Abstract
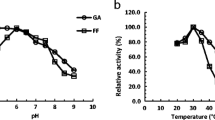
The aldehyde reductases from the short-chain dehydrogenase/reductase (SDR) family were identified as a series of critical enzymes for the improved tolerance of Saccharomyces cerevisiae to the aldehydes by catalyzing the detoxification reactions of aldehydes. Herein, we report that a novel aldehyde reductase Ykl107wp deduced from YKL107W from S. cerevisiae belongs to the classical SDR group and can catalyze the reduction reactions of acetaldehyde (AA), glycolaldehyde (GA), furfural (FF), formaldehyde (FA), and propionaldehyde (PA) but cannot reduce the six representative ketones. Ykl107wp displayed the best maximum velocity (Vmax), catalytic rate constant (Kcat), catalytic efficiency (Kcat/Km), and highest affinity (Km) to acetaldehyde. The optimum pH of Ykl107wp was 6.0 for the reduction of AA and 7.0 for the reduction of GA and FF, and the optimum temperatures were 40, 35, and 30 °C for the reduction of AA, GA, and FF, respectively. Ykl107wp for the reduction of AA was greatly affected by metal ions, chemical additives, and salts and showed poor thermal and pH stability, but its stability was slightly affected by a substrate. Ykl107wp was localized in endoplasmic reticulum and prevented the yeast cells from damage caused by furfural via the detoxification of furfural to furfural alcohol. This research provides guidelines for the study of uncharacterized classical SDR aldehyde reductases and exploration of their protective mechanisms on the corresponding organelles.







Similar content being viewed by others
References
Allen SA, Clark W, McCaffery JM, Cai Z, Lanctot A, Slininger PJ, Liu ZL, Gorsich SW (2010) Furfural induces reactive oxygen species accumulation and cellular damage in Saccharomyces cerevisiae. Biotechnol Biofuels 3:2. https://doi.org/10.1186/1754-6834-3-2
Chen CN, Porubleva L, Shearer G, Svrakic M, Holden LG, Dover JL, Johnston M, Chitnis PR, Kohl DH (2003) Associating protein activities with their genes: rapid identification of a gene encoding a methylglyoxal reductase in the yeast Saccharomyces cerevisiae. Yeast 20(6):545–554. https://doi.org/10.1002/yea.979
Drew D, Newstead S, Sonoda Y, Kim H, von Heijne G, Iwata S (2008) GFP-based optimization scheme for the overexpression and purification of eukaryotic membrane proteins in Saccharomyces cerevisiae. Nat Protoc 3(5):784–798. https://doi.org/10.1038/nprot.2008.44
Gietz RD, Schiestl RH, Willems AR, Woods RA (1995) Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 11(4):355–360. https://doi.org/10.1002/yea.320110408
Guo PC, Bao ZZ, Ma XX, Xia Q, Li WF (2014) Structural insights into the cofactor-assisted substrate recognition of yeast methylglyoxal/isovaleraldehyde reductase Gre2. Biochim Biophys Acta 1844(9):1486–1492. https://doi.org/10.1016/j.bbapap.2014.05.008
Gutiérrez T, Buszko ML, Ingram LO, Preston JF (2002) Reduction of furfural to furfuryl alcohol by ethanologenic strains of bacteria and its effect on ethanol production from xylose. Appl Biochem Biotechnol 98–100(1–9):327–340. https://doi.org/10.1385/abab:98-100:1-9:327
Hauser M, Horn P, Tournu H, Hauser NC, Hoheisel JD, Brown AJ, Dickinson JR (2007) A transcriptome analysis of isoamyl alcohol-induced filamentation in yeast reveals a novel role for Gre2p as isovaleraldehyde reductase. FEMS Yeast Res 7(1):84–92. https://doi.org/10.1111/j.1567-1364.2006.00151.x
Hazelwood LA, Daran JM, van Maris AJ, Pronk JT, Dickinson JR (2008) The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism. Appl Environ Microbiol 74(8):2259–2266. https://doi.org/10.1128/AEM.02625-07
Huh WK, Falvo JV, Gerke LC, Carroll AS, Howson RW, Weissman JS, O'Shea EK (2003) Global analysis of protein localization in budding yeast. Nature 425(6959):686–691. https://doi.org/10.1038/nature02026
Jayakody LN, Horie K, Hayashi N, Kitagaki H (2013) Engineering redox cofactor utilization for detoxification of glycolaldehyde, a key inhibitor of bioethanol production, in yeast Saccharomyces cerevisiae. Appl Microbiol Biotechnol 97(14):6589–6600. https://doi.org/10.1007/s00253-013-4997-4
Jornvall H, Landreh M, Östberg LJ (2015) Alcohol dehydrogenase, SDR and MDR structural stages, present update and altered era. Chem Biol Interact 234:75–79. https://doi.org/10.1016/j.cbi.2014.10.017
Kallberg Y, Oppermann U, Jornvall H, Persson B (2002) Short-chain dehydrogenases/reductases (SDRs): coenzyme-based functional assignments in completed genomes. Eur J Biochem 269(18):4409–4417. https://doi.org/10.1046/j.1432-1033.2002.03130.x
Kavanagh KL, Jornvall H, Persson B, Oppermann U (2008) The SDR superfamily: functional and structural diversity within a family of metabolic and regulatory enzymes. Cell Mol Life Sci 65:3895–3906. https://doi.org/10.1007/s00018-008-8588-y
Kikugawa K (1986) Fluorescent products derived from the reaction of primary amines and compounds in peroxidized lipids. Adv Free Radic Biol Med 2:389–417. https://doi.org/10.1016/S8755-9668(86)80020-5
Larroy C, Fernández M, González E, Parés X, Biosca JA (2002a) Characterization of the Saccharomyces cerevisiae YMR318C (ADH6) gene product as a broad specificity NADPH-dependent alcohol dehydrogenase: relevance in aldehyde reduction. Biochem J 361(Pt 1):163–172. https://doi.org/10.1042/0264-6021:3610163
Larroy C, Parés X, Biosca JA (2002b) Characterization of a Saccharomyces cerevisiae NADP(H)-dependent alcohol dehydrogenase (ADHVII), a member of the cinnamyl alcohol dehydrogenase family. Eur J Biochem 269(22):5738–5745. https://doi.org/10.1046/j.1432-1033.2002.03296.x
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc 56(3):658–666. https://doi.org/10.1021/ja01318a036
Liu ZL (2011) Molecular mechanisms of yeast tolerance and in situ detoxification of lignocellulose hydrolysates. Appl Microbiol Biotechnol 90(3):809–825. https://doi.org/10.1007/s00253-011-3167-9
Liu ZL (2018) Understanding the tolerance of the industrial yeast Saccharomyces cerevisiae against a major class of toxic aldehyde compounds. Appl Microbiol Biotechnol 102(13):5369–5390. https://doi.org/10.1007/s00253-018-8993-6
Liu ZL, Moon J (2009) A novel NADPH-dependent aldehyde reductase gene from Saccharomyces cerevisiae NRRL Y-12632 involved in the detoxification of aldehyde inhibitors derived from lignocellulosic biomass conversion. Gene 446(1):1–10. https://doi.org/10.1016/j.gene.2009.06.018
Liu ZL, Slininger PJ, Dien BS, Berhow MA, Kurtzman CP, Gorsich SW (2004) Adaptive response of yeasts to furfural and 5-hydroxymethylfurfural and new chemical evidence for HMF conversion to 2,5-bis-hydroxymethylfuran. J Ind Microbiol Biotechnol 31(8):345–352. https://doi.org/10.1007/s10295-004-0148-3
Liu ZL, Moon J, Andersh BJ, Slininger PJ, Weber S (2008) Multiple gene-mediated NAD(P)H-dependent aldehyde reduction is a mechanism of in situ detoxification of furfural and 5-hydroxymethylfurfural by Saccharomyces cerevisiae. Appl Microbiol Biotechnol 81(4):743–753. https://doi.org/10.1007/s00253-008-1702-0
Lopez-Villar E, Monteoliva L, Larsen MR, Sachon E, Shabaz M, Pardo M, Pla J, Gil C, Roepstorff P, Nombela C (2006) Genetic and proteomic evidences support the localization of yeast enolase in the cell surface. Proteomics 6(Suppl 1):S107–S118. https://doi.org/10.1002/pmic.200500479
Ma M, Wang X, Zhang X, Zhao X (2013) Alcohol dehydrogenases from Scheffersomyces stipitis involved in the detoxification of aldehyde inhibitors derived from lignocellulosic biomass conversion. Appl Microbiol Biotechnol 97(18):8411–8425. https://doi.org/10.1007/s00253-013-5110-8
Marchler-Bauer A, Zheng C, Chitsaz F, Derbyshire MK, Geer LY, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Lanczycki CJ, Lu F, Lu S, Marchler GH, Song JS, Thanki N, Yamashita RA, Zhang D, Bryant SH (2013) CDD: conserved domains and protein three-dimensional structure. Nucleic Acids Res 41(Database issue:D348–D352. https://doi.org/10.1093/nar/gks1243
Marchler-Bauer A, Derbyshire MK, Gonzales NR, Lu S, Chitsaz F, Geer LY, Geer RC, He J, Gwadz M, Hurwitz DI, Lanczycki CJ, Lu F, Marchler GH, Song JS, Thanki N, Wang Z, Yamashita RA, Zhang D, Zheng C, Bryant SH (2015) CDD: NCBI's conserved domain database. Nucleic Acids Res 43(Database issue):D222–D226. https://doi.org/10.1093/nar/gku1221
Marchler-Bauer A, Bo Y, Han L, He J, Lanczycki CJ, Lu S, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Lu F, Marchler GH, Song JS, Thanki N, Wang Z, Yamashita RA, Zhang D, Zheng C, Geer LY, Bryant SH (2017) CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucleic Acids Res 45(D1):D200–D203. https://doi.org/10.1093/nar/gkw1129
Moon J, Liu ZL (2012) Engineered NADH-dependent GRE2 from Saccharomyces cerevisiae by directed enzyme evolution enhances HMF reduction using additional cofactor NADPH. Enzym Microb Technol 50(2):115–120. https://doi.org/10.1016/j.enzmictec.2011.10.007
Moon J, Liu ZL (2015) Direct enzyme assay evidence confirms aldehyde reductase function of Ydr541cp and Ygl039wp from Saccharomyces cerevisiae. Yeast 32(4):399–407. https://doi.org/10.1002/yea.3067
Newstead S, Kim H, von Heijne G, Iwata S, Drew D (2007) High-throughput fluorescent-based optimization of eukaryotic membrane protein overexpression and purification in Saccharomyces cerevisiae. Proc Natl Acad Sci 104(35):13936–13941. https://doi.org/10.1073/pnas.0704546104
Nordling E, Jörnvall H, Persson B (2002) Medium-chain dehydrogenases/reductases (MDR). Family characterizations including genome comparisons and active site modeling. Eur J Biochem 269(17):4267–4276. https://doi.org/10.1046/j.1432-1033.2002.03114.x
Perrault I, Hanein S, Gerber S, Barbet F, Ducroq D, Dollfus H, Hamel C, Dufier JL, Munnich A, Kaplan J, Rozet JM (2004) Retinal dehydrogenase 12 (RDH12) mutations in leber congenital amaurosis. Am J Hum Genet 75(4):639–646. https://doi.org/10.1086/424889
Persson B, Nordling E, Kallberg Y, Lundh D, Oppermann UC, Marschall HU, Jörnvall H (1999) Bioinformatics in studies of SDR and MDR enzymes. Adv Exp Med Biol 463(463):373–377. https://doi.org/10.1007/978-1-4615-4735-8_46
Persson B, Hedlund J, Jornvall H (2008) Medium- and short-chain dehydrogenase/reductase gene and protein families: the MDR superfamily. Cell Mol Life Sci 65(24):3879–3894. https://doi.org/10.1007/s00018-008-8587-z
Persson B, Kallberg Y, Bray JE, Bruford E, Dellaporta SL, Favia AD, Duarte RG, Jörnvall H, Kavanagh KL, Kedishvili N, Kisiela M, Maser E, Mindnich R, Orchard S, Penning TM, Thornton JM, Adamski J, Oppermann U (2009) The SDR (short-chain dehydrogenase/reductase and related enzymes) nomenclature initiative. Chem Biol Interact 178(1–3):94–98. https://doi.org/10.1016/j.cbi.2008.10.040
Petersson A, Almeida JR, Modig T, Karhumaa K, Hahn-Hägerdal B, Gorwa-Grauslund MF, Lidén G (2006) A 5-hydroxymethyl furfural reducing enzyme encoded by the Saccharomyces cerevisiae ADH6 gene conveys HMF tolerance. Yeast 23(6):455–464. https://doi.org/10.1002/yea.1370
Rehbein H, Orlick B (1990) Comparison of the contribution of formaldehyde and lipid oxidation products to protein denaturation and texture deterioration during frozen storage of minced ice-fish fillet (Champsocephalus gunnari and Pseudochaenichthys georgianus). Int J Refrig 13(5):336–341. https://doi.org/10.1016/0140-7007(90)90066-6
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30(12):2725–2729. https://doi.org/10.1093/molbev/mst197
van Loon AP, Young E (1986) Intracellular sorting of alcohol dehydrogenase isoenzymes in yeast: a cytosolic location reflects absence of an amino-terminal targeting sequence for the mitochondrion. EMBO J 5(1):161–165. https://doi.org/10.1002/j.1460-2075.1986.tb04191.x
Vasiliou V, Pappa A, Petersen D (2000) Role of aldehyde dehydrogenases in endogenous and xenobiotic metabolism. Chem Biol Interact 129(1–2):1–19. https://doi.org/10.1016/S0009-2797(00)00211-8
Voulgaridou GP, Anestopoulos I, Franco R, Panayiotidis MI, Pappa A (2011) DNA damage induced by endogenous aldehydes: current state of knowledge. Mutat Res 711(1–2):13–27. https://doi.org/10.1016/j.mrfmmm.2011.03.006
Wang H, Ouyang Y, Zhou C, Xiao D, Guo Y, Wu L, Li X, Gu Y, Xiang Q, Zhao K, Yu X, Zou L, Ma M (2017a) YKL071W from Saccharomyces cerevisiae encodes a novel aldehyde reductase for detoxification of glycolaldehyde and furfural derived from lignocellulose. Appl Microbiol Biotechnol 101(23–24):8405–8418. https://doi.org/10.1007/s00253-017-8567-z
Wang HY, Xiao DF, Zhou C, Wang LL, Wu L, Lu YT, Xiang QJ, Zhao K, Li X, Ma M (2017b) YLL056C from Saccharomyces cerevisiae encodes a novel protein with aldehyde reductase activity. Appl Microbiol Biotechnol 101(11):4507–4520. https://doi.org/10.1007/s00253-017-8209-5
Wang H, Li Q, Kuang X, Xiao D, Han X, Hu X, Li X, Ma M (2018) Functions of aldehyde reductases from Saccharomyces cerevisiae in detoxification of aldehyde inhibitors and their biotechnological applications. Appl Microbiol Biotechnol 102(24):10439–10456. https://doi.org/10.1007/s00253-018-9425-3
Yamazaki S, Nomata J, Fujita Y (2006) Differential operation of dual protochlorophyllide reductases for chlorophyll biosynthesis in response to environmental oxygen levels in the cyanobacterium Leptolyngbya boryana. Plant Physiol 142(3):911–922. https://doi.org/10.1104/pp.106.086090
Yofe I, Weill U, Meurer M, Chuartzman S, Zalckvar E, Goldman O, Ben-Dor S, Schütze C, Wiedemann N, Knop M, Khmelinskii A, Schuldiner M (2016) One library to make them all: streamlining the creation of yeast libraries via a SWAp-Tag strategy. Nat Methods 13(4):371–378. https://doi.org/10.1038/nmeth.3795
Zhao X, Tang J, Wang X, Yang R, Zhang X, Gu Y, Li X, Ma M (2015) YNL134C from Saccharomyces cerevisiae encodes a novel protein with aldehyde reductase activity for detoxification of furfural derived from lignocellulosic biomass. Yeast 32(5):409–422. https://doi.org/10.1002/yea.3068
Funding
This work was supported by the National Natural Science Foundation of China (No. 31570086), the 2011 Collaborative Innovation Center for Farmland Protection and Agricultural Product Safety in Sichuan Province, and the Talent Introduction Fund of Sichuan Agricultural University (No. 01426100).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 168 kb)
Rights and permissions
About this article
Cite this article
Wang, H., Li, Q., Zhang, Z. et al. YKL107W from Saccharomyces cerevisiae encodes a novel aldehyde reductase for detoxification of acetaldehyde, glycolaldehyde, and furfural. Appl Microbiol Biotechnol 103, 5699–5713 (2019). https://doi.org/10.1007/s00253-019-09885-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-09885-x