Abstract
Purpose
Positron emission tomography (PET) agents targeting the prostate-specific membrane antigen (PSMA) are currently under broad clinical and scientific investigation. 68Ga-PSMA HBED-CC constitutes the first 68Ga-labelled PSMA-inhibitor and has evolved as a promising agent for imaging PSMA expression in vivo. The aim of this study was to evaluate the whole-body distribution and radiation dosimetry of this new probe.
Methods
Five patients with a history or high suspicion of prostate cancer were injected intravenously with a mean of 139.8 ± 13.7 MBq of 68Ga-PSMA HBED-CC (range 120–158 MBq). Four static skull to mid-thigh scans using a whole-body fully integrated PET/MR-system were performed 10 min, 60 min, 130 min, and 175 min after the tracer injection. Time-dependent changes of the injected activity per organ were determined. Mean organ-absorbed doses and effective doses (ED) were calculated using OLINDA/EXM.
Results
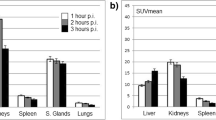
Injection of a standard activity of 150 MBq 68Ga-PSMA HBED-CC resulted in a median effective dose of 2.37 mSv (Range 1.08E-02 – 2.46E-02 mSv/MBq). The urinary bladder wall (median absorbed dose 1.64E-01 mGv/MBq; range 8.76E-02 – 2.91E-01 mGv/MBq) was the critical organ, followed by the kidneys (median absorbed dose 1.21E-01 mGv/MBq; range 7.16E-02 – 1.75E-01), spleen (median absorbed dose 4.13E-02 mGv/MBq; range 1.57E-02 – 7.32E-02 mGv/MBq) and liver (median absorbed dose 2.07E-02 mGv/MBq; range 1.80E-02 – 2.57E-02 mGv/MBq). No drug-related pharmacological effects occurred.
Conclusion
The use of 68Ga-PSMA HBED-CC results in a relatively low radiation exposure, delivering organ doses that are comparable to those of other 68Ga-labelled PSMA-inhibitors used for PET-imaging. Total effective dose is lower than for other PET-agents used for prostate cancer imaging (e.g. 11C- and 18F-Choline).



Similar content being viewed by others
References
Walker RC, Smith GT, Liu E, Moore B, Clanton J, Stabin M. Measured human dosimetry of 68Ga-DOTATATE. J Nucl Med Off Publ Soc Nucl Med. 2013;54(6):855–60.
Breeman WAP, Chan HS, de Zanger RMS, Konijnenberg MK, Blois E de. Overview of Development and Formulation of 177Lu-DOTA-TATE for PRRT. Curr Radiopharm. 2015
Hofman MS, Lau WFE, Hicks RJ. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiogr Rev Publ Radiol Soc North Am Inc. 2015;35(2):500–16.
Notni J, Pohle K, Wester H-J. Comparative gallium-68 labeling of TRAP-, NOTA-, and DOTA-peptides: practical consequences for the future of gallium-68-PET. EJNMMI Res. 2012;2(1):28.
Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res Off J Am Assoc Cancer Res. 1997;3(1):81–5.
Bander NH. Technology insight: monoclonal antibody imaging of prostate cancer. Nat Clin Pract Urol. 2006;3(4):216–25.
Eder M, Schäfer M, Bauder-Wüst U, Hull W-E, Wängler C, Mier W, et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem. 2012;23(4):688–97.
Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42(2):197–209.
Afshar-Oromieh A, Haberkorn U, Schlemmer HP, Fenchel M, Eder M, Eisenhut M, et al. Comparison of PET/CT and PET/MRI hybrid systems using a 68Ga-labelled PSMA ligand for the diagnosis of recurrent prostate cancer: initial experience. Eur J Nucl Med Mol Imaging. 2014;41(5):887–97.
Afshar-Oromieh A, Malcher A, Eder M, Eisenhut M, Linhart HG, Hadaschik BA, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013;40(4):486–95.
Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of hybrid 68Ga-PSMA-ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med Off Publ Soc Nucl Med. 2015
Zechmann CM, Afshar-Oromieh A, Armor T, Stubbs JB, Mier W, Hadaschik B, et al. Radiation dosimetry and first therapy results with a (124)I/(131)I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur J Nucl Med Mol Imaging. 2014;41(7):1280–92.
Afshar-Oromieh A, Hetzheim H, Kratochwil C, Benesova M, Eder M, Neels OC, et al. The novel theranostic PSMA-ligand PSMA-617 in the diagnosis of prostate cancer by PET/CT: biodistribution in humans, radiation dosimetry and first evaluation of tumor lesions. J Nucl Med Off Publ Soc Nucl Med. 2015
Herrmann K, Bluemel C, Weineisen M, Schottelius M, Wester H-J, Czernin J, et al. Biodistribution and radiation dosimetry for a probe targeting prostate-specific membrane antigen for imaging and therapy. J Nucl Med Off Publ Soc Nucl Med. 2015;56(6):855–61.
Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol [Internet]. 2016 Feb 23 [cited 2016 Mar 10];advance online publication. Available from: http://www.nature.com/nrurol/journal/vaop/ncurrent/abs/nrurol.2016.26.html
Martin R, Jüttler S, Müller M, Wester H-J. Cationic eluate pretreatment for automated synthesis of [68Ga]CPCR4.2. Nucl Med Biol. 2014;41(1):84–9.
Schafer M, Bauder-Wust U, Leotta K, Zoller F, Mier W, Haberkorn U, et al. A dimerized urea-based inhibitor of the prostate-specific membrane antigen for 68Ga-PET imaging of prostate cancer. EJNMMI Res. 2012;2:23.
Delso G, Fürst S, Jakoby B, Ladebeck R, Ganter C, Nekolla SG, et al. Performance measurements of the Siemens mMR integrated whole-body PET/MR scanner. J Nucl Med Off Publ Soc Nucl Med. 2011;52(12):1914–22.
Martinez-Möller A, Nekolla SG. Attenuation correction for PET/MR: problems, novel approaches and practical solutions. Z Für Med Phys. 2012;22(4):299–310.
Martinez-Möller A, Eiber M, Nekolla SG, Souvatzoglou M, Drzezga A, Ziegler S, et al. Workflow and scan protocol considerations for integrated whole-body PET/MRI in oncology. J Nucl Med Off Publ Soc Nucl Med. 2012;53(9):1415–26.
Souvatzoglou M, Eiber M, Martinez-Moeller A, Fürst S, Holzapfel K, Maurer T, et al. PET/MR in prostate cancer: technical aspects and potential diagnostic value. Eur J Nucl Med Mol Imaging. 2013;40 Suppl 1:S79–88.
Martinez-Möller A, Souvatzoglou M, Delso G, Bundschuh RA, Chefd’hotel C, Ziegler SI, et al. Tissue classification as a potential approach for attenuation correction in whole-body PET/MRI: evaluation with PET/CT data. J Nucl Med Off Publ Soc Nucl Med. 2009;50(4):520–6.
Soderlund AT, Chaal J, Tjio G, Totman JJ, Conti M, Townsend DW. Beyond 18F-FDG: characterization of PET/CT and PET/MR scanners for a comprehensive Set of positron emitters of growing application—18F, 11C, 89Zr, 124I, 68Ga, and 90Y. J Nucl Med. 2015;56(8):1285–91.
Siegel JA, Stabin MG. Radar commentary: use of linear no-threshold hypothesis in radiation protection regulation in the United States. Health Phys. 2012;102(1):90–9.
Stabin MG, Xu XG, Emmons MA, Segars WP, Shi C, Fernald MJ. RADAR reference adult, pediatric, and pregnant female phantom series for internal and external dosimetry. J Nucl Med Off Publ Soc Nucl Med. 2012;53(11):1807–13.
Stabin MG, Sharkey RM, Siegel JA. RADAR commentary: evolution and current status of dosimetry in nuclear medicine. J Nucl Med Off Publ Soc Nucl Med. 2011;52(7):1156–61.
Stabin MG. Uncertainties in internal dose calculations for radiopharmaceuticals. J Nucl Med Off Publ Soc Nucl Med. 2008;49(5):853–60.
Loevinger R, Budinger TF, Watson EE. MIRD Primer for Absorbed Dose Calculations. Soc Nucl Med. 1988. 152.
Siegel JA, Thomas SR, Stubbs JB, Stabin MG, Hays MT, Koral KF, et al. MIRD pamphlet no. 16: techniques for quantitative radiopharmaceutical Biodistribution data acquisition and analysis for use in human radiation dose estimates. J Nucl Med Off Publ Soc Nucl Med. 1999;40(2):37S–61S.
Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol Bethesda Md 1985. 2000;89(1):81–8.
Sgouros G. Bone marrow dosimetry for radioimmunotherapy: theoretical considerations. J Nucl Med Off Publ Soc Nucl Med. 1993;34(4):689–94.
Shen S, DeNardo GL, Sgouros G, O’Donnell RT, DeNardo SJ. Practical determination of patient-specific marrow dose using radioactivity concentration in blood and body. J Nucl Med Off Publ Soc Nucl Med. 1999;40(12):2102–6.
Ferrer L, Kraeber-Bodéré F, Bodet-Milin C, Rousseau C, Le Gouill S, Wegener WA, et al. Three methods assessing red marrow dosimetry in lymphoma patients treated with radioimmunotherapy. Cancer. 2010;116(4 Suppl):1093–100.
Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med Off Publ Soc Nucl Med. 2005;46(6):1023–7.
Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998;52(4):637–40.
Mannweiler S, Amersdorfer P, Trajanoski S, Terrett JA, King D, Mehes G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol Oncol Res. 2009;15(2):167–72.
Maria T, Panagiotis A, Marina C, Eleni K, Ioanna V, Georgia M, et al. How prostate-specific membrane antigen level may be correlated with stemness in prostate cancer stem cell-like cell populations? J Cancer Res Ther. 2014;10(1):133–41.
Weineisen M, Schottelius M, Simecek J, Eiber M, Schwaiger M, Wester H. Development and first in human evaluation of PSMA I&T - A ligand for diagnostic imaging and endoradiotherapy of prostate cancer. J Nucl Med. 2014;55(supplement 1):1083–3.
Benešová M, Schäfer M, Bauder-Wüst U, Afshar-Oromieh A, Kratochwil C, Mier W, et al. Preclinical evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J Nucl Med Off Publ Soc Nucl Med. 2015;56(6):914–20.
Szabo Z, Mena E, Rowe SP, Plyku D, Nidal R, Eisenberger MA, et al. Initial evaluation of [(18)F]DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol MIB Off Publ Acad Mol Imaging. 2015;17(4):565–74.
Werner RA, Bluemel C, Allen-Auerbach MS, Higuchi T, Herrmann K. 68Gallium- and 90Yttrium-/177Lutetium: “theranostic twins” for diagnosis and treatment of NETs. Ann Nucl Med. 2015;29(1):1–7.
ICRP. Radiation dose to patients from radiopharmaceuticals. Addendum 3 to ICRP publication 53. ICRP publication 106. Approved by the commission in october 2007. Ann ICRP. 2008;38(1–2):1–197.
Giussani A, Janzen T, Uusijärvi-Lizana H, Tavola F, Zankl M, Sydoff M, et al. A compartmental model for biokinetics and dosimetry of 18F-choline in prostate cancer patients. J Nucl Med. 2012;53(6):985–93.
Tolvanen T, Yli-Kerttula T, Ujula T, Autio A, Lehikoinen P, Minn H, et al. Biodistribution and radiation dosimetry of [(11)C]choline: a comparison between rat and human data. Eur J Nucl Med Mol Imaging. 2010;37(5):874–83.
Smolarz K, Krause BJ, Graner FP, Wagner FM, Wester H-J, Sell T, et al. Biodistribution and radiation dosimetry in healthy volunteers of a novel tumour-specific probe for PET/CT imaging: BAY 85–8050. Eur J Nucl Med Mol Imaging. 2013;40(12):1861–8.
Eiber M, Takei T, Souvatzoglou M, Mayerhoefer ME, Fürst S, Gaertner FC, et al. Performance of whole-body integrated 18F-FDG PET/MR in comparison to PET/CT for evaluation of malignant bone lesions. J Nucl Med Off Publ Soc Nucl Med. 2014;55(2):191–7.
Hofmann M, Pichler B, Schölkopf B, Beyer T. Towards quantitative PET/MRI: a review of MR-based attenuation correction techniques. Eur J Nucl Med Mol Imaging. 2009;36 Suppl 1:S93–104.
Hitz S, Habekost C, Fürst S, Delso G, Förster S, Ziegler S, et al. Systematic comparison of the performance of integrated whole-body PET/MR imaging to conventional PET/CT for 18F-FDG brain imaging in patients examined for suspected dementia. J Nucl Med Off Publ Soc Nucl Med. 2014;55(6):923–31.
Gaertner FC, Beer AJ, Souvatzoglou M, Eiber M, Fürst S, Ziegler SI, et al. Evaluation of feasibility and image quality of 68Ga-DOTATOC positron emission tomography/magnetic resonance in comparison with positron emission tomography/computed tomography in patients with neuroendocrine tumors. Invest Radiol. 2013;48(5):263–72.
Souvatzoglou M, Eiber M, Takei T, Fürst S, Maurer T, Gaertner F, et al. Comparison of integrated whole-body [11C]choline PET/MR with PET/CT in patients with prostate cancer. Eur J Nucl Med Mol Imaging. 2013;40(10):1486–99.
Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, et al. Diagnostic Efficacy of (68) Gallium-PSMA Positron Emission Tomography Compared to Conventional Imaging in Lymph Node Staging of 130 Consecutive Patients with Intermediate to High Risk Prostate Cancer. J Urol. 2015
Verburg FA, Krohn T, Heinzel A, Mottaghy FM, Behrendt FF. First evidence of PSMA expression in differentiated thyroid cancer using [68Ga]PSMA-HBED-CC PET/CT. Eur J Nucl Med Mol Imaging. 2015;42(10):1622–3.
Eiber M, Nekolla SG, Maurer T, Weirich G, Wester H-J, Schwaiger M. (68)Ga-PSMA PET/MR with multimodality image analysis for primary prostate cancer. Abdom Imaging. 2015;40(6):1769–71.
Maurer T, Weirich G, Schottelius M, Weineisen M, Frisch B, Okur A, et al. Prostate-specific membrane antigen-radioguided surgery for metastatic lymph nodes in prostate cancer. Eur Urol. 2015;68(3):530–4.
Maurer T, Kübler H, Gschwend JE, Eiber M. Positron-emission tomography in urooncology. Urol Ausg. 2015;54(7):983–91.
Boubaker A, Prior JO, Willi J-P, Champendal M, Kosinski M, Bischof Delaloye A, et al. Biokinetics and dosimetry of 111In-DOTA-NOC-ATE compared with 111In-DTPA-octreotide. Eur J Nucl Med Mol Imaging. 2012;39(12):1868–75.
The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP. 2007;37(2–4):1–332.
Herrmann K, Lapa C, Wester H-J, Schottelius M, Schiepers C, Eberlein U, et al. Biodistribution and radiation dosimetry for the chemokine receptor CXCR4-targeting probe 68Ga-pentixafor. J Nucl Med Off Publ Soc Nucl Med. 2015;56(3):410–6.
Acknowledgments
We thank Daniela Hiob for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Markus Schwaiger has received funding from the European Union Seventh Framework Program (FP7) under Grant Agreement No. 294582 ERC Grant MUMI. The development of 68Ga-PSMA HBED-CC synthesis was supported by SFB 824 (DFG Sonderforschungsbereich 824, Project Z1) from the Deutsche Forschungsgemeinschaft, Bonn, Germany. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The analysis of patient data was approved by the Ethics Committee of the Technische Universität München (permit 5665/13). Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Pfob, C.H., Ziegler, S., Graner, F.P. et al. Biodistribution and radiation dosimetry of 68Ga-PSMA HBED CC—a PSMA specific probe for PET imaging of prostate cancer. Eur J Nucl Med Mol Imaging 43, 1962–1970 (2016). https://doi.org/10.1007/s00259-016-3424-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3424-3