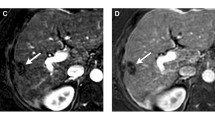
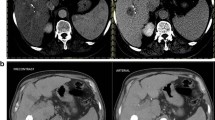
Abstract
Background
The Liver Imaging Reporting and Data System (LI-RADS) Treatment Response Algorithm (TRA) (LI-RADS TRA) is used for assessing response of HCC to locoregional therapy (LRT), however, the value of ancillary features (AFs) for TACE-treated HCCs has not been extensively investigated on extracellular agent MRI (ECA-MRI).
Purpose
To evaluate the diagnostic performance of LI-RADS v2018 TRA on ECA-MRI for HCC treated with transarterial chemoembolization (TACE) and the value of ancillary features.
Methods
This retrospective study included patients who underwent TACE for HCC and then followed by hepatic surgery between January 2019 and June 2023 with both pre- and post-TACE contrast-enhanced MRI available. Two radiologists independently evaluated the post-treated lesions on MRI using LI-RADS treatment response (TR) (LR-TR) algorithm and modified LR-TR (mLR-TR) algorithm in which ancillary features (restricted diffusion and intermediate T2-weighted hyperintensity) were added, respectively. Lesions were categorized as complete pathologic necrosis (100%, CPN) and non-complete pathologic necrosis (< 100%, non-CPN) on the basis of surgical pathology. The diagnostic performance in predicting viable and non-viable tumors based on LR-TR and mLR-TR algorithms was compared using the McNemar test. Interreader agreement was calculated by using Cohen’s weighted and unweighted κ.
Results
A total of 61 patients [mean age 59 years ± 10 (standard deviation); 47 men] with 79 lesions (57 pathologically viable) were included. For non-CPN prediction, the sensitivity, specificity of LR-TR viable and mLR-TR viable category were 75% (43 of 57), 82% (18 of 22) and 88% (50 of 57), 77% (17 of 22), respectively, the sensitivity of mLR-TR was significantly higher than that of LR-TR (P = 0.016) without difference in specificity (P = 1.000). Interreader agreement for LR-TR and mLR-TR category was moderate (k = 0.50, 95% confidence interval 0.33, 0.67, k = 0.42, 95% confidence interval 0.20, 0.63). The sensitivity of both LR-TR and mLR-TR algorithms in predicting viable tumors between conventional TACE (cTACE) and drug-eluting beads TACE (DEB-TACE) did not have significant difference (cTACE: 76%, 89% vs. DEB-TACE: 73%, 82%).
Conclusions
On ECA-MRI, applying ancillary features to LI-RADS v2018 TRA can improve the sensitivity in predicting pathologic tumor viability in patients treated with TACE for hepatocellular carcinoma with no significant difference in specificity.



Similar content being viewed by others
Abbreviations
- AF:
-
Ancillary feature
- APHE:
-
Arterial phase hyperenhancement
- cTACE:
-
Conventional TACE
- CPN:
-
Complete pathologic necrosis
- DEB-TACE:
-
Drug-eluting beads TACE
- ECA:
-
Extracellular contrast agent
- HCC:
-
Hepatocellular carcinoma
- LI-RADS:
-
Liver Imaging Reporting and Data System
- LRT:
-
Locoregional therapy
- LR-TR:
-
LI-RADS treatment response
- NMLIT:
-
Nodular, masslike, or irregular thick tissue in or along the treated lesions
- TACE:
-
Transarterial chemoembolization
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021 May;71(3):209–249. https://doi.org/10.3322/caac.21660
Kielar, A., Fowler, K. J., Lewis, S., Yaghmai, V., Miller, F. H., Yarmohammadi, H., Kim, C., Chernyak, V., Yokoo, T., Meyer, J., Newton, I., & Do, R. K. (2018). Locoregional therapies for hepatocellular carcinoma and the new LI-RADS treatment response algorithm. Abdominal Radiology (New York), 43(1), 218–230. https://doi.org/https://doi.org/10.1007/s00261-017-1281-6
Reig, M., Forner, A., Rimola, J., Ferrer-Fàbrega, J., Burrel, M., Garcia-Criado, Á., Kelley, R. K., Galle, P. R., Mazzaferro, V., Salem, R., Sangro, B., Singal, A. G., Vogel, A., Fuster, J., Ayuso, C., & Bruix, J. (2022). BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. Journal of Hepatology, 76(3), 681–693. https://doi.org/https://doi.org/10.1016/j.jhep.2021.11.018
Chernyak V, Sirlin CB (eds) (2018) The LI-RADS® v2018 Manual. American College of Radiology Committee on LI-RADS®. American College of Radiology, Virginia. https://www.acr.org/-/media/ACR/Files/Clinical-Resources/LIRADS/LI-RADS-2018-Manual-5Dec18.pdf
Shropshire, E. L., Chaudhry, M., Miller, C. M., Allen, B. C., Bozdogan, E., Cardona, D. M., King, L. Y., Janas, G. L., Do, R. K., Kim, C. Y., Ronald, J., & Bashir, M. R. (2019). LI-RADS Treatment Response Algorithm: Performance and Diagnostic Accuracy. Radiology, 292(1), 226–234. https://doi.org/https://doi.org/10.1148/radiol.2019182135
Mendiratta-Lala, M., Aslam, A., Maturen, K. E., Westerhoff, M., Maurino, C., Parikh, N. D., Sun, Y., Sonnenday, C. J., Stein, E. B., Shampain, K. L., Kaza, R. K., Cuneo, K., Masch, W., Do, R. K. G., Lawrence, T. S., & Owen, D. (2022). LI-RADS Treatment Response Algorithm: Performance and Diagnostic Accuracy with Radiologic–Pathologic Explant Correlation in Patients with SBRT-Treated Hepatocellular Carcinoma. International Journal of Radiation Oncology, Biology, Physics, 112(3), 704–714. https://doi.org/https://doi.org/10.1016/j.ijrobp.2021.10.006
Chaudhry, M., McGinty, K. A., Mervak, B., Lerebours, R., Li, C., Shropshire, E., Ronald, J., Commander, L., Hertel, J., Luo, S., Bashir, M. R., & Burke, L. M. B. (2020). The LI-RADS Version 2018 MRI Treatment Response Algorithm: Evaluation of Ablated Hepatocellular Carcinoma. Radiology, 294(2), 320–326. https://doi.org/https://doi.org/10.1148/radiol.2019191581
Park, S., Joo, I., Lee, D. H., Bae, J. S., Yoo, J., Kim, S. W., & Lee, J. M. (2020). Diagnostic Performance of LI-RADS Treatment Response Algorithm for Hepatocellular Carcinoma: Adding Ancillary Features to MRI Compared with Enhancement Patterns at CT and MRI. Radiology, 296(3), 554–561. https://doi.org/https://doi.org/10.1148/radiol.2020192797
Kim, S. W., Joo, I., Kim, H. C., Ahn, S. J., Kang, H. J., Jeon, S. K., & Lee, J. M. (2020). LI-RADS treatment response categorization on gadoxetic acid-enhanced MRI: diagnostic performance compared to mRECIST and added value of ancillary features. European Radiology, 30(5), 2861–2870. https://doi.org/https://doi.org/10.1007/s00330-019-06623-9
Kim, Y. Y., Kim, M. J., Yoon, J. K., Shin, J., & Roh, Y. H. (2022). Incorporation of Ancillary MRI Features into the LI-RADS Treatment Response Algorithm: Impact on Diagnostic Performance After Locoregional Treatment of Hepatocellular Carcinoma. American Journal of Roentgenology, 218(3), 484–493. https://doi.org/https://doi.org/10.2214/AJR.21.26677
Kierans, A. S., Najjar, M., Dutruel, S. P., Gavlin, A., Chen, C., Lee, M. J., Askin, G., & Halazun, K. J. (2021). Evaluation of the LI-RADS treatment response algorithm in hepatocellular carcinoma after trans-arterial chemoembolization. Clinical Imaging, 80, 117–122. https://doi.org/https://doi.org/10.1016/j.clinimag.2021.06.009
Motosugi, U., Bannas, P., Bookwalter, C. A., Sano, K., & Reeder, S. B. (2016). An Investigation of Transient Severe Motion Related to Gadoxetic Acid-Enhanced MR Imaging. Radiology, 279(1), 93–102. https://doi.org/https://doi.org/10.1148/radiol.2015150642
Huh, Y. J., Kim, D. H., Kim, B., Choi, J. I., & Rha, S. E. (2021). Per-Feature Accuracy of Liver Imaging Reporting and Data System Locoregional Treatment Response Algorithm: A Systematic Review and Meta-Analysis. Cancers, 13(17), 4432. https://doi.org/https://doi.org/10.3390/cancers13174432
Bae, J. S., Lee, J. M., Yoon, J. H., Kang, H. J., Jeon, S. K., Joo, I., Lee, K. B., & Kim, H. (2021). Evaluation of LI-RADS Version 2018 Treatment Response Algorithm for Hepatocellular Carcinoma in Liver Transplant Candidates: Intraindividual Comparison between CT and Hepatobiliary Agent-Enhanced MRI. Radiology, 299(2), 336–345. https://doi.org/https://doi.org/10.1148/radiol.2021203537
Polikoff, A., Wessner, C. E., Balasubramanya, R., Dulka, S., Liu, J. B., Machado, P., Savsani, E., Lyshchik, A., Shaw, C. M., & Eisenbrey, J. R. (2022). Imaging appearance of residual HCC following incomplete trans-arterial chemoembolization on contrast-enhanced imaging. Abdominal Radiology (New York), 47(1), 152–160. https://doi.org/https://doi.org/10.1007/s00261-021-03298-z
Seo, N., Kim, M. S., Park, M. S., Choi, J. Y., Do, R. K. G., Han, K., & Kim, M. J. (2020). Evaluation of treatment response in hepatocellular carcinoma in the explanted liver with Liver Imaging Reporting and Data System version 2017. European Radiology, 30(1), 261–271. https://doi.org/https://doi.org/10.1007/s00330-019-06376-5
Kim TH, Woo S, Joo I, Bashir MR, Park MS, Burke LMB, Mendiratta-Lala M, Do RKG. LI-RADS treatment response algorithm for detecting incomplete necrosis in hepatocellular carcinoma after locoregional treatment: a systematic review and meta-analysis using individual patient data. Abdom Radiol (NY). 2021 Aug;46(8):3717–3728. doi: https://doi.org/10.1007/s00261-021-03122-8
Voizard, N., Cerny, M., Assad, A., Billiard, J. S., Olivié, D., Perreault, P., Kielar, A., Do, R. K. G., Yokoo, T., Sirlin, C. B., & Tang, A. (2019). Assessment of hepatocellular carcinoma treatment response with LI-RADS: a pictorial review. Insights into Imaging, 10(1), 121. https://doi.org/https://doi.org/10.1186/s13244-019-0801-z
Golfieri, R., Giampalma, E., Renzulli, M., Cioni, R., Bargellini, I., Bartolozzi, C., Breatta, A. D., Gandini, G., Nani, R., Gasparini, D., Cucchetti, A., Bolondi, L., Trevisani, F., & Precision Italia Study Group (2014). Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. British Journal of Cancer, 111(2), 255–264. https://doi.org/10.1038/bjc.2014.199
Kwan, S. W., Fidelman, N., Ma, E., Kerlan, R. K., Jr, & Yao, F. Y. (2012). Imaging predictors of the response to transarterial chemoembolization in patients with hepatocellular carcinoma: a radiological–pathological correlation. Liver Transplantation: Official Publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society, 18(6), 727–736. https://doi.org/https://doi.org/10.1002/lt.23413
Funding
This study was funded by Shanghai 2022 “Science and Technology Innovation Action Plan” Medical Innovation Research Special Project (Grant Number 22Y11910900), and Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-074C).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval was waived by the Local Ethics Committee of Zhongshan Hospital Fudan University in view of the retrospective nature of the study and all the procedures being performed were part of the routine care.
Informed consent
The Institutional Review Board approved this study and waived informed consent because of retrospective study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, D., Zhang, Y., Lyu, R. et al. LI-RADS version 2018 treatment response algorithm on extracellular contrast-enhanced MRI in patients treated with transarterial chemoembolization for hepatocellular carcinoma: diagnostic performance and the added value of ancillary features. Abdom Radiol (2024). https://doi.org/10.1007/s00261-024-04275-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00261-024-04275-y