Abstract
Streptomyces peucetius ATCC 27952 is known to produce a variety of secondary metabolites, including two important antitumor anthracyclines: daunorubicin and doxorubicin. Identification of peucemycin and 25-hydroxy peucemycin (peucemycin A), as well as their biosynthetic pathway, has expanded its biosynthetic potential. In this study, we isolated a new peucemycin derivative and identified it as 19-hydroxy peucemycin (peucemycin B). Its antibacterial activity was lower than those of peucemycin and peucemycin A. On the other hand, this newly identified peucemycin derivative had higher anticancer activity than the other two compounds for MKN45, NCI-H1650, and MDA-MB-231 cancer cell lines with IC50 values of 76.97 µM, 99.68 µM, and 135.2 µM, respectively. Peucemycin biosynthetic gene cluster revealed the presence of a SARP regulator named PeuR whose role was unknown. The presence of the TTA codon in the peuR and the absence of global regulator BldA in S. peucetius reduced its ability to regulate the peucemycin biosynthetic gene cluster. Hence, different mutants harboring these genes were prepared. S. peucetius bldA25 harboring bldA produced 1.75 times and 1.77 times more peucemycin A (11.8 mg/L) and peucemycin B (21.2 mg/L), respectively, than the wild type. On the other hand, S. peucetius R25 harboring peuR produced 1.86 and 1.79 times more peucemycin A (12.5 mg/L) and peucemycin B (21.5 mg/L), respectively, than the wild type. Finally, strain S. peucetius bldAR25 carrying bldA and peuR produced roughly 3.52 and 2.63 times more peucemycin A (23.8 mg/L) and peucemycin B (31.5 mg/L), respectively, than the wild type.
Key points
• This study identifies a new peucemycin derivative, 19-hydroxy peucemycin (peucemycin B).
• The SARP regulator (PeuR) acts as a positive regulator of the peucemycin biosynthetic gene cluster.
• The overexpression of peuR and heterologous expression of bldA increase the production of peucemycin derivatives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Streptomyces peucetius produces a number of bioactive secondary metabolites including daunorubicin (DNR), doxorubicin (DXR) (Arcamone and Cassinelli 1998; Arcamone et al. 1969), flaviolin (Ghimire et al. 2008), geosmin (Singh et al. 2009), peucechelin (Park et al. 2013) (Kodani et al. 2015), and hopene (Ghimire et al. 2015). Furthermore, a new 14-membered macrolide (peucemycin) with a γ-pyrone ring was purified and assigned its structure (Pham et al. 2021). The compound exhibited antibacterial activities against some pathogens and suppressed the viability of various cancer cell lines (Pham et al. 2021). Recently, a new peucemycin derivative and its putative biosynthetic gene cluster were identified, providing insight into the biosynthesis of peucemycin and its derivatives (Magar et al. 2023). The newly identified derivative was 25-hydroxy peucemycin (peucemycin A) and had antibacterial activities against some pathogens (Magar et al. 2023).
The peucemycin biosynthetic gene cluster (Peu BGC) has two cluster-situated regulators (pathway-specific regulators). Among the two, PeuR was identified as a Streptomyces antibiotic regulator protein (SARP) regulator having a helix-turn-helix (HTH) DNA-binding domain motif at the N-terminal and a bacterial transcriptional activator domain (BTAD) at their C terminus (Hindra and Elliot 2010; Magar et al. 2023). It is currently unknown how PeuR controls the production of peucemycin and its derivatives.
The regulation of biosynthetic pathways by regulatory genes functions as pathway-specific regulators (also known as cluster-situated regulators) and/or pleiotropic (or global) regulators (Lu et al. 2017). Some biosynthetic gene clusters necessitate the participation of both regulators, such as pathway-specific regulatory gene actII-ORF4 and global regulatory gene cprB (Fernández-Moreno et al. 1991) in the production of actinorhodin (ACT) from S. coelicolor (Onaka et al. 1998). Similarly, the production of polyketide can be improved by controlling the regulatory mechanism (Chen et al. 2010). For instance, overexpression of SARP regulator in Streptomyces sp. KCCM11116P resulted in a higher production titer of tacrolimus (FK506) compared to the wild type (Chen et al. 2015). Furthermore, overexpression of the SARP regulator can activate a silent gene cluster. One example is the activation of the undecylprodigiosin (Red) gene cluster by the overexpression of the papR2 from S. pristinaespiralis in S. lividans (Krause et al. 2020).
In addition to the pathway-specific regulator, a global regulator BldA also participates in the regulation of biosynthetic pathways. The bldA encodes tRNA required for the translation of a rare UUA codon for leucine (Chater and Chandra 2008). Hence, the BldA affects the translation of genes having this rare codon. It also plays an important role in the regulation of antibiotic production (Hackl and Bechthold 2015) like undecylprodigiosin (RED) biosynthesis in S. coelicolor (White and Bibb 1997) and landomycin E in S. globisporus 1912 (Rebets et al. 2006).
In this study, we successfully isolated another peucemycin derivative and determined its structure. The impact of overexpressing peuR, a SARP regulator, on biosynthesis of peucemycin and its derivatives was also thoroughly investigated. Furthermore, the effect of heterologous expression of bldA, a pleiotropic regulator, on biosynthesis of peucemycin and its derivatives was investigated.
Materials and methods
Bacterial strains, media, and culture conditions
Escherichia coli XL1-Blue MRF (Stratagene) was used as the cloning host, while E. coli ET12567 (John Innes Centre, UK) was used as the demethylating host. They were cultured in Luria–Bertani (LB) media at 37 °C with ampicillin (100 µg/mL) when necessary. For cloning PCR fragments, pGEM®–T Easy vector (Promega) was used. As an expression vector for S. peucetius, pIBR25 (Sthapit et al. 2004) was used. Standard protocols were used for manipulating DNA in E. coli (Sambrook and Russell 2001). S. peucetius and its derived strains were grown in R2YE media at 28 °C with thiostrepton (15 µg/mL) when necessary. Their protoplasts were prepared in tryptic soya broth (TSB) following the standard protocol (Kieser et al. 2000). Peucemycin and its derivatives were produced by growing S. peucetius strains in Hickey-Tresner (HT) media at 18 °C for 3 days (Hickey and Tresner 1952). All wild-type and recombinant strains along with vectors developed during this study are listed in Table S1.
Construction of recombinant vectors and generation of mutant strains
Standard protocols were followed for DNA manipulations and the production of mutants (Kieser et al. 2000; Sambrook and Russell 2001). The peuR and bldA were amplified from genomic DNAs of S. peucetius DM07 and S. coelicolor A3, respectively, using primer pair listed in Table S2. Conditions for PCR amplification were 95 °C for 7 min; by 30 cycles of 95 °C for 30 s, 55–68 °C for 30 s, 72 °C for 60–90 s; and final extension at 72 °C for 7 min. DNA amplification was performed using PrimeSTAR HS DNA Polymerase (Takara) according to the manufacturer’s instructions. Following ligation into the pGEM-T Easy vector, sequences were verified.
After peuR and bldA were ligated separately into pIBR25 at XbaI-HindIII and BamHI-XbaI sites, respectively, the combination of both genes was produced using BamHI-HindIII sites of pIBR25. All recombinant vectors were demethylated by E. coli ET12567 and subsequently transferred into S. peucetius using polyethylene glycol (PEG)-mediated protoplast transformation (Malla et al. 2009). Transformants were selected using R2YE plates with thiostrepton. Confirmation of the transformation was done by isolating recombinant vectors from positive clones that were resistant to thiostrepton. A single colony from each mutant was taken to study the production of peucemycin and its derivatives.
Analysis of growth rate and production of peucemycin and its derivatives
To determine the optimal time for producing 19-hydroxypeucemycin (peucemycin B), a seed culture was prepared by growing S. peucetius DM07 in R2YE media for 48 h at 28 °C. Then 1 mL seed culture was added to 50 mL HT media and cultured for 168 h at 18 °C. At every 24-h time interval, 1 mL of culture broth was collected for 168 h. The remaining culture broths were used for quantification of peucemycin B. Samples were centrifuged at 12,000 rpm and washed twice with sterile distilled water. Pellets were finally dried at 70 °C for 4 days, and dry cell weight (DCW) for each sample was measured. Measurements were taken for three biological replicates. Peucemycin B was quantified after the remaining culture broths were extracted with ethyl acetate at double volume. The organic phase was dried using a rotary evaporator under reduced pressure. Each extract was then dissolved in 1 mL of methanol.
Samples were analyzed by Thermo HPLC series 1100 with a Thermo-C18 column (5 µm, 4.6 mm × 250 mm) which was equilibrated with 90% solvent A (water + 0.1% trifluoroactetic acid (TFA)) and 10% acetonitrile (ACN). The condition for analysis was a linear gradient of 1–25 min with 10% to 90% B, 25–28 min with 90% to 50% B, and 28 − 30 min with 50% to 10% B at a flow rate of 1 mL/min with UV detection at 268 nm. A standard curve was prepared with different concentrations of peucemycin B. Concentration of peucemycin B was calculated based on the peak area. Compound mass was analyzed by ultra-high-performance liquid chromatography electrospray ionization quadrupole time-of-flight high-resolution mass spectrometry (UPLC − ESI − Q − TOF − HRMS) using an Acquity column (UPLC; Waters Corp., Milford, MA, USA) coupled with a SYNAPT G2-S (Waters Corp) with a gradient of solvent mobile phase of 0.1% TFA in water and 100% ACN (0 to 12 min) at 35 °C. The sample volume used for injection was 10 µL. LC − MS analysis was performed using a high-resolution mass spectrometer equipped with an electrospray ionization source under the following conditions: 3 kV of capillary voltage, 300 °C of desolvation gas temperature, and 600 L/h of desolvation gas flow rate.
Growth rate and peucemycin production for all other mutants were studied by taking samples for 3 days and performing similar steps as mentioned above. Calibration curves were prepared for peucemycin and peucemycin A. All the data were generated from three experimental samples, and a student t-test was performed with GraphPad Prism 6 for significance tests.
Isolation and purification of peucemycin B
For sample preparation, S. peucetius DM07 was grown in HT media for 72 h at 18 °C in a 4 L fermenter. A total of 12 L of HT medium was used to isolate peucemycin B. Double volume of ethyl acetate was added to the sample and the organic phase was dried using a rotary evaporator under reduced pressure. This crude extract was washed twice with autoclaved distilled water followed by washing once with hexane. The sample was then washed with dichloromethane and dried. The final extract was dissolved in 150 mL of methanol. The extract was filtered and purified using Dionex Ultimate 3000 UPLC (Thermo Fisher Scientific) with a C18 column (YMC − Pack ODS − Aq, 150 × 20 mm2) connected to a UV detector (220 and 268 nm). The condition for binary gradient of mobile phase was 100% water (solvent A) and 100% acetonitrile (solvent B) (0–5 min, 0 to 20% B; 5–10 min, 20 to 40% B, 10–20 min, 40 to 60% B; 20–28 min, 60 to 100% B; 28–30 min, 100% B; and 30–35 min, 100 to 0% B) at a flow rate of 10 mL/min. The purified compound was dried, lyophilized, and dissolved in dimethyl sulfoxide (DMSO-d6), and then subjected to 700 MHz analyses using a Bruker BioSpin nuclear magnetic resonance (NMR) spectrometer (Billerica, USA), including one-dimensional (1D) 1H- NMR,13C-NMR, two-dimensional (2D) correlated spectroscopy (COSY), rotational frame nuclear overhauser effect (NOE) spectroscopy (ROESY), heteronuclear single quantum correlation (HSQC) analysis, and heteronuclear multiple bond correlation (HMBC) analysis (Ochang, Republic of Korea).
Transcriptional level analysis
Total RNAs were isolated from 48 h bacterial cultures using an RNeasy Mini Kit (Qiagen Inc., USA) following the manufacturer’s protocols. DNA contamination was removed using RNase-Free DNase (Qiagen Inc., USA). Total RNA concentration and purity were determined using a spectrophotometer at 260 nm. Finally, cDNA was prepared by QuantiTect® Reverse Transcription kit (Qiagen) with an equal amount of RNA. RT-PCR conditions were cDNA synthesis at 50 °C for 30 min; initial denaturation at 95 °C for 10 min followed by 45 cycles of denaturation for 1 min at 95 °C, annealing at 1 min for 64 °C, and elongation at 72 °C for 1 min for PCR. Finally, PCR products were visualized using 1% agarose gel. Primers used for this study are listed in Table S2.
Biological assays
The paper disk method was used to evaluate the antibacterial activities of peucemycin B against Gram-positive bacteria including both MRSA and MSSA strains of Staphylococcus aureus and Gram-negative bacteria Proteus hauseri NBRC 3851. The compound dissolved in DMSO was used for the test along with DMSO as a negative control and erythromycin as a positive control. The antibacterial effect was assessed after incubating plates at 37 °C for 16 − 24 h (Weinstein et al. 2018; Poudel et al. 2022).
Five different human cancer cell lines were obtained from the Korean Cell Line Bank (Seoul, Korea). MKN45 gastric cancer and NCI-H1650 lung cancer cell lines were grown in RPMI-1640 media (Corning Cellgro, Manassas, VA, USA) supplemented with 10% fetal bovine serum (FBS; R&D systems, Minneapolis, MN, USA). Hep3B liver cancer cells, MDA-MB-231 breast cancer cells, and MRC-5 lung normal cells were grown in DMEM medium (Corning Cellgro) supplemented with 10% FBS. U87MG brain cancer cell line was cultured with MEM medium (Corning Cellgro) supplemented with 10% FBS. All cells were cultured at 37 °C in a humidified CO2 incubator with 5% CO2 (Thermo Fisher Scientific, Vantaa, Finland). For cell viability assay, a CellTiter-Glo® luminescent assay system (Promega, Madison, WI, USA) was used according to the manufacturer’s instructions. Briefly, cells were seeded into a 96-white well culture plate at a density of 3 × 103 cells/well and treated with peucemycin or its derivative at 0–400 µM for 72 h. After adding 20 µL of substrate solution to each well, culture plates were shaken for 2 min. They were then incubated at room temperature in the dark for 10 min. Luminescence was measured using a multimode microplate reader. Finally, IC50 values were determined with acquired data utilizing GraphPad Prism 6.
Results
Identification of a new peucemycin derivative
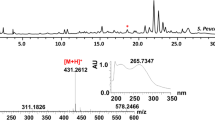
Mass analysis and UV absorption pattern for the peak at 15.31 min indicated that it was likely a derivative of peucemycin (Fig. 1). The structure was supported by LC–MS/MS analysis of the compound with m/z 431.2071 [M + H]+ (expected mass m/z 431.2064), m/z 413.1956 [M-OH + H]+ (expected mass m/z 413.1959), m/z 175.1104 [M-B + H]+ (expected mass m/z 175.1117), and m/z 239.0910 [M-A + H]+ (expected mass m/z 239.0914) (Fig. S1). These findings led to the conclusion that the newly discovered compound was hydroxylated peucemycin. Furthermore, the compound had absorbance maxima of 200.7346, 215.7346, and 265.7347 nm similar to peucemycin and peucemycin A (Magar et al. 2023; Pham et al. 2021) (Fig. 1).
Growth rate analysis and production of peucemycin B
The maximum peucemycin B production occurred at 72 h, while the maximum growth occurred at 144 h (Fig. S2). At 72 h, the yield of peucemycin B was found to be 11.48 mg/L. Therefore, S. peucetius DM07 was cultured for 72 h to isolate the compound and determine its structure.
Structure elucidation of peucemycin B
The compound was isolated as a yellow powder that could readily dissolve in dimethylsulfoxide (DMSO). The structure of the compound was examined using NMR spectroscopy. Results are summarized in Table 1, Fig. 2, and Fig. S3. Compared to the structure of peucemycin (Pham et al. 2021), a chemical shift (δC) of C-19 from 21.24 ppm of peucemycin to 62.89 ppm of peucemycin B was found. Similarly, a chemical shift (δC) of C-20 from 14.01 ppm of peucemycin to 24.35 ppm of peucemycin B was found. 2D NMR data further supported the structure of peucemycin B. Correlation between carbon and proton of peucemycin B is summarized in Fig. 2. COSY correlations were observed between H-25/Me-26, H23/H24, H-19/Me-20, H-21/Me-22, and H-19/H-18 (Fig. S3c). Similarly, HMBC correlations were found between Me-20 to C-19, 18, Me-22 to C-21, C-17, and Me-26 to C-25, C24 (Fig. S3f). These results revealed the existence of three methyl groups in the structure. The presence of methine groups was identified from COSY correlations as seen among H-7 and H-8, and H-23 and H-24 while similar HMBC correlations were seen among H-23 and H-24 to C-25 and, H-7 and H-8 to H-9 and H-6. ROESY correlations among protons of C-9, C-8, and C-7 revealed that they were all present on the same side. Figure S3d gives information on additionally observed correlations. All of these results supported that the structure was 19-hydroxy peucemycin (peucemycin B).
Biological activities
Antibacterial activities of peucemycin B were weaker than those of peucemycin and peucemycin A. For Proteus hauseri NBRC 3851, peucemycin produced about two times higher antibacterial effect than peucemycin B when equivalent amounts of compounds were used (Fig. S4b). The growth of some S. aureus strains like S. aureus CCARM 0204 (MSSA) and S. aureus CCARM 3090 (MRSA) was inhibited by the compound at a high amount (Fig. S4a). These data showed that the compound had less antibacterial effects than peucemycin and peucemycin A.
Five different cancer cell lines were used to evaluate the anticancer activities of the compound (Fig. 3). IC50 values for MKN45 and NCI-H1650 were found to be 76.97 µM and 99.68 µM, respectively (Table S3). These results showed that the compound has mild activity against these cell lines. Similarly, for three other cell lines MDA-MB-231, U87MG, and Hep3B, its IC50 values were found to be 135.2 µM, 150.0 µM, and 175.4 µM, respectively (Table S3). These results showed that the compound was weakly active against these cell lines.
Heterologous expression of bldA and overexpression of peuR
The nucleotide sequence of peuR was found to have three rare TTA codons at the 13th, 52nd, and 74th codon positions that code for leucine (Fig. S6). S. peucetius does not have tRNA for this codon. Hence, bldA from S. coelicolor was heterologously expressed along with overexpression of peuR. At first, 873 bp bldA was amplified from S. coelicolor and ligated into pIBR25 at BamHI and XbaI sites to generate pbldA25 (Fig. S7). Then, 963 bp peuR was amplified from S. peucetius DM07 and ligated into pIBR25 at XbaI and HindIII sites to generate pR25 (Fig. S7). Finally, pbldAR25 vector having both genes was constructed by ligating peuR at XbaI and HindIII sites of pbldA25. The observation of a fragment with a size of 1836 bp in the gel after digesting with BamHI and HindIII confirmed the presence of both genes in pIBR25. Transformation of these vectors resulted in mutants S. peucetius R25, S. peucetius bldA25, S. peucetius bldAR25, and S. peucetius P25.
Analysis of the production of peucemycin and its derivatives as well as growth rate
The impact of heterologously expressed bldA and overexpressed peuR on the production of peucemycin and its derivatives were examined in all five strains (S. peucetius DM07, S. peucetius R25, S. peucetius bldA25, S. peucetius bldAR25, and S. peucetius P25) (Fig. 4). There was no significant difference in the production of peucemycin or its derivatives between S. peucetius DM07 and S. peucetius P25 as evidenced by their HPLC profiles (Fig. 4). With less variation in the production of peucemycin than the wild type, S. peucetius bldA25 strain produced peucemycin A (11.8 mg/L) and peucemycin B (21.2 mg/L) at 1.75 and 1.77 times higher levels than the wild type, respectively. Similarly, S. peucetius R25 produced a 1.86 times higher level of peucemycin A (12.5 mg/L) and 1.79 times higher level of peucemycin B (21.5 mg/L) with less variation in production of peucemycin (Fig. 5). On the other hand, S. peucetius bldAR25 significantly altered the production of peucemycin and its derivatives when compared with the wild type (Fig. 5). This strain produced 3.52 times more peucemycin A (23.8 mg/L) and 2.63 times more peucemycin B (31.5 mg/L) than the wild type. In addition, the amount of peucemycin (5.8 mg/L) in S. peucetius bldAR25 was 3.92 times lower than the wild type, indicating that, in contrast to the other four strains, peucemycin was converted to its derivatives at a significant rate in this strain (Fig. 5).
Comparison of the production of peucemycin and its derivatives. DM07, S. peucetius DM07; P25, S. peucetius P25; bldA25, S. peucetius bldA25; R25, S. peucetius R25; bldAR25, S. peucetius bldAR25. Standard deviation was calculated and represented as error bars from three experimental replicates for each sample. Student’s t-test is used to determine the p-values. Statistical significance: ** p < 0.01, *** p < 0.001
Next, we sought to determine how genes affected bacterial growth. When compared to the wild type, S. peucetius P25 did not exhibit any significant growth differences. Among the five strains, S. peucetius bldA25 exhibited reduced growth (Fig. 6). On the other hand, S. peucetius R25 strain had a slight reduction in growth, whereas S. peucetius bldAR25 grew similarly to the wild type (Fig. 6).
RT-PCR analysis of different strains
Transcriptional levels of peuA, peuG, and peuJ in several strains were examined. RNA polymerase subunit gene (rpoB) was used as a positive internal control. S. peucetius DM07 and S. peucetius P25 showed lower transcriptional levels of peuA, peuG, and peuJ (Fig. 7). On the other hand, heterologous expression of bldA resulted in higher transcriptional levels of peuA, peuG, and peuJ in S. peucetius bldA25 as compared with the wild-type strain (Fig. 7). Similarly, overexpression of peuR produced a higher transcriptional level of peuA as seen for S. peucetius R25 while showing similar transcriptional levels of other genes as that of S. peucetius bldA25 (Fig. 7). Finally, the highest transcriptional levels of peuG and peuJ were seen in S. peucetius bldAR25, while similar transcriptional levels were seen for peuA and peuR as compared with S. peucetius R25 (Fig. 7).
Discussion
In this work, a new peucemycin derivative (peucemycin B) was isolated, and both the pathway-specific regulator (PeuR) and global regulator (BldA) were investigated as targets for the improvement of the production of peucemycin and its derivatives. The newly identified derivative is synthesized from the hydroxylation at C-19 of peucemycin suggesting a separate hydroxylation step distinct from the C-25 hydroxylation of peucemycin A biosynthesis (Magar et al. 2023). The Peu BGC analysis provides insight into the likelihood that the PeuK is involved in the biosynthesis of peucemycin B (Magar et al. 2023).
The peucemycin B produced higher anticancer activity towards some cancer cell lines when compared to peucemycin and peucemycin A (Fig. 3 and Fig. S5) (Pham et al. 2021; Magar et al. 2023). The IC50 values of peucemycin B for MKN45 and NCI-H1650 cell lines were less than 100 µM, whereas those for MDA-MB-231, Hep3B, and U87MG cancer cell lines were higher than 100 µM. In comparison with some anticancer drugs that have very low IC50 values (Table S4), the compound is characterized as mildly active to weakly active against these cell lines (Table S3) (Jin et al. 2016; Abbasi et al. 2020; Wan et al. 2021; Choi et al. 2008; Zhan et al. 2018). The reduced solubility of the compound could be the cause of low anticancer activity. Therefore, enhancing solubility might help to improve activity.
Analysis of peuR revealed three TTA codons. TTA codon is regarded as a rare codon since the tRNA required for this UUA (leucine) is very rare in Streptomyces (Chater and Chandra 2008; Zaburannyy et al. 2009). The S. peucetius was found to be deficient in tRNA required for the UUA codon (Chater and Chandra 2008; Pokhrel et al. 2016). Hence, the combined effects of overexpression of peuR and heterologous expression of bldA were studied.
Similar patterns of peucemycin and its derivatives production were seen in the S. peucetius bldA25 and S. peucetius R25 strains, both of which had higher titers than the S. peucetius DM07 and S. peucetius P25 (Fig. 4 and Fig. 5). The results were also supported by the transcription level of some genes (Fig. 7). As predicted, overexpression of SARP gene contributed to higher production titer (Chen et al. 2015). Furthermore, the production results of S. peucetius DM07 and S. peucetius P25 suggest that a mistranslation of peuR mRNA may have resulted in a lower level of functional PeuR that regulates Peu BGC.
The final strain, S. peucetius bldAR25 produced the highest level of peucemycin derivatives (Fig. 4 and Fig. 5). The strain had the highest transcriptional levels of peuA, peuG, and peuJ, indicating that a functional copy of PeuR was synthesized in the presence of BldA (Fig. 7). Similarly, the results conclude that higher expression of peuJ is linked to the highest level of peucemycin A production. However, the gene responsible for the hydroxylation of peucemycin to peucemycin B is yet unknown and hence, the reason for the higher titer cannot be concluded from the existing results. All of these findings ultimately lead to the conclusion that the peuR is responsible for the regulation of Peu BGC, while BldA helps in producing a functional copy of PeuR.
In summary, we identified another peucemycin derivative and named it peucemycin B, 19-hydroxy peucemycin. Compared to peucemycin, the production of peucemycin derivatives was lower. Hence, in this study, we increased the production of peucemycin derivatives by using two regulators, a pathway-specific SARP regulator (PeuR) and a global regulator (BldA). We are now working on the gene responsible for the formation of peucemycin B.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Abbasi A, Hajialyani M, Hosseinzadeh L, Jalilian F, Yaghmaei P, Navid SJ, Motamed H (2020) Evaluation of the cytotoxic and apoptogenic effects of cinnamaldehyde on U87MG cells alone and in combination with doxorubicin. Res Pharm Sci 15:26–35. https://doi.org/10.4103/1735-5362.278712
Arcamone F, Cassinelli G (1998) Biosynthetic anthracyclines. Curr Med Chem 5:391–419
Arcamone F, Cassinelli G, Faktini G, Grein A, Orezzi P, Pol C, Spalla C (1969) Adriamycin, 14-hydroxydaunomycin, a new antitumor antibiotic from S. peucetius var. caesius. Biotechnol Bioeng 11:1101–1110. https://doi.org/10.1002/bit.260110607
Chater KF, Chandra G (2008) The use of the rare UUA codon to define “expression space” for genes involved in secondary metabolism, development and environmental adaptation in Streptomyces. J Microbiol 46:1–11. https://doi.org/10.1007/s12275-007-0233-1
Chen Y, Smanski MJ, Shen B (2010) Improvement of secondary metabolite production in Streptomyces by manipulating pathway regulation. Appl Microbiol Biotechnol 86:19–25. https://doi.org/10.1007/s00253-009-2428-3
Chen C, Zhao X, Chen L, Jin Y, Zhao ZK, Suh JW (2015) Effect of overexpression of endogenous and exogenous Streptomyces antibiotic regulatory proteins on tacrolimus (FK506) production in Streptomyces sp. KCCM11116P. RSC Adv 5:15756–15762. https://doi.org/10.1039/c4ra15038d
Choi J, Yip-Schneider M, Albertin F, Wiesenauer C, Wang Y, Schmidt CM (2008) The effect of doxorubicin on MEK-ERK signaling predicts its efficacy in HCC. J Surg Res 150:219–226. https://doi.org/10.1016/j.jss.2008.01.029
Fernández-Moreno MA, Caballero JL, Hopwood DA, Malpartida F (1991) The act cluster contains regulatory and antibiotic export genes, direct targets for translational control by the bldA tRNA gene of Streptomyces. Cell 66:769–780. https://doi.org/10.1016/0092-8674(91)90120-N
Ghimire GP, Oh TJ, Liou K, Sohng JK (2008) Identification of a cryptic type III polyketide synthase (1,3,6,8-tetrahydroxynaphthalene synthase) from Streptomyces peucetius ATCC 27952. Mol Cells 26:362–367
Ghimire GP, Koirala N, Sohng JK (2015) Activation of cryptic hop genes from Streptomyces peucetius ATCC 27952 involved in hopanoid biosynthesis. J Microbiol Biotechnol 25:658–661. https://doi.org/10.4014/jmb.1408.08058
Hackl S, Bechthold A (2015) The gene bldA, a regulator of morphological differentiation and antibiotic production in Streptomyces. Arch Pharm (weinheim) 348:455–462. https://doi.org/10.1002/ardp.201500073
Hickey RJ, Tresner HD (1952) A cobalt-containing medium for sporulation of Streptomyces species. J Bacteriol 64:891–892. https://doi.org/10.1128/jb.64.6.891-892.1952
Hindra PP, Elliot MA (2010) Regulation of a novel gene cluster involved in secondary metabolite production in Streptomyces coelicolor. J Bacteriol 192:4973–4982. https://doi.org/10.1128/JB.00681-10
Jin P, Wong CC, Mei S, He X, Qian Y, Sun L (2016) MK-2206 co-treatment with 5-fluorouracil or doxorubicin enhances chemosensitivity and apoptosis in gastric cancer by attenuation of akt phosphorylation. Onco Targets Ther 9:4387–4396. https://doi.org/10.2147/OTT.S106303
Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA (2000) Practical Streptomyces genetics. John Innes Foundation, Norwich
Kodani S, Komaki H, Suzuki M, Kobayakawa F, Hemmi H (2015) Structure determination of a siderophore peucechelin from Streptomyces peucetius. Biometals 28:791–801. https://doi.org/10.1007/s10534-015-9866-4
Krause J, Handayani I, Blin K, Kulik A, Mast Y (2020) Disclosing the potential of the SARP-type regulator PapR2 for the activation of antibiotic gene clusters in Streptomycetes. Front Microbiol 11:1–17. https://doi.org/10.3389/fmicb.2020.00225
Lu F, Hou Y, Zhang H, Chu Y, Xia H, Tian Y (2017) Regulatory genes and their roles for improvement of antibiotic biosynthesis in Streptomyces. 3 Biotech 7:1–15. https://doi.org/10.1007/s13205-017-0875-6
Magar RT, Pham VTT, Poudel PB, Nguyen HT, Bridget AF, Sohng JK (2023) Biosynthetic pathway of peucemycin and identification of its derivative from Streptomyces peucetius. Appl Microbiol Biotechnol 107:1217–1231. https://doi.org/10.1007/s00253-023-12385-8
Malla S, Niraula NP, Liou K, Sohng JK (2009) Enhancement of doxorubicin production by expression of structural sugar biosynthesis and glycosyltransferase genes in Streptomyces peucetius. J Biosci Bioeng 108:92–98. https://doi.org/10.1016/j.jbiosc.2009.03.002
Onaka H, Nakagawa T, Horinouchi S (1998) Involvement of two A-factor receptor homologues in Streptomyces coelicolor A3(2) in the regulation of secondary metabolism and morphogenesis. Mol Microbiol 28:743–753. https://doi.org/10.1046/j.1365-29581998.00832.x
Park HM, Kim BG, Chang D, Malla S, Joo HS, Kim EJ, Park SJ, Sohng JK, Kim PI (2013) Genome-based cryptic gene discovery and functional identification of NRPS siderophore peptide in Streptomyces peucetius. Appl Microbiol Biotechnol 97:1213–1222. https://doi.org/10.1007/s00253-012-4268-9
Pham VTT, Nguyen HT, Nguyen CT, Choi YS, Dhakal D, Kim TS, Jung HJ, Yamaguchi T, Sohng JK (2021) Identification and enhancing production of a novel macrolide compound in engineered Streptomyces peucetius. RSC Adv 11:3168–3173. https://doi.org/10.1039/d0ra06099b
Pokhrel AR, Chaudhary AK, Nguyen HT, Dhakal D, Le TT, Shrestha A, Liou K, Sohng JK (2016) Overexpression of a pathway specific negative regulator enhances production of daunorubicin in bldA deficient Streptomyces peucetius ATCC 27952. Microbiol Res 192:96–102. https://doi.org/10.1016/j.micres.2016.06.009
Poudel PB, Dhakal D, Magar RT, Sohng JK (2022) Microbial biosynthesis of chrysazin derivatives in recombinant Escherichia coli and their biological activities. Molecules 27:5554. https://doi.org/10.3390/molecules27175554
Rebets YV, Ostash BO, Fukuhara M, Nakamura T, Fedorenko VO (2006) Expression of the regulatory protein LndI for landomycin E production in Streptomyces globisporus 1912 is controlled by the availability of tRNA for the rare UUA codon. FEMS Microbiol Lett 256:30–37. https://doi.org/10.1111/j.1574-6968.2005.00087.x
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Singh B, Oh TJ, Sohng JK (2009) Exploration of geosmin synthase from Streptomyces peucetius ATCC 27952 by deletion of doxorubicin biosynthetic gene cluster. J Ind Microbiol Biotechnol 36:1257–1265. https://doi.org/10.1007/s10295-009-0605-0
Sthapit B, Oh TJ, Lamichhane R, Liou K, Lee HC, Kim CG, Sohng JK (2004) Neocarzinostatin naphthoate synthase: an unique iterative type I PKS from neocarzinostatin producer Streptomyces carzinostaticus. FEBS Lett 566:201–206. https://doi.org/10.1016/j.febslet.2004.04.033
Wan X, Hou J, Liu S, Zhang Y, Li W, Zhang Y, Ding Y (2021) Estrogen receptor α mediates doxorubicin sensitivity in breast cancer cells by regulating E-Cadherin. Front Cell Dev Biol 9. https://doi.org/10.3389/fcell.2021.583572
Weinstein MP, Patel JB, Campeau S, Eliopoulos GM, Galas MF, Humphries RM, Jenkins SG, Lewis JS II, Limbago B, Mathers AJ, Mazzulli T, Patel R, Richter SS, Satlin M, Swenson JM, Zimmer BL (2018) Performance standards for antimicrobial disk susceptibility tests, 28th edn. Clinical and laboratory standards institute, Wayne, PA
White J, Bibb M (1997) bldA dependence of undecylprodigiosin production in Streptomyces coelicolor A3(2) involves a pathway-specific regulatory cascade. J Bacteriol 179:627–633. https://doi.org/10.1128/jb.179.3.627-633.1997
Zaburannyy N, Ostash B, Fedorenko V (2009) TTA Lynx: a web-based service for analysis of actinomycete genes containing rare TTA codon. Bioinformatics 25:2432–2433. https://doi.org/10.1093/bioinformatics/btp402
Zhan Y, Wang K, Li Q, Zou Y, Chen B, Gong Q, Ho HI, Yin T, Zhang F, Lu Y, Wu W, Zhang Y, Tan Y, Du B, Liu X, Xiao J (2018) The novel autophagy inhibitor alpha-hederin promoted paclitaxel cytotoxicity by increasing reactive oxygen species accumulation in non-small cell lung cancer cells. Int J Mol Sci 19:1–15. https://doi.org/10.3390/ijms19103221
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (NRF-2021R1A2C2004775).
Author information
Authors and Affiliations
Contributions
RTM conceived and conducted the research and wrote the manuscript. RTM and VTTP designed the research. PBP assisted with carrying out the antimicrobial test. AFB helped in preparing some reagents and media. JKS conceived and supervised the research, helped in writing the manuscript, and edited the manuscript to the final version.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Magar, R.T., Pham, V.T.T., Poudel, P.B. et al. A new peucemycin derivative and impacts of peuR and bldA on peucemycin biosynthesis in Streptomyces peucetius. Appl Microbiol Biotechnol 108, 107 (2024). https://doi.org/10.1007/s00253-023-12923-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-023-12923-4