Abstract
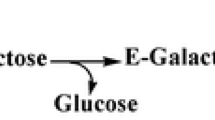
Galacto-oligosaccharides (GOS), which can be produced by enzymatic transgalactosylation of lactose with β-galactosidases, have attracted much attention in recent years because of their prebiotic functions and wide uses in infant formula, infant foods, livestock feed, and pet food industries. In this study, a novel β-galactosidase-producing Klebsiella oxytoca ZJUH1705, identified by its 16S rRNA sequence (GenBank accession no. MH981243), was isolated. Two β-galactosidase genes, bga 1 encoding a 2058-bp fragment (GenBank accession no. MH986613) and bga 2 encoding a 3108-bp fragment (GenBank accession no. MN182756), were cloned from K. oxytoca ZJUH1705 and expressed in E. coli. The purified β-gal 1 and β-gal 2 had the specific activity of 217.56 U mg−1 and 57.9 U mg−1, respectively, at the optimal pH of 7.0. The reaction kinetic parameters Km, Vmax, and Kcat with oNPG as the substrate at 40 °C were 5.62 mM, 167.1 μmol mg−1 min−1, and 218.1 s−1, respectively, for β-gal 1 and 3.91 mM, 14.6 μmol mg−1 min−1, and 28.9 s−1, respectively, for β-gal 2. Although β-gal 1 had a higher enzyme activity for lactose hydrolysis, only β-gal 2 had a high transgalactosylation capacity. Using β-gal 2 with the addition ratio of ~ 2.5 U g−1 lactose, a high GOS yield of 45.5 ± 2.3% (w/w−1) was obtained from lactose (40% w/w−1 or 480 g L−1) in a phosphate buffer (100 mM, pH 7.0) at 40 °C in 48 h. Thus, the β-gal 2 from K. oxytoca ZJUH1705 would be a promising biocatalyst for GOS production from lactose.
Key Points • A novel bacterial β-galactosidase producer was isolated and identified. • β-Galactosidases were cloned and expressed in heterologous strain and characterized. • Both enzymes have hydrolytic activity but only one have transglycosilation activity. • The developed process with β-gal 2 could provide an alternative for GOS production. |





Similar content being viewed by others
References
Aburto C, Castillo C, Cornejo F, Arenas-Salinas M, Vasquez C, Guerrero C, Arenas F, Illanes A, Vera C (2019) Beta-galactosidase from Exiguobacterium acetylicum: cloning, expression, purification and characterization. Bioresour Technol 277:211–215. https://doi.org/10.1016/j.biortech.2019.01.005
Albayrak N, Yang ST (2002a) Production of galacto-oligosaccharides from lactose by Aspergillus oryzae β-galactosidase immobilized on cotton cloth. Biotechnol Bioeng 77:8–19. https://doi.org/10.1002/bit.1195
Albayrak N, Yang ST (2002b) Immobilization of β-galactosidase on fibrous matrix by polyethylenimine for production of galacto-oligosaccharides from lactose. Biotechnol Prog 18:240–251. https://doi.org/10.1021/bp010167b
Bazhanov DP, Yatsevich KK, Bazhanova AA (2010) Phylogenetic identification of three strains of rhizosphere bacteria based on the results of 16s rRNA gene analysis and genetic typing. Microbiology 79:374–384. https://doi.org/10.1134/S0026261710030148
Burn SF (2012) Detection of beta-galactosidase activity: x-gal staining. Methods Mol Biol 886:241–250. https://doi.org/10.1007/978-1-61779-851-1_21
Carevic M, Vukasinovic-Sekulic M, Corovic M, Rogniaux H, Ropartz D, Velickovic D, Bezbradica D (2018) Evaluation of beta-galactosidase from Lactobacillus acidophilus as biocatalyst for galacto-oligosaccharides synthesis: product structural characterization and enzyme immobilization. J Biosci Bioeng 126:697–704. https://doi.org/10.1016/j.jbiosc.2018.06.003
Claus D (1992) A standardized gram staining procedure. World J Microb Biot 8:451–452. https://doi.org/10.1007/BF01198764
Cruz R, Cruz VD, Belote JG, Khenayfes MD, Dorta C, Oliveira LHD, Ardiles E, Galli A (1999) Production of transgalactosylated oligosaccharides (TOS) by galactosyltransferase activity from Penicillium simplicissimum. Bioresour Technol 70:165–171. https://doi.org/10.1016/S0960-8524(99)00033-4
Fan Y, Hua X, Zhang Y, Feng Y, Shen Q, Dong J, Zhao W, Zhang W, Jin Z, Yang R (2015) Cloning, expression and structural stability of a cold-adapted beta-galactosidase from Rahnella sp. R3. Protein Expres Purif 115:158–164. https://doi.org/10.1016/j.pep.2015.07.001
Frenzel M, Zerge K, Clawin-Rädecker I, Lorenzen PC (2015) Comparison of the galacto-oligosaccharide forming activity of different β-galactosidases. LWT Food Sci Technol 60:1068–1071. https://doi.org/10.1016/j.lwt.2014.10.064
Ghosh M, Pulicherla KK, Rekha VP, Raja PK, Rao KRSS (2012) Cold active beta-galactosidase from Thalassospira sp. 3SC-21 to use in milk lactose hydrolysis: a novel source from deep waters of Bay-of-Bengal. World J Microb Biot 28:2859–2869. https://doi.org/10.1007/s11274-012-1097-z
Guerrero C, Vera C, Illanes A (2013) Optimisation of synthesis of oligosaccharides derived from lactulose (fructosyl-galacto-oligosaccharides) with beta-galactosidases of different origin. Food Chem 138:2225–2232. https://doi.org/10.1016/j.foodchem.2012.10.128
Hidaka M, Fushinobu S, Ohtsu N, Motoshima H, Matsuzawa H, Shoun H, Wakagi T (2002) Trimeric crystal structure of the glycoside hydrolase family 42 beta-galactosidase from Thermus thermophilus A4 and the structure of its complex with galactose. J Mol Biol 322:79–91. https://doi.org/10.1016/S0022-2836(02)00746-5
Iqbal S, Nguyen TH, Nguyen HA, Nguyen TT, Maischberger T, Kittl R, Haltrich D (2011) Characterization of a heterodimeric GH2 β-galactosidase from Lactobacillus sakei Lb790 and formation of prebiotic galacto-oligosaccharides. J Agr Food Chem 59:3803–3811. https://doi.org/10.1021/jf103832q
Katrolia P, Zhang M, Yan Q, Jiang Z, Song C, Li L (2011) Characterisation of a thermostable family 42 β-galactosidase (BgalC) family from Thermotoga maritima showing efficient lactose hydrolysis. Food Chem 125:614–621. https://doi.org/10.1016/j.foodchem.2010.08.075
Li L, Li G, Cao LC, Ren GH, Kong W, Wang SD, Guo GS, Liu YH (2015) Characterization of the cross-linked enzyme aggregates of a novel beta-galactosidase, a potential catalyst for the synthesis of galacto-oligosaccharides. J Agr Food Chem 63:894–901. https://doi.org/10.1021/jf504473k
Mahoney RR (1998) Galactosyl-oligosaccharide formation during lactose hydrolysis: a review. Food Chem 63:147–154. https://doi.org/10.1016/S0308-8146(98)00020-X
Maischberger T, Leitner E, Nitisinprasert S, Juajun O, Yamabhai M, Nguyen TH, Haltrich D (2010) Beta-Galactosidase from Lactobacillus pentosus: purification, characterization and formation of galacto-oligosaccharides. Biotechnol J 5:838–847. https://doi.org/10.1002/biot.201000126
Manning TS, Gibson GR (2004) Prebiotics. Best Pract Res Cl Ga 2:287–298. https://doi.org/10.1053/ybega.2004.445
Martins GN, Ureta MM, Tymczyszyn EE, Castilho PC, Gomez-Zavaglia G (2019) Technological aspects of the production of fructo and galacto-oligosaccharides. Enzymatic synthesis and hydrolysis. Front Nutrition 6:78. https://doi.org/10.3389/fnut.2019.00078
Nguyen T, Splechtna B, Steinbock M, Kneifel W, Lettner HP, Kulbe KD, Haltrich D (2006) Purification and characterization of two novel β-galactosidases from Lactobacillus reuteri. J Agrc Food Chem 64:4989–4998. https://doi.org/10.1016/j.bej.2016.04.003
Osman A, Tzortzis G, Rastall RA, Charalampopoulos D (2012) BbgIV is an important Bifidobacterium beta-galactosidase for the synthesis of prebiotic galactooligosaccharides at high temperatures. J Agrc Food Chem 60:740–748. https://doi.org/10.1021/jf204719w
Park AR, Oh DK (2010) Galacto-oligosaccharide production using microbial β-galactosidase: current state and perspectives. Appl Microbiol Biotechnol 85:1279–1286. https://doi.org/10.1007/s00253-009-2356-2
Sun HS, You SP, Wang MF, Qi W, Su RX, He ZM (2016) Recyclable strategy for the production of high-purity galacto-oligosaccharides by Kluyveromyces lactis. J Agr Food Chem 64:5679–5685. https://doi.org/10.1021/acs.jafc.6b01531
Talens-Perales D, Gorska A, Huson DH, Polaina J, Marin-Navarro J (2016) Analysis of domain architecture and phylogenetics of family 2 glycoside hydrolases (GH2). PLoS One 11:1–17. https://doi.org/10.1371/journal.pone.0168035
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. https://doi.org/10.1093/molbev/mst197
Tokuda K, Hattori N, Donpou M, Shibayama K, Kuriyama T (1996) Beta-galactosidase. JP3556704B2
Torres DPM, Gonçalves MP, Teixeira JA, Rodrigues LR (2010) Galacto-oligosaccharides: production, properties, applications, and significance as prebiotics. Compr Rev Food Sci F 9:438–454. https://doi.org/10.1111/j.1541-4337.2010.00119.x
Vera C, Guerrero C, Conejeros R, Illanes A (2012) Synthesis of galacto-oligosaccharides by beta-galactosidase from Aspergillus oryzae using partially dissolved and supersaturated solution of lactose. Enzym Microb Technol 50:188–194. https://doi.org/10.1016/j.enzmictec.2011.12.003
Vera C, Cordova A, Aburto C, Guerrero C, Suarez S, Illanes A (2016) Synthesis and purification of galacto-oligosaccharides: state of the art. World J Microb Biot 32:197. https://doi.org/10.1007/s11274-016-2159-4
Wang J, Tian S, Yu H, Wang J, Zhu W (2019) Response of colonic mucosa-associated microbiota composition, mucosal immune homeostasis, and barrier function to early life galactooligosaccharides intervention in suckling piglets. J Agric Food Chem 67:578–588. https://doi.org/10.1021/acs.jafc.8b05679
Wu YF, Yuan SG, Chen S, Wu D, Chen J, Wu J (2013) Enhancing the production of galacto-oligosaccharides by mutagenesis of Sulfolobus solfataricus beta-galactosidase. Food Chem 138:1588–1595. https://doi.org/10.1016/j.foodchem.2012.11.052
Zhu YP, Li XT, Li JL, Teng C, Fan GS, Xu LY, Li F (2016) A beta-galactosidase-producing strain Klebsiella and application thereof. CN 201610928979:8
Funding
This study was supported by the Yunhe Program of Zhejiang University of Technology and the foundation (No.ZXKF20180204) of Tianjin Engineering Research Center of Microbial Metabolism and Fermentation Process Control, P.R. China.
Author information
Authors and Affiliations
Contributions
J H and SQ Z conceived and designed the research. LQ Z and L C conducted the experiments. MN D contributed new reagents or analytical tools. J H and ST Y analyzed the data. J H and SQ Z wrote the manuscript. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 840 kb)
Rights and permissions
About this article
Cite this article
Huang, J., Zhu, S., Zhao, L. et al. A novel β-galactosidase from Klebsiella oxytoca ZJUH1705 for efficient production of galacto-oligosaccharides from lactose. Appl Microbiol Biotechnol 104, 6161–6172 (2020). https://doi.org/10.1007/s00253-020-10679-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10679-9