Abstract
Purpose
To compare the efficacies of glass and resin-based Yttrium-90 microspheres by comparing absorbed tumor dose (TD) with both tumor response (TR) and overall survival (OS) in patients with chemorefractory intrahepatic cholangiocarcinoma (ICC).
Methods
Post-Y90 treatment bremsstrahlung SPECT/CT of 38 consecutive patients receiving 45 treatments (21 resin microspheres, 24 glass microspheres) were analyzed retrospectively. MIM software v6.9.4 (MIM Software Inc, Cleveland, OH) was used to calculate targeted tumors’ dose volume histogram. Modified Response Evaluation Criteria in Solid Tumors was used to evaluate tumor response 3 months post-treatment. Kaplan Meier estimation was used for survival analysis. T-test was used to compare the devices on various dosimetric parameters.
Results
Thresholds for TD to predict TR with ≥ 80% specificity were as follows: mean TD (Resin: 78.9 Gy; Glass: 254.7 Gy), maximum TD (Resin: 162.9 Gy; Glass: 591 Gy), minimum TD (Resin: 53.7 Gy; Glass: 149.1 Gy).
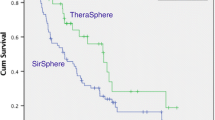
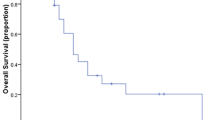
Microsphere type had no effect on survival from first Y90 (Resin: 11.2 mo; Glass 10.9 mo [p = 0.548]). In patients receiving resin microspheres, mean TD ≥ 75 Gy or maximum TD ≥ 150 Gy was associated with median OS of 20.2 mo compared to 6.5 mo for those receiving less (p = 0.001, 0.002, respectively). For patients treated with glass microspheres, those receiving a mean TD ≥ 150 Gy had a median OS of 14.6 mo vs. 2.6 mo for those receiving less (p = 0.031).
Conclusion
TD thresholds predictive of TR and OS differ significantly between glass and resin microspheres. However, microsphere type has no impact on survival in patients with chemorefractory ICC.
Level of Evidence
Level 3, Retrospective Study.






Similar content being viewed by others
References
Ibrahim SM, Nikolaidis P, Miller FH, Lewandowski RJ, Ryu RK, Sato KT, et al. Radiologic findings following Y90 radioembolization for primary liver malignancies. Abdom Imaging. 2009;34(5):566–81. https://doi.org/10.1007/s00261-008-9454-y.
Jia Z, Paz-Fumagalli R, Frey G, Sella DM, McKinney JM, Wang W. Resin-based Yttrium-90 microspheres for unresectable and failed first-line chemotherapy intrahepatic cholangiocarcinoma: preliminary results. J Cancer Res Clin Oncol. 2017;143(3):481–9. https://doi.org/10.1007/s00432-016-2291-4.
Ni Q, Shen W, Zhang M, Yang C, Cai W, Wu M, et al. Prognostic analysis of radical resection for intrahepatic cholangiocarcinoma: a retrospective cohort study. Oncotarget. 2017;8(43):75627–37. https://doi.org/10.18632/oncotarget.18465.
Helms RA, Quan DJ. Textbook of therapeutics: drug and disease management. Lippincott Williams & Wilkins (2006).
Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383(9935):2168–79. https://doi.org/10.1016/S0140-6736(13)61903-0.
Feisthammel J, Schoppmeyer K, Mössner J, Schulze M, Caca K, Wiedmann M. Irinotecan with 5-FU/FA in advanced biliary tract adenocarcinomas: a multicenter phase II trial. Am J Clin Oncol. 2007;30(3):319–24.
de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29(23):3140–5. https://doi.org/10.1200/jco.2011.35.6519.
Marsh RdW, Alonzo M, Bajaj S, Baker M, Elton E, Farrell TA, et al. Comprehensive review of the diagnosis and treatment of biliary tract cancer Diagnosis-clinical staging and pathology. J SurgOncol. 2012;106(3):332–8. https://doi.org/10.1002/jso.23028.
Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96(6):896–902. https://doi.org/10.1038/sj.bjc.6603648.
Bhangoo MS, Karnani DR, Hein PN, Giap H, Knowles H, Issa C, et al. Radioembolization with Yttrium-90 microspheres for patients with unresectable hepatocellular carcinoma. J Gastrointest Oncol. 2015;6(5):469–78. https://doi.org/10.3978/j.issn.2078-6891.2015.056.
Gangi A, Shah J, Hatfield N, Smith J, Sweeney J, Choi J, et al. Intrahepatic cholangiocarcinoma treated with transarterial Yttrium-90 glass microsphere radioembolization: results of a single institution retrospective study. J VascIntervRadiol. 2018;29(8):1101–8. https://doi.org/10.1016/j.jvir.2018.04.001.
Piscaglia F, Ogasawara S. Patient selection for transarterial chemoembolization in hepatocellular carcinoma: importance of benefit/risk assessment. Liver Cancer. 2018;7(1):104–19. https://doi.org/10.1159/000485471.
Cremonesi M, Chiesa C, Strigari L, Ferrari M, Botta F, Guerriero F, et al. Radioembolization of hepatic lesions from a radiobiology and dosimetric perspective. Front Oncol. 2014. https://doi.org/10.3389/fonc.2014.00210.
Salem R, Thurston KG. Radioembolization with 90Yttrium Microspheres: a state-of-the-art brachytherapy treatment for primary and secondary Liver malignancies: Part 1: technical and methodologic considerations. J VascIntervRadiol. 2006;17(8):1251–78. https://doi.org/10.1097/01.RVI.0000233785.75257.9A.
Kokabi N, Galt JR, Xing M, Camacho JC, Barron BJ, Schuster DM, et al. A simple method for estimating dose delivered to hepatocellular carcinoma after Yttrium-90 Glass-based radioembolization therapy: preliminary results of a proof of concept study. J VascIntervRadiol. 2014;25(2):277–87. https://doi.org/10.1016/j.jvir.2013.11.007.
Camacho JC, Kokabi N, Xing M, Prajapati HJ, El-Rayes B, Kim HS. Modified response evaluation criteria in solid tumors and European association for the study of the liver criteria using delayed-phase imaging at an early time point predict survival in patients with unresectable intrahepatic cholangiocarcinoma following Yttrium-90 radioembolization. J VascIntervRadiol. 2014;25(2):256–65. https://doi.org/10.1016/j.jvir.2013.10.056.
NationalCancerInstitute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. (2017).
Elsayed M, Ermentrout RM, Sethi I, Bercu ZL, Galt JR, Whitmore M, et al. Incidence of radioembolization-induced liver disease and liver toxicity following repeat 90Y-radioembolization: outcomes at a large tertiary care center. ClinNucl Med. 2020;45(2):100–4. https://doi.org/10.1097/rlu.0000000000002828.
Edeline J, Touchefeu Y, Guiu B, Farge O, Tougeron D, Baumgaertner I, et al. Radioembolization Plus chemotherapy for first-line treatment of locally advanced intrahepatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2020;6(1):51–9. https://doi.org/10.1001/jamaoncol.2019.3702.
Zhen Y, Liu B, Chang Z, Ren H, Liu Z, Zheng J. A pooled analysis of transarterialradioembolization with Yttrium-90 microspheres for the treatment of unresectable intrahepatic cholangiocarcinoma. Onco Targets Ther. 2019;12:4489–98. https://doi.org/10.2147/OTT.S202875.
Piana PM, Bar V, Doyle L, Anne R, Sato T, Eschelman DJ, et al. Early arterial stasis during resin-based Yttrium-90 radioembolization: incidence and preliminary outcomes. HPB (Oxford). 2014;16(4):336–41. https://doi.org/10.1111/hpb.12135.
Zhang Z, Fardanesh MR, Machac J, Heiba S, Knesaurek K, Zaretsky V, et al. Comparison of therapeutic response using RECIST criteria: Y-90 SIR-Spheres and TheraSphere treatment of unresectable hepatocellular carcinoma. J Nucl Med. 2013;54(supplement 2):224.
Horsman MR, Overgaard J. The impact of hypoxia and its modification of the outcome of radiotherapy. J Radiat Res. 2016;57(Suppl 1(Suppl 1)):i90–8. https://doi.org/10.1093/jrr/rrw007.
Bilbao JI, de Martino A, de Luis E, Díaz-Dorronsoro L, Alonso-Burgos A, Martínez de la Cuesta A, et al. Biocompatibility inflammatory response and recannalization characteristics of nonradioactive resin microspheres histological findings. CardioVascIntervenRadiol. 2009;32(4):727–36. https://doi.org/10.1007/s00270-009-9592-9.
Miller G, Kim A. Rate of stasis and disease response after Y-90 radioembolization. J Vasc Interv Radiol. 2017;28(2):S119–20. https://doi.org/10.1016/j.jvir.2016.12.887.
Nezami N, Kokabi N, Camacho JC, Schuster DM, Xing M, Kim HS. (90)Y radioembolization dosimetry using a simple semi-quantitative method in intrahepatic cholangiocarcinoma: glass versus resin microspheres. Nucl Med Biol. 2018;59:22–8. https://doi.org/10.1016/j.nucmedbio.2018.01.001.
Srinivas SM, Nasr EC, Kunam VK, Bullen JA, Purysko AS. Administered activity and outcomes of glass versus resin (90)Y microsphere radioembolization in patients with colorectal liver metastases. J Gastrointest Oncol. 2016;7(4):530–9. https://doi.org/10.21037/jgo.2016.03.09.
Garin E, Rolland Y, Edeline J, Icard N, Lenoir L, Laffont S, et al. Personalized dosimetry with intensification using 90Y-loaded glass microsphere radioembolization induces prolonged overall survival in hepatocellular carcinoma patients with portal vein thrombosis. J Nucl Med. 2015;56(3):339–46. https://doi.org/10.2967/jnumed.114.145177.
Demirelli S, Erkilic M, Oner AO, Budak ES, Gunduz S, Ozgur O, et al. Evaluation of factors affecting tumor response and survival in patients with primary and metastatic liver cancer treated with microspheres. Nucl Med Commun. 2015;36(4):340–9. https://doi.org/10.1097/mnm.0000000000000257.
Kao YH, Steinberg JD, Tay YS, Lim GK, Yan J, Townsend DW, et al. Post-radioembolization Yttrium-90 PET/CT - part 2: dose-response and tumor predictive dosimetry for resin microspheres. EJNMMI Res. 2013;3(1):57. https://doi.org/10.1186/2191-219x-3-57.
Chung YE, Kim M-J, Park YN, Choi J-Y, Pyo JY, Kim YC, et al. Varying appearances of cholangiocarcinoma: radiologic-pathologic correlation. Radiographics. 2009;29(3):683–700. https://doi.org/10.1148/rg.293085729.
Köhler M, Harders F, Lohöfer F, Paprottka PM, Schaarschmidt BM, Theysohn J, et al. Prognostic factors for overall survival in advanced intrahepatic cholangiocarcinoma treated with Yttrium-90 radioembolization. J Clin Med. 2019;9(1):56. https://doi.org/10.3390/jcm9010056.
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Nima Kokabi conducts research partially funded by Sirtex Medical Ltd. No grant funding has been received for the development of this manuscript. The remaining authors have no financial disclosures.
Ethical Approval
For this type of study formal consent is not required.
Informed Consent
This study has obtained approval from our tertiary center’s IRB and the need for informed consent was waived.
Consent for Publication
For this type of study consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cheng, B., Villalobos, A., Sethi, I. et al. Determination of Tumor Dose Response Thresholds in Patients with Chemorefractory Intrahepatic Cholangiocarcinoma Treated with Resin and Glass-based Y90 Radioembolization. Cardiovasc Intervent Radiol 44, 1194–1203 (2021). https://doi.org/10.1007/s00270-021-02834-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-021-02834-0