimi F, McGreivy J, Bray SL, Hei Y, Feigal EG, Loh E, Fuchs CS (2012) A randomized, placebo-controlled phase 2 study of ganitumab (AMG 479) or conatumumab (AMG 655) in combination with gemcitabine in patients with metastatic pancreatic cancer. Ann Oncol 23:2834–2842. https://doi.org/10.1093/annonc/mds142
Forero-Torres A, Infante JR, Waterhouse D, Wong L, Vickers S, Arrowsmith E, He AR, Hart L, Trent D, Wade J, Jin XP, Wang Q, Austin T, Rosen M, Beckman R, von Roemeling R, Greenberg J, Saleh M (2013) Phase 2, multicenter, open-label study of tigatuzumab (CS-1008), a humanized monoclonal antibody targeting death receptor 5, in combination with gemcitabine in chemotherapy-naive patients with unresectable or metastatic pancreatic cancer. Cancer Med 2:925–932. https://doi.org/10.1002/cam4.137
Hassan R, Alewine C, Mian I, Spreafico A, Siu LL, Gomez-Roca C, Delord JP, Italiano A, Lassen U, Soria JC, Bahleda R, Thomas A, Steinberg SM, Peer CJ, Figg WD, Niederfellner G, Naegelen VM, Pastan I (2020) Phase 1 study of the immunotoxin LMB-100 in patients with mesothelioma and other solid tumors expressing mesothelin. Cancer 126:4936–4947. https://doi.org/10.1002/cncr.33145
Hassan R, Blumenschein GR, Moore KN, Santin AD, Kindler HL, Nemunaitis JJ, Seward SM, Thomas A, Kim SK, Rajagopalan P, Walter AO, Laurent D, Childs BH, Sarapa N, Elbi C, Bendell JC (2020) First-in-human, multicenter, phase i dose-escalation and expansion study of anti-mesothelin antibody-drug conjugate anetumab ravtansine in advanced or metastatic solid tumors. J Clin Oncol 38:1824. https://doi.org/10.1200/jco.19.02085
Berlin JD, Feng Y, Catalano P, Abbruzzese JL, Philip PA, McWilliams RR, Lowy AM, Benson A, Blackstock AW (2018) An intergroup randomized phase II study of bevacizumab or cetuximab in combination with gemcitabine and in combination with chemoradiation in patients with resected pancreatic carcinoma: a trial of the ECOG-ACRIN cancer research group (E2204). Oncology 94:39–46. https://doi.org/10.1159/000480295
Sen S, Kato S, Agarwal R, Piha-Paul S, Hess K, Karp D, Janku F, Fu SQ, Naing A, Pant S, Falchook G, Tang C, Wu XF, Ye YQ, Tsimberidou A, Subbiah V, Kurzrock R, Byers L, Westin S, Lim J, Bean S, Bass A, Nguyen L, Meric-Bernstam F, Hong D (2018) Phase I study of nab-paclitaxel, gemcitabine, and bevacizumab in patients with advanced cancers. Br J Cancer 118:1419–1424. https://doi.org/10.1038/s41416-018-0068-z
Sahai V, Saif MW, Kalyan A, Philip PA, Rocha-Lima CM, Ocean A, Ondovik MS, Simeone DM, Banerjee S, Bhore R, Louis CU, Picozzi V (2019) A phase I/II open-label multicenter single-arm study of FABLOx (metronomic 5-fluorouracil plus nab-paclitaxel, bevacizumab, leucovorin, and oxaliplatin) in patients with metastatic pancreatic cancer. J Pancreatic Cancer 5:35–42. https://doi.org/10.1089/pancan.2019.0012
Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, Solt S, Dorman A, Wamwea A, Yager A, Laheru D, Wolfgang CL, Wang J, Hruban RH, Anders RA, Jaffee EM, Zheng L (2014) Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res 2:616–631. https://doi.org/10.1158/2326-6066.CIR-14-0027
Zheng L, Ding D, Edil BH, Judkins C, Durham JN, Thomas DL, Bever KM, Mo GL, Solt SE, Hoare JA, Bhattacharya R, Zhu QF, Osipov A, Onner B, Purtell KA, Cai HY, Parkinson R, Hacker-Prietz A, Herman JM, Le DT, Azad NS, De Jesus-Acosta AMC, Blair AB, Kim V, Soares KC, Manos L, Cameron JL, Makary MA, Weiss MJ, Schulick RD, He J, Wolfgang CL, Thompson ED, Anders RA, Sugar E, Jaffee EM, Laheru DA (2021) Vaccine-induced intratumoral lymphoid aggregates correlate with survival following treatment with a neoadjuvant and adjuvant vaccine in patients with resectable pancreatic adenocarcinoma. Clin Cancer Res 27:1278–1286. https://doi.org/10.1158/1078-0432.Ccr-20-2974
Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G, Morse M, Zeh H, Cohen D, Fine RL, Onners B, Uram JN, Laheru DA, Lutz ER, Solt S, Murphy AL, Skoble J, Lemmens E, Grous J, Dubensky T, Brockstedt DG, Jaffee EM (2015) Safety and survival with gvax pancreas prime and listeria monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol 33:1325. https://doi.org/10.1200/jco.2014.57.4244
Le DT, Picozzi VJ, Ko AH, Wainberg ZA, Kindler H, Wang-Gillam A, Oberstein P, Morse MA, Zeh HJ, Weekes C, Reid T, Borazanci E, Crocenzi T, LoConte NK, Musher B, Laheru D, Murphy A, Whiting C, Nair N, Enstrom A, Ferber S, Brockstedt DG, Jaffee EM (2019) Results from a phase iib, randomized, multicenter study of GVAX pancreas and CRS-207 compared with chemotherapy in adults with previously treated metastatic pancreatic adenocarcinoma (ECLIPSE Study). Clin Cancer Res 25:5493–5502. https://doi.org/10.1158/1078-0432.Ccr-18-2992
Nair N, Chen SY, Lemmens E, Chang S, Le DT, Jaffee EM, Murphy A, Whiting C, Muller T, Brockstedt DG (2020) Single-cell immune competency signatures associate with survival in phase II GVAX and CRS-207 randomized studies in patients with metastatic pancreatic cancer. Cancer Immunol Res 8:609–617. https://doi.org/10.1158/2326-6066.Cir-19-0650
Soares KC, Rucki AA, Wu AA, Olino K, Xiao Q, Chai Y, Wamwea A, Bigelow E, Lutz E, Liu LD, Yao S, Anders RA, Laheru D, Wolfgang CL, Edil BH, Schulick RD, Jaffee EM, Zheng L (2015) PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J Immunother 38:1–11
Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, Diaz LA, Donehower RC, Jaffee EM, Laheru DA (1997) Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer, Journal of Immunotherapy (Hagerstown. MD 36(2013):382–389. https://doi.org/10.1097/CJI.0b013e31829fb7a2
Tsujikawa T, Crocenzi T, Durham JN, Sugar EA, Wu AA, Onners B, Nauroth JM, Anders RA, Fertig EJ, Laheru DA, Reiss K, Vonderheide RH, Ko AH, Tempero MA, Fisher GA, Considine M, Danilova L, Brockstedt DG, Coussens LM, Jaffee EM, Le DT (2020) Evaluation of cyclophosphamide/GVAX pancreas followed by listeria-mesothelin (CRS-207) with or without nivolumab in patients with pancreatic. Clin Cancer Res 26:3578–3588. https://doi.org/10.1158/1078-0432.Ccr-19-3978
Heumann T, Judkins C, Li K, Lim SJ, Hoare J, Parkinson R, Cao H, Zhang T, Gai J, Celiker B, Zhu Q, McPhaul T, Durham J, Purtell K, Klein R, Laheru D, De Jesus-Acosta A, Le DT, Narang A, Anders R, Burkhart R, Burns W, Soares K, Wolfgang C, Thompson E, Jaffee E, Wang H, He J, Zheng L (2023) A platform trial of neoadjuvant and adjuvant antitumor vaccination alone or in combination with PD-1 antagonist and CD137 agonist antibodies in patients with resectable pancreatic adenocarcinoma. Nat Commun 14:3650. https://doi.org/10.1038/s41467-023-39196-9
Wu AA, Bever KM, Ho WJ, Fertig EJ, Niu N, Zheng L, Parkinson RM, Durham JN, Onners B, Ferguson AK, Wilt C, Ko AH, Wang-Gillam A, Laheru DA, Anders RA, Thompson ED, Sugar EA, Jaffee EM, Le DT (2020) A phase II study of allogeneic GM-CSF-transfected pancreatic tumor vaccine (GVAX) with ipilimumab as maintenance treatment for metastatic pancreatic cancer. Clin Cancer Res 26:5129–5139. https://doi.org/10.1158/1078-0432.Ccr-20-1025
Gao T, Cen Q, Lei H (2020) A review on development of MUC1-based cancer vaccine. Biomed Pharmacother 132. https://doi.org/10.1016/j.biopha.2020.110888
Hollingsworth MA, Swanson BJ (2004) Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer 4:45–60
Nath S, Mukherjee P (2014) MUC1: a multifaceted oncoprotein with a key role in cancer progression. Trends Mol Med 20:332–342. https://doi.org/10.1016/j.molmed.2014.02.007
Rong Y, Qin X, Jin D, Lou W, Wu L, Wang D, Wu W, Ni X, Mao Z, Kuang T, Zang YQ, Qin X (2012) A phase I pilot trial of MUC1-peptide-pulsed dendritic cells in the treatment of advanced pancreatic cancer. Clin Exp Med 12:173–180. https://doi.org/10.1007/s10238-011-0159-0
Lau SP, Klaase L, Vink M, Dumas J, Bezemer K, van Krimpen A, van der Breggen R, Wismans LV, Doukas M, de Koning W, Stubbs AP, Mustafa DAM, Vroman H, Stadhouders R, Nunes JB, Stingl C, de Miranda NFCC, Luider TM, van der Burg SH, Aerts JG, van Eijck CHJ (1990) Autologous dendritic cells pulsed with allogeneic tumour cell lysate induce tumour-reactive T-cell responses in patients with pancreatic cancer: a phase I study. Eur J Cancer (Oxford, England) 169(2022):20–31. https://doi.org/10.1016/j.ejca.2022.03.015
Hirooka Y, Kawashima H, Ohno E, Ishikawa T, Kamigaki T, Goto S, Takahara M, Goto H (2018) Comprehensive immunotherapy combined with intratumoral injection of zoledronate-pulsed dendritic cells, intravenous adoptive activated T lymphocyte and gemcitabine in unresectable locally advanced pancreatic carcinoma: a phase I/II trial. Oncotarget 9:2838–2847. https://doi.org/10.18632/oncotarget.2297
Kimura Y, Tsukada J, Tomoda T, Takahashi H, Imai K, Shimamura K, Sunamura M, Yonemitsu Y, Shimodaira S, Koido S, Homma S, Okamoto M (2012) Clinical and immunologic evaluation of dendritic cell-based immunotherapy in combination with gemcitabine and/or S-1 in patients with advanced pancreatic carcinoma. Pancreas 41:195–205. https://doi.org/10.1097/MPA.0b013e31822398c6
Mehrotra S, Britten CD, Chin S, Garrett-Mayer E, Cloud CA, Li M, Scurti G, Salem ML, Nelson MH, Thomas MB, Paulos CM, Salazar AM, Nishimura MI, Rubinstein MP, Li Z, Cole DJ (2017) Vaccination with poly(IC:LC) and peptide-pulsed autologous dendritic cells in patients with pancreatic cancer. J Hematol Oncol 10:82. https://doi.org/10.1186/s13045-017-0459-2
Bassani-Sternberg M, Digklia A, Huber F, Wagner D, Sempoux C, Stevenson BJ, Thierry AC, Michaux J, Pak H, Racle J, Boudousquie C, Balint K, Coukos G, Gfeller D, Lluesma SM, Harari A, Demartines N, Kandalaft LE (2019) A phase Ib study of the combination of personalized autologous dendritic cell vaccine, aspirin, and standard of care adjuvant chemotherapy followed by nivolumab for resected pancreatic adenocarcinoma-a proof of antigen discovery feasibility in three patients. Front Immunol 10:1832. https://doi.org/10.3389/fimmu.2019.01832
Chen Z, Zhang S, Han N, Jiang J, Xu Y, Ma D, Lu L, Guo X, Qiu M, Huang Q, Wang H, Mo F, Chen S, Yang L (2021) A neoantigen-based peptide vaccine for patients with advanced pancreatic cancer refractory to standard treatment. Front Immunol 12:691605. https://doi.org/10.3389/fimmu.2021.691605
Koido S, Homma S, Okamoto M, Takakura K, Mori M, Yoshizaki S, Tsukinaga S, Odahara S, Koyama S, Imazu H, Uchiyama K, Kajihara M, Arakawa H, Misawa T, Toyama Y, Yanagisawa S, Ikegami M, Kan S, Hayashi K, Komita H, Kamata Y, Ito M, Ishidao T, Yusa S-I, Shimodaira S, Gong J, Sugiyama H, Ohkusa T, Tajiri H (2014) Treatment with chemotherapy and dendritic cells pulsed with multiple Wilms’ tumor 1 (WT1)-specific MHC class I/II-restricted epitopes for pancreatic cancer. Clin Cancer Res 20:4228–4239. https://doi.org/10.1158/1078-0432.CCR-14-0314
Koido S, Homma S, Okamoto M, Takakura K, Gong J, Sugiyama H, Ohkusa T, Tajiri H (2014) Chemoimmunotherapy targeting Wilms’ tumor 1 (WT1)-specific cytotoxic T lymphocyte and helper T cell responses for patients with pancreatic cancer. Oncoimmunology 3:e958950
Yanagisawa R, Koizumi T, Koya T, Sano K, Koido S, Nagai K, Kobayashi M, Okamoto M, Sugiyama H, Shimodaira S (2018) WT1-pulsed dendritic cell vaccine combined with chemotherapy for resected pancreatic cancer in a phase I study. Anticancer Res 38:2217–2225
Asahara S, Takeda K, Yamao K, Maguchi H, Yamaue H (2013) Phase I/II clinical trial using HLA-A24-restricted peptide vaccine derived from KIF20A for patients with advanced pancreatic cancer. J Transl Med 11:291. https://doi.org/10.1186/1479-5876-11-291
Shima H, Tsurita G, Wada S, Hirohashi Y, Yasui H, Hayashi H, Miyakoshi T, Watanabe K, Murai A, Asanuma H, Tokita S, Kubo T, Nakatsugawa M, Kanaseki T, Tsukahara T, Nakae Y, Sugita O, Ito YM, Ota Y, Kimura Y, Kutomi G, Hirata K, Mizuguchi T, Imai K, Takemasa I, Sato N, Torigoe T (2019) Randomized phase II trial of survivin 2B peptide vaccination for patients with HLA-A24-positive pancreatic adenocarcinoma. Cancer Sci 110:2378–2385. https://doi.org/10.1111/cas.14106
Miyazawa M, Katsuda M, Maguchi H, Katanuma A, Ishii H, Ozaka M, Yamao K, Imaoka H, Kawai M, Hirono S, Okada K-I, Yamaue H (2017) Phase II clinical trial using novel peptide cocktail vaccine as a postoperative adjuvant treatment for surgically resected pancreatic cancer patients. Int J Cancer 140:973–982. https://doi.org/10.1002/ijc.30510
Hardacre JM, Mulcahy M, Small W, Talamonti M, Obel J, Krishnamurthi S, Rocha-Lima CS, Safran H, Lenz H-J, Chiorean EG (2013) Addition of algenpantucel-L immunotherapy to standard adjuvant therapy for pancreatic cancer: a phase 2 study. J Gastrointest Surg Official J Soc Surg Aliment Tract 17:6. https://doi.org/10.1007/s11605-012-2064-6
Hewitt DB, Nissen N, Hatoum H, Musher B, Seng J, Coveler AL, Al-Rajabi R, Yeo CJ, Leiby B, Banks J, Balducci L, Vaccaro G, LoConte N, George TJ, Brenner W, Elquza E, Vahanian N, Rossi G, Kennedy E, Link C, Lavu H (2022) A phase 3 randomized clinical trial of chemotherapy with or without algenpantucel-L (hyperacute-pancreas) immunotherapy in subjects with borderline resectable or locally advanced unresectable pancreatic cancer. Ann Surg 275:45–53. https://doi.org/10.1097/SLA.0000000000004669
Vonderheide RH, Kraynyak KA, Shields AF, McRee AJ, Johnson JM, Sun W, Chintakuntlawar AV, Pawlicki J, Sylvester AJ, McMullan T, Samuels R, Kim JJ, Weiner D, Boyer JD, Morrow MP, Humeau L, Skolnik JM (2021) Phase 1 study of safety, tolerability and immunogenicity of the human telomerase (hTERT)-encoded DNA plasmids INO-1400 and INO-1401 with or without IL-12 DNA plasmid INO-9012 in adult patients with solid tumors. J Immunother Cancer 9:3019. https://doi.org/10.1136/jitc-2021-003019
Rojas LA, Sethna Z, Soares KC, Olcese C, Pang N, Patterson E, Lihm J, Ceglia N, Guasp P, Chu A, Yu R, Chandra AK, Waters T, Ruan J, Amisaki M, Zebboudj A, Odgerel Z, Payne G, Derhovanessian E, Müller F, Rhee I, Yadav M, Dobrin A, Sadelain M, Łuksza M, Cohen N, Tang L, Basturk O, Gönen M, Katz S, Do RK, Epstein AS, Momtaz P, Park W, Sugarman R, Varghese AM, Won E, Desai A, Wei AC, D’Angelica MI, Kingham TP, Mellman I, Merghoub T, Wolchok JD, Sahin U, Türeci Ö, Greenbaum BD, Jarnagin WR, Drebin J, O’Reilly EM, Balachandran VP (2023) Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 618:144–150. https://doi.org/10.1038/s41586-023-06063-y
Huff AL, Zaidi N (2023) Vaccine boosts T cells that target pancreatic tumours. Nature 618:37–38. https://doi.org/10.1038/d41586-023-01526-8
Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L (2020) “Off-the-shelf” allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov 19:185–199. https://doi.org/10.1038/s41573-019-0051-2
Ramos CA, Dotti G (2011) Chimeric antigen receptor (CAR)-engineered lymphocytes for cancer therapy. Expert Opin Biol Ther 11:855–873. https://doi.org/10.1517/14712598.2011.573476
Glumac PM, LeBeau AM (2018) The role of CD133 in cancer: a concise review. Clin Transl Med 7:18. https://doi.org/10.1186/s40169-018-0198-1
Wang Y, Chen M, Wu Z, Tong C, Dai H, Guo Y, Liu Y, Huang J, Lv H, Luo C, Feng K-C, Yang Q-M, Li X-L, Han W (2018) CD133-directed CAR T cells for advanced metastasis malignancies: A phase I trial. Oncoimmunology 7:e1440169. https://doi.org/10.1080/2162402X.2018.1440169
Beatty GL, O’Hara MH, Lacey SF, Torigian DA, Nazimuddin F, Chen F, Kulikovskaya IM, Soulen MC, McGarvey M, Nelson AM, Gladney WL, Levine BL, Melenhorst JJ, Plesa G, June CH (2018) Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenterology 155:29–32. https://doi.org/10.1053/j.gastro.2018.03.029
Haas AR, Tanyi JL, O’Hara MH, Gladney WL, Lacey SF, Torigian DA, Soulen MC, Tian L, McGarvey M, Nelson AM, Farabaugh CS, Moon E, Levine BL, Melenhorst JJ, Plesa G, June CH, Albelda SM, Beatty GL (2019) Phase I study of lentiviral-transduced chimeric antigen receptor-modified T Cells recognizing mesothelin in advanced solid cancers. Mol Therapy J Am Soc Gene Therapy 27:1919–1929. https://doi.org/10.1016/j.ymthe.2019.07.015
Feng K, Liu Y, Guo Y, Qiu J, Wu Z, Dai H, Yang Q, Wang Y, Han W (2018) Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers. Protein Cell 9:838–847. https://doi.org/10.1007/s13238-017-0440-4
Liu Y, Guo Y, Wu Z, Feng K, Tong C, Wang Y, Dai H, Shi F, Yang Q, Han W (2020) Anti-EGFR chimeric antigen receptor-modified T cells in metastatic pancreatic carcinoma: a phase I clinical trial. Cytotherapy 22:573–580. https://doi.org/10.1016/j.jcyt.2020.04.088
Lum LG, Thakur A, Choi M, Deol A, Kondadasula V, Schalk D, Fields K, Dufrense M, Philip P, Dyson G, Aon HD, Shields AF (2020) Clinical and immune responses to anti-CD3 x anti-EGFR bispecific antibody armed activated T cells (EGFR BATs) in pancreatic cancer patients. Oncoimmunology 9:1773201. https://doi.org/10.1080/2162402x.2020.1773201
Daher M, Rezvani K (2021) Outlook for new CAR-based therapies with a focus on CAR NK cells: what lies beyond CAR-engineered T cells in the race against cancer. Cancer Discov 11:45–58. https://doi.org/10.1158/2159-8290.CD-20-0556
Klichinsky M, Ruella M, Shestova O, Lu XM, Best A, Zeeman M, Schmierer M, Gabrusiewicz K, Anderson NR, Petty NE, Cummins KD, Shen F, Shan X, Veliz K, Blouch K, Yashiro-Ohtani Y, Kenderian SS, Kim MY, O’Connor RS, Wallace SR, Kozlowski MS, Marchione DM, Shestov M, Garcia BA, June CH, Gill S (2020) Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol 38:947–953. https://doi.org/10.1038/s41587-020-0462-y
Zheng L, Qin S, Si W, Wang A, Xing B, Gao R, Ren X, Wang L, Wu X, Zhang J, Wu N, Zhang N, Zheng H, Ouyang H, Chen K, Bu Z, Hu X, Ji J, Zhang Z (2021) Pan-cancer single-cell landscape of tumor-infiltrating T cells. Science 374:abe6474. https://doi.org/10.1126/science.abe6474
Han J, DePinho RA, Maitra A (2021) Single-cell RNA sequencing in pancreatic cancer. Nat Rev Gastroenterol Hepatol 18:451–452. https://doi.org/10.1038/s41575-021-00471-z
Mahajan UM, Langhoff E, Goni E, Costello E, Greenhalf W, Halloran C, Ormanns S, Kruger S, Boeck S, Ribback S, Beyer G, Dombroswki F, Weiss FU, Neoptolemos JP, Werner J, D’Haese JG, Bazhin A, Peterhansl J, Pichlmeier S, Büchler MW, Kleeff J, Ganeh P, Sendler M, Palmer DH, Kohlmann T, Rad R, Regel I, Lerch MM, Mayerle J (2018) Immune cell and stromal signature associated with progression-free survival of patients with resected pancreatic ductal adenocarcinoma. Gastroenterology 155:1625-1639.e1622. https://doi.org/10.1053/j.gastro.2018.08.009
Sadozai H, Acharjee A, Eppenberger-Castori S, Gloor B, Gruber T, Schenk M, Karamitopoulou E (2021) Distinct stromal and immune features collectively contribute to long-term survival in pancreatic cancer. Front Immunol 12:643529. https://doi.org/10.3389/fimmu.2021.643529
Wartenberg M, Cibin S, Zlobec I, Vassella E, Eppenberger-Castori S, Terracciano L, Eichmann MD, Worni M, Gloor B, Perren A, Karamitopoulou E (2018) Integrated genomic and immunophenotypic classification of pancreatic cancer reveals three distinct subtypes with prognostic/predictive significance. Clin Cancer Res 24:4444–4454. https://doi.org/10.1158/1078-0432.Ccr-17-3401
de Santiago I, Yau C, Heij L, Middleton MR, Markowetz F, Grabsch HI, Dustin ML, Sivakumar S (2019) Immunophenotypes of pancreatic ductal adenocarcinoma: meta-analysis of transcriptional subtypes. Int J Cancer 145:1125–1137. https://doi.org/10.1002/ijc.32186
Montagne JM, Jaffee EM, Fertig EJ (2023) Multiomics empowers predictive pancreatic cancer immunotherapy. J Immunol 210:859–868. https://doi.org/10.4049/jimmunol.2200660
Ahmed J, Chard LS, Yuan M, Wang J, Howells A, Li Y, Li H, Zhang Z, Lu S, Gao D, Wang P, Chu Y, Al Yaghchi C, Schwartz J, Alusi G, Lemoine N, Wang Y (2020) A new oncolytic Vacciniavirus augments antitumor immune responses to prevent tumor recurrence and metastasis after surgery. J Immunother Cancer 8:415. https://doi.org/10.1136/jitc-2019-000415
Chen X, Ma H, Mo S, Zhang Y, Lu Z, Yu S, Chen J (2022) Analysis of the OX40/OX40L immunoregulatory axis combined with alternative immune checkpoint molecules in pancreatic ductal adenocarcinoma. Front Immunol 13:942154. https://doi.org/10.3389/fimmu.2022.942154
Lau SP, van Montfoort N, Kinderman P, Lukkes M, Klaase L, van Nimwegen M, van Gulijk M, Dumas J, Mustafa DAM, Lievense SLA, Groeneveldt C, Stadhouders R, Li Y, Stubbs A, Marijt KA, Vroman H, van der Burg SH, Aerts J, van Hall T, Dammeijer F, van Eijck CHJ (2020) Dendritic cell vaccination and CD40-agonist combination therapy licenses T cell-dependent antitumor immunity in a pancreatic carcinoma murine model. J Immunother Cancer 8:772. https://doi.org/10.1136/jitc-2020-000772
Muth ST, Saung MT, Blair AB, Henderson MG, Thomas DL, Zheng L (2021) CD137 agonist-based combination immunotherapy enhances activated, effector memory T cells and prolongs survival in pancreatic adenocarcinoma. Cancer Lett 499:99–108. https://doi.org/10.1016/j.canlet.2020.11.041
Blair AB, Wang J, Davelaar J, Baker A, Li K, Niu N, Wang J, Shao Y, Funes V, Li P, Pachter JA, Maneval DC, Dezem F, Plummer J, Chan KS, Gong J, Hendifar AE, Pandol SJ, Burkhart R, Zhang Y, Zheng L, Osipov A (2022) Dual stromal targeting sensitizes pancreatic adenocarcinoma for anti-programmed cell death protein 1 therapy. Gastroenterology 163:1267-1280.e1267. https://doi.org/10.1053/j.gastro.2022.06.027
Xiao Z, Todd L, Huang L, Noguera-Ortega E, Lu Z, Huang L, Kopp M, Li Y, Pattada N, Zhong W, Guo W, Scholler J, Liousia M, Assenmacher CA, June CH, Albelda SM, Puré E (2023) Desmoplastic stroma restricts T cell extravasation and mediates immune exclusion and immunosuppression in solid tumors. Nat Commun 14:5110. https://doi.org/10.1038/s41467-023-40850-5
Fujisawa T, Tsuchiya T, Kato M, Mizuide M, Takakura K, Nishimura M, Kutsumi H, Matsuda Y, Arai T, Ryozawa S, Itoi T, Isayama H, Saya H, Yahagi N (2023) STNM01, the RNA oligonucleotide targeting carbohydrate sulfotransferase 15, as second-line therapy for chemotherapy-refractory patients with unresectable pancreatic cancer: an open label, phase I/IIa trial. EClinical Medicine 55:101731. https://doi.org/10.1016/j.eclinm.2022.101731
Kinker GS, Vitiello GAF, Diniz AB, Cabral-Piccin MP, Pereira PHB, Carvalho MLR, Ferreira WAS, Chaves AS, Rondinelli A, Gusmão AF, Defelicibus A, Dos Santos GO, Nunes WA, Claro LCL, Bernardo TM, Nishio RT, Pacheco AM, Laus AC, Arantes L, Fleck JL, de Jesus VHF, de Moricz A, Weinlich R, Coimbra FJF, de Lima VCC, Medina TDS (2023) Mature tertiary lymphoid structures are key niches of tumour-specific immune responses in pancreatic ductal adenocarcinomas. Gut 72:1927–1941. https://doi.org/10.1136/gutjnl-2022-328697
Mirji G, Worth A, Bhat SA, El Sayed M, Kannan T, Goldman AR, Tang HY, Liu Q, Auslander N, Dang CV, Abdel-Mohsen M, Kossenkov A, Stanger BZ, Shinde RS (2022) The microbiome-derived metabolite TMAO drives immune activation and boosts responses to immune checkpoint blockade in pancreatic cancer. Sci Immunol 7:704. https://doi.org/10.1126/sciimmunol.abn0704
Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, Dong W, Quesada P, Sahin I, Chandra V, San Lucas A, Scheet P, Xu H, Hanash SM, Feng L, Burks JK, Do KA, Peterson CB, Nejman D, Tzeng CD, Kim MP, Sears CL, Ajami N, Petrosino J, Wood LD, Maitra A, Straussman R, Katz M, White JR, Jenq R, Wargo J, McAllister F (2019) Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell 178:795–806. https://doi.org/10.1016/j.cell.2019.07.008
Yu Q, Newsome RC, Beveridge M, Hernandez MC, Gharaibeh RZ, Jobin C, Thomas RM (2022) Intestinal microbiota modulates pancreatic carcinogenesis through intratumoral natural killer cells. Gut Microbes 14:2112881. https://doi.org/10.1080/19490976.2022.2112881
Funding
This work was supported by the National Natural Science Foundation of China (#82171783, # 81871284, # 82241216, # 32270963, # 31870993), Research Funds of Center for Advanced Interdisciplinary Science and Biomedicine of IHM (Reference Numbers: QYZD20220008), Anhui key research and development plan (Reference Numbers: 2023z04020011), and the Fundamental Research Funds for the Central Universities (WK9110000005).
Author information
Authors and Affiliations
Contributions
HM and XY wrote the manuscript; YY and XZ provided important advice and suggestions; HM and XZ supervised the writing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ye, X., Yu, Y., Zheng, X. et al. Clinical immunotherapy in pancreatic cancer. Cancer Immunol Immunother 73, 64 (2024). https://doi.org/10.1007/s00262-024-03632-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00262-024-03632-6