Abstract
Background
In radioembolization, response is achieved through the irradiation and damaging of tumor DNA. For hepatic metastases of neuroendocrine tumors, a dose–response relationship has not been established yet. This study assesses whether increasing tumor-absorbed doses lead to increased response rates.
Methods
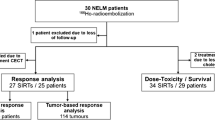
We included all patients who underwent yttrium-90 (90Y) glass microspheres radioembolization in our center if both pre- and post-treatment contrast-enhanced CT and post-injection PET/CT were available. Up to five hepatic tumors and the healthy hepatic tissue were delineated, and absorbed dose was quantified using post-injection PET/CT. Response was measured according to RECIST 1.1 on patient and tumor level. Linear mixed models were used to study the relationship between absorbed dose and response on tumor level. Logistic regression analysis was used on patient level to study dose–response and hepatic dose-toxicity relationships.
Results
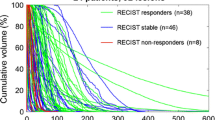
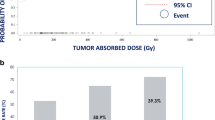
A total of 128 tumors in 26 patients (31 procedures) were included in the response analysis. While correcting for confounding by tumor volume, a significant effect of response on dose was found (p = 0.0465). Geometric mean of absorbed dose for responding tumors was 170 Gy, for stable disease 101 Gy, and for progressive disease 67 Gy. No significant dose-toxicity relationship could be identified.
Conclusion
In patients with neuroendocrine tumor liver metastases, treated with 90Y-radioembolization, a clear dose–response relationship was found. We propose to perform 90Y-radioembolization with an absolute minimum planned tumor-absorbed dose of 150 Gy.




Similar content being viewed by others
Data availability
The data that support the findings of this study and the code used to perform analyses are available from the corresponding author upon reasonable request.
References
Hermann AL, Dieudonné A, Ronot M, Sanchez M, Pereira H, Chatellier G, et al. Relationship of tumor radiation–absorbed dose to survival and response in hepatocellular carcinoma treated with transarterial radioembolization with 90Y in the SARAH study. Radiology. 2020;296:673–84. https://doi.org/10.1148/radiol.2020191606.
Alsultan AA, van Roekel C, Barentsz MW, Smits MLJ, Kunnen B, Koopman M, et al. Dose-response and dose-toxicity relationships for yttrium-90 glass radioembolization in patients with colorectal cancer liver metastases. J Nucl Med. 2021;jnumed.120.255745. https://doi.org/10.2967/jnumed.120.255745.
Chansanti O, Jahangiri Y, Matsui Y, Adachi A, Geeratikun Y, Kaufman JA, et al. Tumor dose response in yttrium-90 resin microsphere embolization for neuroendocrine liver metastases: a tumor-specific analysis with dose estimation using SPECT-CT. J Vasc Interv Radiol. 2017;28:1528–35. https://doi.org/10.1016/j.jvir.2017.07.008.
Cheng B, Villalobos A, Sethi I, Wagstaff W, Galt J, Brandon D, et al. Determination of tumor dose response thresholds in patients with chemorefractory intrahepatic cholangiocarcinoma treated with resin and glass-based Y90 radioembolization. Cardiovasc Intervent Radiol. 2021;44:1194–203. https://doi.org/10.1007/s00270-021-02834-0.
Barat M, Cottereau A-S, Kedra A, Dermine S, Palmieri L-J, Coriat R, et al. The role of interventional radiology for the treatment of hepatic metastases from neuroendocrine tumor: an updated review. J Clin Med. 2020;9:2302. https://doi.org/10.3390/jcm9072302.
Braat AJAT, Kappadath SC, Ahmadzadehfar H, Stothers CL, Frilling A, Deroose CM, et al. Radioembolization with 90Y resin microspheres of neuroendocrine liver metastases: international multicenter study on efficacy and toxicity. Cardiovasc Intervent Radiol. 2019;42:413–25. https://doi.org/10.1007/s00270-018-2148-0.
Parliament MB, Murray D. Single nucleotide polymorphisms of DNA repair genes as predictors of radioresponse. Semin Radiat Oncol. 2010;20:232–40. https://doi.org/10.1016/j.semradonc.2010.05.003.
Kappadath SC, Mikell J, Balagopal A, Baladandayuthapani V, Kaseb A, Mahvash A. Hepatocellular carcinoma tumor dose response after 90Y-radioembolization with glass microspheres using 90Y-SPECT/CT-based voxel dosimetry. Int J Radiat Oncol Biol Phys. 2018;102:451–61. https://doi.org/10.1016/j.ijrobp.2018.05.062.
Willowson KP, Eslick EM, Bailey DL. Individualised dosimetry and safety of SIRT for intrahepatic cholangiocarcinoma. EJNMMI Phys. 2021;8:65. https://doi.org/10.1186/s40658-021-00406-2.
Garin E, Tselikas L, Guiu B, Chalaye J, Edeline J, De Baere T, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6:17–29. https://doi.org/10.1016/S2468-1253(20)30290-9.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
Braat MNGJA, Van Erpecum KJ, Zonnenberg BA, Van Den Bosch MAJ, Lam MGEH. Radioembolization-induced liver disease: a systematic review. Eur J Gastroenterol Hepatol. 2017;29:144–52. https://doi.org/10.1097/MEG.0000000000000772.
Haste P, Tann M, Persohn S, LaRoche T, Aaron V, Mauxion T, et al. Correlation of technetium-99m macroaggregated albumin and yttrium-90 glass microsphere biodistribution in hepatocellular carcinoma: a retrospective review of pretreatment single photon emission CT and posttreatment positron emission tomography/CT. J Vasc Interv Radiol. 2017;28:722–730.e1. https://doi.org/10.1016/j.jvir.2016.12.1221.
Jadoul A, Bernard C, Lovinfosse P, Gérard L, Lilet H, Cornet O, et al. Comparative dosimetry between 99mTc-MAA SPECT/CT and 90Y PET/CT in primary and metastatic liver tumors. Eur J Nucl Med Mol Imaging. 2020;47:828–37. https://doi.org/10.1007/s00259-019-04465-7.
Gnesin S, Canetti L, Adib S, Cherbuin N, Silva Monteiro M, Bize P, et al. Partition model-based 99mTc-MAA SPECT/CT Predictive dosimetry compared with 90Y TOF PET/CT posttreatment dosimetry in radioembolization of hepatocellular carcinoma: a quantitative agreement comparison. J Nucl Med. 2016;57:1672–8. https://doi.org/10.2967/jnumed.116.173104.
Smits MLJ, Dassen MG, Prince JF, Braat AJAT, Beijst C, Bruijnen RCG, et al. The superior predictive value of Ho-scout compared with mTc-macroaggregated albumin prior to Ho-microspheres radioembolization in patients with liver metastases. Eur J Nucl Med Mol Imaging. 2019;47:798–806. https://doi.org/10.1007/S00259-019-04460-Y.
Chiesa C, Maccauro M. 166Ho microsphere scout dose for more accurate radioembolization treatment planning. Eur J Nucl Med Mol Imaging. 2019;47:744–7. https://doi.org/10.1007/S00259-019-04617-9.
Chiesa C, Mira M, Bhoori S, Bormolini G, Maccauro M, Spreafico C, et al. Radioembolization of hepatocarcinoma with 90Y glass microspheres: treatment optimization using the dose-toxicity relationship. Eur J Nucl Med Mol Imaging. 2020;47:3018–32. https://doi.org/10.1007/S00259-020-04845-4.
van Roekel C, Bastiaannet R, Smits MLJ, Bruijnen RC, Braat AJAT, de Jong HWAM, et al. Dose-effect relationships of 166Ho radioembolization in colorectal cancer. J Nucl Med. 2021;62:272–9. https://doi.org/10.2967/jnumed.120.243832.
Van Den Hoven AF, Rosenbaum CENM, Elias SG, De Jong HWAM, Koopman M, Verkooijen HM, et al. Insights into the dose-response relationship of radioembolization with resin Y-microspheres: a prospective cohort study in patients with colorectal cancer liver metastases. J Nucl Med. 2016;57:1014–9. https://doi.org/10.2967/jnumed.115.166942.
Tomozawa Y, Jahangiri Y, Pathak P, Kolbeck KJ, Schenning RC, Kaufman JA, et al. long-term toxicity after transarterial radioembolization with yttrium-90 using resin microspheres for neuroendocrine tumor liver metastases. J Vasc Interv Radiol. 2018;29:858–65. https://doi.org/10.1016/j.jvir.2018.02.002.
Currie BM, Hoteit MA, Ben-Josef E, Nadolski GJ, Soulen MC. Radioembolization-induced chronic hepatotoxicity: a single-center cohort analysis. J Vasc Interv Radiol. 2019;30:1915–23. https://doi.org/10.1016/j.jvir.2019.06.003.
Braat MNGJA, Braat AJAT, Lam MGEH. Personalized dosimetry: the way to limit hepatotoxicity. J Vasc Interv Radiol. 2020;31:515–6. https://doi.org/10.1016/j.jvir.2019.11.038.
Devcic Z, Rosenberg J, Braat AJA, Techasith T, Banerjee A, Sze DY, et al. The efficacy of hepatic 90Y resin radioembolization for metastatic neuroendocrine tumors: a meta-analysis. J Nucl Med. 2014;55:1404–10. https://doi.org/10.2967/jnumed.113.135855.
Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–35. https://doi.org/10.1056/NEJMoa1607427.
Pavel M, O’Toole D, Costa F, Capdevila J, Gross D, Kianmanesh R, et al. ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology. 2016;103:172–85. https://doi.org/10.1159/000443167.
Pavel M, Öberg K, Falconi M, Krenning EPP, Sundin A, Perren A, et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. 2020;31:844–60. https://doi.org/10.1016/j.annonc.2020.03.304.
Egger ME, Armstrong E, Martin RC, Scoggins CR, Philips P, Shah M, et al. Transarterial chemoembolization vs radioembolization for neuroendocrine liver metastases: a multi-institutional analysis. J Am Coll Surg. 2020;230:363–70. https://doi.org/10.1016/j.jamcollsurg.2019.12.026.
Currie BM, Nadolski G, Mondschein J, Dagli M, Sudheendra D, Stavropoulos SW, et al. Chronic hepatotoxicity in patients with metastatic neuroendocrine tumor: transarterial chemoembolization versus transarterial radioembolization. J Vasc Interv Radiol. 2020;31:1627–35. https://doi.org/10.1016/j.jvir.2020.05.019.
Currie BM, Nadolski G, Soulen MC. Response Letter to Correspondence Regarding “Chronic Hepatotoxicity in Patients with Metastatic Neuroendocrine Tumor: transarterial chemoembolization versus transarterial radioembolization.” J Vasc Interv Radiol. 2021;32:483–4. https://doi.org/10.1016/j.jvir.2020.11.019.
Padia SA. Radioembolization versus chemoembolization for neuroendocrine metastases. J Vasc Interv Radiol. 2021;32:482–3. https://doi.org/10.1016/j.jvir.2020.11.017.
Braat AJAT, Bruijnen RCG, van Rooij R, Braat MNGJA, Wessels FJ, van Leeuwaarde RS, et al. Additional holmium-166 radioembolisation after lutetium-177-dotatate in patients with neuroendocrine tumour liver metastases (HEPAR PLuS): a single-centre, single-arm, open-label, phase 2 study. Lancet Oncol. 2020;21:561–70. https://doi.org/10.1016/S1470-2045(20)30027-9.
Soulen MC, van Houten D, Teitelbaum UR, Damjanov N, Cengel KA, Metz DC. Safety and feasibility of integrating yttrium-90 radioembolization with capecitabine-temozolomide for grade 2 liver-dominant metastatic neuroendocrine tumors. Pancreas. 2018;47:980–4. https://doi.org/10.1097/MPA.0000000000001115.
Kim HS, Shaib WL, Zhang C, Nagaraju GP, Wu C, Alese OB, et al. Phase 1b study of pasireotide, everolimus, and selective internal radioembolization therapy for unresectable neuroendocrine tumors with hepatic metastases. Cancer. 2018;124:1992–2000. https://doi.org/10.1002/cncr.31192.
Study of lanreotide in patients with metastatic gastrointestinal neuroendocrine tumors who are undergoing liver-directed radioembolization with yttrium-90 microspheres - full text view - ClinicalTrials.gov [Internet]. https://clinicaltrials.gov/ct2/show/NCT02859064. Accessed 4 Aug 2021.
Strosberg J, Kunz PL, Hendifar A, Yao J, Bushnell D, Kulke MH, et al. Impact of liver tumour burden, alkaline phosphatase elevation, and target lesion size on treatment outcomes with 177Lu-Dotatate: an analysis of the NETTER-1 study. Eur J Nucl Med Mol Imaging. 2020;1–11. https://doi.org/10.1007/s00259-020-04709-x.
Author information
Authors and Affiliations
Contributions
ML and AB were in charge of medical care of the treated patients. AB and SE were responsible for data collection. SE and CR performed data analysis. Data analysis was supervised by MWB and AB. MNB reviewed patient’s imaging. SE is the main author of this manuscript; all authors reviewed the manuscript and gave consent for publication of the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
By the retrospective and anonymous nature of the study, the need for approval by an ethics committee was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Dosimetry.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ebbers, S.C., van Roekel, C., Braat, M.N.G.J.A. et al. Dose–response relationship after yttrium-90-radioembolization with glass microspheres in patients with neuroendocrine tumor liver metastases. Eur J Nucl Med Mol Imaging 49, 1700–1710 (2022). https://doi.org/10.1007/s00259-021-05642-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05642-3