Abstract
Objectives
To prospectively assess the utility of transabdominal ultrasound in surveillance of known pancreatic cystic lesions (PCL) using same day MRI as reference standard.
Methods
In an IRB-approved study with written informed consent, patients with known PCL underwent pancreas US on same day as surveillance MRI. US was performed blinded to same date MRI results. Transverse (TR), antero-posterior (AP), cranio-caudal (CC), and longest any plane diameter, were measured for each PCL at US and MRI. Visualization was correlated with patient (weight, abdominal diameter, thickness of abdominal fat, sex) and cyst (location, size, internal complexity) factors.
Results
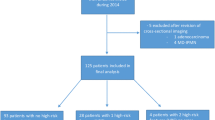
252 PCLs evaluated in 57 subjects (39 females; mean age 67 (range 39–86) yrs). Mean maximum PCL diameter 8.5 (range 2–92) mm. US identified 100% (5/5) of cysts ≥3 cm; 92% (12/13) ≥2 and <3 cm; 78% (43/55) ≥1 and <2 cm; 35% (27/78) ≥5 mm and <1 cm; and 16% (16/101) <5 mm. US visualization correlated with PCL location (<0.0001), size (p < 0.0001), patient gender (p = 0.005), participation of attending radiologist (p = 0.03); inversely with patient weight (p = 0.012) and AP abdominal diameter (p = 0.01).
Conclusion
Many PCLs are visualized and accurately measured at follow-up with transabdominal ultrasound. Visualization correlates with lesion size, location, patient sex, weight, and abdominal diameter.








Similar content being viewed by others
References
Laffan TA, Horton KM, Klein AP, et al. (2008) Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol 191(3):802–807
Lee KS, Sekhar A, Rofsky NM, Pedrosa I (2010) Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol 105(9):2079–2084
Ahn DW, Lee SH, Kim J, et al. (2012) Long-term outcome of cystic lesions in the pancreas: a retrospective cohort study. Gut Liver 6(4):493–500
Tanaka M, Fernandez-del Castillo C, Adsay V, et al. (2012) International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 12(3):183–197
Tanaka M, Chari S, Adsay V, et al. (2006) International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 6(1–2):17–32
Abbott DE, Ahmad SA (2014) Comparison of the Sendai and Fukuoka consensus guidelines for the management of mucinous cystic lesions of the pancreas: are we making progress? Ann Surg Oncol 21(6):1770–1772
Lee SH, Shin CM, Park JK, et al. (2007) Outcomes of cystic lesions in the pancreas after extended follow-up. Dig Dis Sci 52(10):2653–2659
Maguchi H, Tanno S, Mizuno N, et al. (2011) Natural history of branch duct intraductal papillary mucinous neoplasms of the pancreas: a multicenter study in Japan. Pancreas 40(3):364–370
Khannoussi W, Vullierme MP, Rebours V, et al. (2012) The long term risk of malignancy in patients with branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreatology 12(3):198–202
Handrich SJ, Hough DM, Fletcher JG, Sarr MG (2005) The natural history of the incidentally discovered small simple pancreatic cyst: long-term follow-up and clinical implications. AJR Am J Roentgenol 184(1):20–23
Lee CJ, Scheiman J, Anderson MA, et al. (2008) Risk of malignancy in resected cystic tumors of the pancreas < or =3 cm in size: is it safe to observe asymptomatic patients? A multi-institutional report. J Gastrointest Surg 12(2):234–242
Wu BU, Sampath K, Berberian CE, et al. (2014) Prediction of malignancy in cystic neoplasms of the pancreas: a population-based cohort study. Am J Gastroenterol 109(1):121–129; quiz 30.
Brook OR, Beddy P, Pahade J, et al. (2015) Delayed growth in incidental pancreatic cysts: are the current American College of Radiology recommendations for follow-up appropriate? Radiology 278(3):752–761
Pinho DF, Rofsky NM, Pedrosa I (2014) Incidental pancreatic cysts: role of magnetic resonance imaging. Top Magn Reson Imaging 23(2):117–128
Del Chiaro M, Verbeke C, Salvia R, et al. (2013) European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis 45(9):703–711
Hruban RH, Takaori K, Klimstra DS, et al. (2004) An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol 28(8):977–987
Italian Association of Hospital G, Endoscopists, Italian Association for the Study of the Pancreas, et al. (2014) Italian consensus guidelines for the diagnostic work-up and follow-up of cystic pancreatic neoplasms. Dig Liver Dis 46(6):479–493
Society for Surgery of the Alimentary Tract. SSAT Patient Care Guidelines (2007) Cystic neoplasms of the pancreas. J Gastrointest Surg 11(9):1225–1227
Falconi M, Crippa S, Chari S, et al. (2015) Quality assessment of the guidelines on cystic neoplasms of the pancreas. Pancreatology 15(5):463–469
Budde C, Beyer G, Kuhn JP, Lerch MM, Mayerle J (2015) The clinical and socio-economic relevance of increased IPMN detection rates and management choices. Viszeralmedizin 31(1):47–52
Yu MH, Lee JY, Kim JH, Han JK, Choi BI (2013) Value of near-isovoxel ultrasound for evaluation of ductal communications with pancreatic cystic lesions: correlation with magnetic resonance cholangiopancreatography. Ultrasound Med Biol 39(12):2279–2284
Soroida Y, Sato M, Hikita H, et al. (2016) Pancreatic cysts in general population on ultrasonography: Prevalence and development of risk score. J Gastroenterol 51(12):1133–1140
Ikeda M, Sato T, Morozumi A, et al. (1994) Morphologic changes in the pancreas detected by screening ultrasonography in a mass survey, with special reference to main duct dilatation, cyst formation, and calcification. Pancreas 9(4):508–512
Das A, Wells CD, Nguyen CC (2008) Incidental cystic neoplasms of pancreas: what is the optimal interval of imaging surveillance? Am J Gastroenterol 103(7):1657–1662
Sumi H, Itoh A, Kawashima H, et al. (2014) Preliminary study on evaluation of the pancreatic tail observable limit of transabdominal ultrasonography using a position sensor and CT-fusion image. Eur J Radiol 83(8):1324–1331
Dunn D, Brook O, Brook A, et al. (2016) Measurement of pancreatic cystic lesions on magnetic resonance imaging: efficacy of standards in reducing inter-observer variability. Abdom Radiol 41(3):500–507
Megibow AJ, Baker ME, Morgan DE, et al. (2017) Management of incidental pancreatic cysts: a white paper of the ACR incidental findings committee. J Am Coll Radiol 14(7):911–923
Yamaguchi K, Ohuchida J, Ohtsuka T, Nakano K, Tanaka M (2002) Intraductal papillary-mucinous tumor of the pancreas concomitant with ductal carcinoma of the pancreas. Pancreatology 2(5):484–490
Yamaguchi K, Kanemitsu S, Hatori T, et al. (2011) Pancreatic ductal adenocarcinoma derived from IPMN and pancreatic ductal adenocarcinoma concomitant with IPMN. Pancreas 40(4):571–580
Tanno S, Nakano Y, Koizumi K, et al. (2010) Pancreatic ductal adenocarcinomas in long-term follow-up patients with branch duct intraductal papillary mucinous neoplasms. Pancreas 39(1):36–40
Tanaka M (2011) Controversies in the management of pancreatic IPMN. Nat Rev Gastroenterol Hepatol 8(1):56–60
Tanno S, Nakano Y, Sugiyama Y, et al. (2010) Incidence of synchronous and metachronous pancreatic carcinoma in 168 patients with branch duct intraductal papillary mucinous neoplasm. Pancreatology 10(2–3):173–178
Sofuni A, Itoi T, Itokawa F, et al. (2013) Real-time virtual sonography visualization and its clinical application in biliopancreatic disease. World J Gastroenterol 19(42):7419–7425
Fan Z, Yan K, Wang Y, et al. (2015) Application of contrast-enhanced ultrasound in cystic pancreatic lesions using a simplified classification diagnostic criterion. Biomed Res Int 2015:974621
D’Onofrio M, Biagioli E, Gerardi C, et al. (2014) Diagnostic performance of contrast-enhanced ultrasound (CEUS) and contrast-enhanced endoscopic ultrasound (ECEUS) for the differentiation of pancreatic lesions: a systematic review and meta-analysis. Ultraschall Med 35(6):515–521
Xu M, Xie XY, Liu GJ, et al. (2012) The application value of contrast-enhanced ultrasound in the differential diagnosis of pancreatic solid-cystic lesions. Eur J Radiol 81(7):1432–1437
Acknowledgements
The authors would like to acknowledge Laurie Sammons, RDMS and Lisa Napolitano, RDMS, for assistance with ultrasound examinations, as well as Lauren O’Loughlin, Bridget Giarusso, Amy Callahan, and Kelly Roth, for administrative support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No grants or funding were associated with this study.
Conflict of interest
There are no disclosures relevant to the content of this manuscript.
Appendices
Appendix 1
See Fig. 9.
US machines and transducers used. Initial scanning was performed with curvilinear array 5-1 MHz transducers on IU-22 units, and with C9-2 transducers on EPIQ units; additional linear array (L9-3 and L12-5) and sector (S4-1) transducers, could be subsequently utilized per operator discretion. US unit selection was based upon the site of service and availability of machines
Appendix 2
See Table 2.
Rights and permissions
About this article
Cite this article
Sun, M.R.M., Strickland, C.D., Tamjeedi, B. et al. Utility of transabdominal ultrasound for surveillance of known pancreatic cystic lesions: prospective evaluation with MRI as reference standard. Abdom Radiol 43, 1180–1192 (2018). https://doi.org/10.1007/s00261-017-1269-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-017-1269-2