Abstract
Devastating outbreaks of porcine epidemic diarrhea (PED) started in China in late 2010 and rapidly spread to North America and Asia causing severe diarrhea and high mortality in neonatal piglets, indicating that a new generation of vaccine against porcine epidemic diarrhea virus (PEDV) is urgently needed. In the present study, to mimic the native spike (S) glycoprotein, a stable cell line producing the trimeric ectodomain of S glycoprotein of the PEDV Pintung-52 (PEDV-PT) strain was successfully established by incorporating T4 bacteriophage foldon sequence of fibritin trimerization domains at the C-terminal end and replacing the signal peptide of S protein with the tissue plasminogen activator signal peptide sequence at the N terminal end. The trimeric structure, bio-reactivity to PEDV-specific antibodies, and the N-glycosylation level of the recombinant S protein were characterized. To induce systemic and mucosal immunity, conventional 5-week-old piglets were immunized with the trimeric S glycoprotein combined with the B subunit of Escherichia coli heat-labile enterotoxin (LTB) by the intramuscular (IM) route. As compared with the control group, all piglets in the S protein-LTB immunized (IM PEDV S-LTB) group generated systemic PEDV S-specific IgG and neutralizing antibody in blood but a low level of fecal PEDV-specific IgA and limited protection against challenge of PEDV-PT strain. Our results suggest that the recombinant PEDV trimeric S glycoprotein could be a potential subunit vaccine candidate against PEDV, but IM immunization with LTB as the adjuvant provided insufficient protection. The development of a vaccine regimen for inducing mucosal immunity is an important task for generating a successful subunit vaccine against PEDVs.
Similar content being viewed by others
Introduction
Before 2010, most of the historic type of porcine epidemic diarrhea (PED) strains caused epidemic or sporadic outbreaks with transient diarrhea with minimal effect on piglets. However, devastating PEDV outbreaks started in China in late 2010 and rapidly spread to North America and Asia (Chiou et al. 2017; Li et al. 2012; Park et al. 2011; Temeeyasen et al. 2014; Zhou et al. 2012) causing severe diarrhea and high mortality in neonatal piglets. So far, there are two conditionally licensed PEDV vaccines, alphavirus-based vaccine (Harrisvaccines) and an inactivated vaccine (Zoetis), available in the USA, but not available in other countries. Furthermore, the protective efficacy of these vaccines in the field is still uncertain (Langel et al. 2016). Therefore, a new generation of subunit vaccine against PEDV is still urgently needed.
PEDV belongs to Coronaviridae and contains seven open reading frames (ORFs) which encode for four structural proteins, including spike (S), membrane (M), envelope (E), and nucleocapsid (N), and three non-structural proteins, namely replicases 1a and 1b, and ORF3 (Song and Park 2012). The S protein of PEDV is a group of trimeric glycoproteins present on the viral surface and is composed of S1 and S2 domains (Sun et al. 2006). The S1 domain mediates the interaction between the host cell receptor glycoprotein for viral entry and attachment and induces the neutralizing antibodies in the hosts (Cruz et al. 2008; Sun et al. 2008). The S2 domain is responsible for membrane fusion. Furthermore, the CO-26K equivalent epitope (COE) (aa position 499–638) and N-terminal domain in S1 region have been reported as a neutralizing epitope and potential sugar co-receptor binding area for PEDV, respectively (Deng et al. 2016; Sun et al. 2006). Therefore, S protein has been a major target for PEDV vaccine development (Ge et al. 2012; Makadiya et al. 2016; Oh et al. 2014; Piao et al. 2016). The Taiwan PEDV Pintung-52 (PEDV-PT) strain, which was a high virulent genogroup 2b (G2b) PEDV strain isolated in Taiwan in early 2014, sharing 99.7 to 99.8% nucleotide sequence identity to the USA-Iowa 28- 2013, USA-Kansas 29-2013, USA-Texas 39-2013, or Canada-PEI-023 PEDV strains. The finding suggests that the Taiwan PEDV-PT S glycoprotein might serve as a good subunit vaccine candidate for protection of the G2b PEDVs worldwide (Chang et al. 2017; Chiou et al. 2017).
Recently, several studies using truncated S1 proteins or inactivate S protein combined with commercial adjuvants, such as water/oil/water multiple emulsions or TriAdj adjuvants, as subunit vaccine regimens by intramuscular (IM) administration have been demonstrated being able to induce robust systemic IgG and neutralizing antibody against PEDVs but fail to completely prevent clinical symptoms and fecal viral shedding (Lee et al. 2018; Oh et al. 2014; Subramaniam et al. 2018). Although vaccinations with various adjuvants administered by mucosal routes, such as intranasal or oral, have been intensely explored and appear promising for eliciting protective mucosal immunity (Haan et al. 2001; Marchioro et al. 2014), their application in clinical practice and pig farms has been limited due to technical, inconsistencies of vaccine dosage, and labor-intensive challenges. Intramuscular administration is one of the most convenient and easiest ways for farmers to administer vaccinations in pigs, whereas IM route is acknowledged as being capable to elicit antigen specific systemic immune responses but generally perceived as incapable of generating IgA responses or protective mucosal immunity. Cumulative data from recent studies suggest that systemically administered antigens with B subunit of Escherichia coli heat-labile enterotoxin (LTB) as adjuvant might be able to stimulate systemic and mucosal immunities to provide protection against viral or bacterial pathogens challenge in mice (Haan et al. 2001; Weltzin et al. 2000). However, the parenteral adjuvant activities of the LTB in pigs by IM administration are not explored.
In the present study, an immunogen containing trimeric full-length ectodomain of the Taiwan PEDV-PT S protein was generated. The structure and biological characteristics of the recombinant S protein were characterized. The immunogenicity and the protection efficacy of the recombinant S protein with LTB as a subunit vaccine regimen by IM administration against the PEDV-PT challenge were evaluated in 5-week-old, PEDV-seronegative conventional piglets.
Material and methods
Construction of the trimeric ectodomain of S protein of PEDV
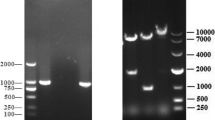
The nucleotide sequence of S ectodomain, derived from the Taiwan G2b PEDV-PT strain (GenBank accession no. KP276252), combined with T4 bacteriophage foldon sequence of fibritin trimerization domains (Tao et al. 1997) in the C-terminal end was codon optimized (GenBank accession no. MH085496) for human cell and synthesized by Genscript Corporation (Piscataway, NJ, USA). In addition, the signal peptide of the PEDV spike protein was replaced by a tissue plasminogen activator signal peptide (tPA-SP) sequence in order to increase the protein production (Wang et al. 2011). The schematic of the construct is shown in Fig. 1. The synthetic gene was inserted into BamHI-NotI restriction sites of the pcDNA™ 3.1/V5-His TOPO® vector (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. The size of the insert and vector were confirmed by gel electrophoresis and purified using QIAquick Gel Extraction Kit (Qiagen, Chatsworth, CA, USA). Ligation was performed using T4 DNA ligase and T4 DNA Ligase Reaction Buffer (New England Biolabs, Beverly, Mass, USA) and incubated at 16 °C overnight. One Shot TOP10 chemically Competent E. coli (Invitrogen) was used for transformation following the manufacturer’s descriptions. Plasmid extraction was performed by using QIAprep® Spin Miniprep Kit (Qiagen) as manufacturer’s protocol. The plasmid was further confirmed by nucleotide sequencing (Tri-I Biotech Inc., Taipei, Taiwan).
The construction schematic of the trimeric ectodomain of the PEDV spike protein. The vector, pcDNA3.1/V5-His-TOPO, contained sequences of human cytomegalovirus promoter (Pcmv), ampicillin and neomycin resistance genes, restriction enzyme sites (BamHI and Not I), V5 epitope, and six histidine tail (His6). PEDV-PT ectodomain sequence with tissue plasminogen activator signal peptide (tPA-SP) sequence at the N-terminal end and foldon domain of phage T4 fibritin (T4 foldon sequence) at the C-terminal end was inserted between the BamHI and Not I restriction enzyme sites of the pcDNA3.1/V5-His-TOPO vector
Cell transfection to establish stable cell line expressing ectodomain of S protein of PEDV
Human embryonic kidney (HEK) 293 cells (ATCC® CRL-1573™) were transfected with the plasmid using GenJet Plus In Vitro DNA Transfection Reagent (SignaGen® Laboratories, MD, USA) according to the manufacturer’s protocol. After 48 h of transfection, the culture medium was replaced with fresh culture medium containing 750 μg/ml Geneticin (G418, Gibco, Thermo Fisher Scientific). The cells were maintained in this medium for 2 weeks to establish a cell line stably expressing the trimeric S ectodomain protein of PEDV. Expression of the S protein was detected based on immunocytochemical staining (ICC) and western blot assay using the anti-V5 antibody (Invitrogen, Thermo Fisher Scientific).
Purification of recombinant S protein
The HEK 293 cells stably expressing full-length S protein were harvested and resuspended in 350 ml of FreeStyle 293 expression medium (Gibco) and were cultured in a CELLSPIN system (INTERGRA bioscience, NH, USA) for 7 days. The supernatant was collected, centrifuged at 1000 rpm for 10 min, and passed through a 0.22-μm pore size filter. Each liter of the supernatant was added to 20 ml of HisPur Cobalt Resin (Thermo Fisher Scientific) following the manufacturer’s protocols. The eluted protein was concentrated using Vivaspin® 20 (GE Healthcare Life Sciences, Uppsala, Sweden) with 100 kDa molecular weight cutoff, added with cOmplete™ EDTA-free Protease Inhibitor Cocktail (Roche Molecular Biochemicals, Laval, Quebec, Canada) and stored at − 20 °C for subsequent use.
Properties of the recombinant S protein
Trimeric conformation and glycoprotein size confirmation of the recombinant S protein
A gradient sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) gel was casted using T-Pro EZ Gel Solution (T-Pro Biotechnology, Taiwan) as per the manufacturer’s protocol. The purified S protein samples were mixed with 4X NuPAGE® LDS Sample Buffer (Thermo Fisher Scientific) and denatured at different temperatures, with or without 10X NuPAGE® Reducing Agent (Thermo Fisher Scientific). The S protein was subjected to either one of following conditions: heating at 65 °C for 10 min without reducing agent, heating at 95 °C for 5 min without reducing agent, or heating at 95 °C for 5 min with reducing agent. For detection of N-glycosylation pattern of PEDV S glycoprotein, the S protein was digested with or without peptide N-glycosidase F (PNGase F, New England Biolabs, MA, USA) according to the manufacturer’s instructions. After electrophoresis and protein transfer, polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA, USA) were blocked with 5% skim milk (diluted with Tris-buffered saline containing 0.05% Tween 20, TBST) for 1 h and washed with TBST. Western blotting was performed by staining with the anti-V5 antibody (1:5000 dilution in TBST buffer; Life Technologies, Novex, USA) and horseradish peroxidase-conjugated goat anti-mouse IgG (1:10000 dilution in blocking buffer; Jackson ImmunoResearch Laboratories, Philadelphia, USA) as primary and secondary antibodies, respectively. Finally, the membranes were developed using Clarity™ Western ECL Blotting Substrates (Bio-Rad) and detected by ChemiDoc™ Imaging Systems (Bio-Rad).
Bioreactivity of the recombinant S protein
The PVDF membranes used to confirm the protein size and trimeric structure of S protein were stripped using Restore™ Western Blot Stripping Buffer (Thermo Fisher Scientific) according to the manufacturer’s protocol, followed by six rounds of TBST washing. The membrane was re-probed with anti-PEDV hyperimmune serum (1:1000 dilution in TBST) and stained by horseradish peroxidase-conjugated goat anti-pig IgG (1:1000 dilution; Kirkegaard & Perry Laboratories, MD, US). Finally, the membranes were developed using Clarity™ Western ECL Blotting Substrates (Bio-Rad) and detected by ChemiDoc™ Imaging Systems (Bio-Rad).
Estimation of the molecular weight of the S glycoprotein
To estimate the protein size of PEDV S glycoprotein, native polyacrylamide gel electrophoresis using a 10% acrylamide/Bis-acrylamide (Bio-Rad) separating gel with 4% acrylamide/Bis-acrylamide stacking gel was performed. The S protein samples were mixed with 2X sample buffer, containing 25% glycerol (Sigma-Aldrich, St. Louis, Missouri, USA) and 1% bromophenol blue (Sigma-Aldrich) in 62.5 mM Tris-HCl at pH 6.8, and heated at 95 °C for 5 min. The protein electrophoresis was performed in running buffer containing 25 mM Tris and 192 mM glycine at 180 V for 4 h. After electrophoresis, the protein gel was stained with Coomassie Brilliant Blue R-250 Staining Solution (Bio-Rad) as per the manufacturer’s protocol and detected by ChemiDoc™ Imaging Systems (Bio-Rad).
Immunogenicity of the recombinant S protein
Immunization with the recombinant S protein and challenge with PEDV-PT strain
Six 5-week-old, PEDV seronegative and fecal PEDV RNA negative, large white x Duroc crossbred piglets were selected from a conventional pig farm. These piglets were randomly evenly divided into intramuscular-immunized (IM PEDV S-LTB; A1-A3, n = 3) and control (B1-B3, n = 3) groups. In the IM PEDV S-LTB group, the piglets were intramuscularly immunized with 50 μg of recombinant S protein, mixed with 10 μg of LTB (Sigma-Aldrich) (Weltzin et al. 2000) as an adjuvant (in a total volume of 1 ml), every 2 weeks (at 5, 7, and 9 weeks of age) for three times. Blood was collected from piglets at each time point of immunization and at 2 weeks after the third immunization (at 5, 7, 9, and 11 weeks of age). Plasma was stored at − 20 °C for antibody testing. After confirming the elevation of systemic PEDV-specific IgG in the S protein-immunized group, both groups of piglets were orally challenged with 5 × 105 TCID50 of PEDV-PT strain at 11 weeks of age as previously described (Chang et al. 2017). The fecal consistency was carefully monitored and recorded daily (Jung et al. 2015). Fecal swabs were collected every day for evaluation of fecal viral shedding and measurement of PEDV-specific IgA antibody titer. The animals were euthanized at 5 days postchallenge (DPC).
Antibody response of the piglets in the IM PEDV S-LTB and control groups
To evaluate the titers of PEDV-specific plasma IgG and fecal IgA, 96-well enzyme-linked immunosorbent assay (ELISA) plates coated with purified recombinant S protein (0.2 μg/well), following the manufacturer’s protocol of Coating Solution Concentrate Kit (Kirkegaard & Perry Laboratories), were used similar to a previous study (Chang et al. 2017). Twenty-fold diluted plasma and 2-fold diluted fecal samples were measured in duplicate for detection of plasma IgG and fecal IgA titers, respectively. The optical density (OD) was read at a wavelength of 405 nm by EMax Plus Microplate Reader (Molecular Devices, Crawley, UK). The result was expressed as sample to positive ratio (S/P ratio), and the cutoff value of both plasma IgG and fecal IgA detection was 0.1 (95% confidence level).
The plasma neutralizing antibody titer was measured as previously described (Chang et al. 2017). Heat-inactivated plasma samples were serially diluted 20-, 40-, 80-, 160-, and 320-fold, and consequently incubated with 500 TCID50 of PEDV-PT strain at 37 °C, 5% CO2 for 1 h. The cytopathogenic effects (CPEs) were monitored in the following 3 days. The neutralizing titer was determined as the last dilution without CPE.
Monitoring the fecal viral shedding after challenge
The feces collected from rectal swabs, ranging from 0.25 to 0.35 g, were suspended in 1 ml of PBS (Jung et al. 2015; Lin et al. 2015). RNA extraction and real-time RT-PCR were conducted to quantify the genomic equivalents (GE) in feces using SYBR® Advantage® qPCR Premix (Clontech, CA, USA) as previously described (Chang et al. 2017; Jung et al. 2014). Each reaction was confirmed by performing the melting curve assay, in which samples with a melting curve located between 84.7–86.1 °C were considered as positive. The efficiency of qPCR ranged from 90.42 to 96.92%.
Statistical analysis
The results of fecal viral shedding and plasma antibody titers were analyzed by one-way analysis of variance (ANOVA) using SAS 9.4 (Statistical Analysis System, SAS Institute Inc., Cary, NC, USA) and statistically compared by Scheffe’s method. A p value of < 0.05 was interpreted as statistically significant.
Result
Expression of the ectodomain of S protein of PEDV
The expression of the V5-tagged ectodomain of the PEDV-PT S protein was evaluated using immunocytochemistry staining (data not shown) and western blotting assay under the denaturing condition (Fig. 2). The molecular weight of the PEDV-PT S ectodomain was about 200–250 kDa, which was larger than the predicted size of 149 kDa. De-N-glycosylation by PNGase was performed to study the posttranslational modification of the S protein. Compared to the non-digested protein sample, a significant reduction in protein size after PNGase F treatment confirmed that the HEK-293-expressed PEDV-PT S protein was highly glycosylated (Fig. 2).
Western blot analysis of the expression of the ectodomain S protein of PEDV-PT strain and effect of enzymatic deglycosylation. The recombinant S protein was treated with peptide N-glycosidase F (PNGase F; lane PNGase F) or left untreated (lane S). Proteins were separated by SDS-PAGE, transferred to PVDF membranes, and incubated with the anti-V5 antibody (1:5000 dilution) as primary antibody and horseradish peroxidase conjugated goat anti-mouse IgG (1:10,000 dilution) as secondary antibody. Lane M, protein ladder
Trimeric conformation and bioactivity of the recombinant S protein
To determine the trimeric structure of the foldon peptide containing PEDV-PT S protein, the stability of the protein was evaluated under different temperature and reduction conditions (Fig. 3a). Under low level of reduction, as the PEDV-PT S protein was heated at 65 °C for 10 min without reducing buffer, the majority of the protein aggregated within the well but the remaining protein formed a thin band of about 200 kDa. When the protein samples were heated at 95 °C for 5 min without reducing buffer, a slow-migrating smeared band of a size larger than the 250 kDa was observed, suggestive of an oligomeric structure of the S protein. Under the complete reduction condition when the PEDV-PT S protein was heated at 95 °C for 5 min with reducing buffer, most of the S protein changed to a monomer of about 200 kDa. Additionally, as the same protein membrane was stripped and re-probed with an anti-PEDV hyperimmune antibody, the oligomeric and monomeric proteins, immunoreactive to the PEDV antibody, were detected (Fig. 3b). To confirm the molecular weight of the native S protein, native PAGE was performed. The presence of a band (> 720 kDa) larger than the predicted size (447 kDa) (Fig. 3c) suggests that the S protein exhibits posttranslational glycosylation and an asymmetric structure.
Illustration of the structure and bioactivity of the recombinant PEDV S protein. a The oligomeric status of the S protein under different levels of reductive treatments was detected by the anti-V5 antibody (1:5000 dilution). Samples in lanes 65 and 95 were respectively heated at 65 for 10 min and 95 °C for 5 min without reducing buffer, and samples in lane 95R were boiled at 95 °C for 5 min with reducing buffer. b The result from the same membrane as used in a when it was re-stained with hyperimmune serum (1: 1000 dilution) from a PEDV-infected pig. c The native form of recombinant trimeric protein in native PAGE gel. M, protein ladder; S, recombinant PEDV spike protein
Evaluation of immunogenicity of the recombinant S protein by detection of S-specific plasma IgG, fecal IgA, and neutralizing antibody titers
The S/P ratio of S-specific IgG titer in the control group remained undetectable during the experiment. As compared to the control group, the S/P ratio of PEDV S-specific IgG titer in the blood of piglets of the IM PEDV S-LTB group slightly increased at 2 weeks postpriming and significantly elevated at 2 weeks postadministration of the first and second boosters (Fig. 4). The S/P ratios of the PEDV S-specific IgG in piglets in IM PEDV S-LTB group were 0.035 ± 0.06, 0.60 ± 0.28, and 1.02 ± 0.37 at 2 weeks after the priming, first, and second boosters, respectively. To evaluate the titer of mucosal IgA, detection of PEDV-specific IgA in feces was performed in these animals. Overall, the mean S/P ratio of fecal IgA in IM PEDV S-LTB group, ranging from 0.031 ± 0.019 to 0.080 ± 0.051, was much lower than those of plasma IgG and had no significant difference from the control group, ranging from 0.038 ± 0.031 to 0.062 ± 0.040.
The changes in PEDV S-specific plasma IgG and fecal IgA titers. Five-week-old conventional piglets were intramuscularly immunized with PBS (control group) or 50 μg of recombinant spike protein mixed with 10 μg of B subunit of Escherichia coli heat-labile enterotoxin (LTB) (IM PEDV S-LTB group) three times with 2-week intervals. The result of the IgG titers was presented as sample/positive (S/P) ratio ± standard error of mean. *Significant difference with the control group (p < 0.05)
To evaluate the neutralizing activity of the elevated plasma antibodies in the IM PEDV S-LTB group, plasma samples taken before the immunization (5-week-old piglets) and 2 weeks after the second booster (11-week-old piglets) were used. As compared to the control group, piglets in the IM PEDV S-LTB group showed 20- to 50-fold higher plasma neutralizing antibody titer (Fig. 5).
The changes in plasma neutralizing antibody titers against PEDV-PT. Five-week-old conventional piglets were intramuscularly immunized with PBS (control group) or 50 μg of recombinant spike protein mixed with 10 μg of B subunit of Escherichia coli heat-labile enterotoxin (LTB) (IM PEDV S-LTB group) three times with 2-week intervals. The plasma neutralizing antibodies of the control and IM PEDV S-LTB groups in piglets at 5 and 9 weeks old are presented as mean values ± standard error of mean (SEM). The black and dash lines indicate the results of IM PEDV S-LTB and control groups, respectively
Evaluation of the efficacy of immunization against the challenge of virulent PEDV-PT strain passage 5
Clinical manifestations in the IM PEDV S-LTB and control groups
During the animal immunization period, none of the piglets had PEDV-associated diarrhea and other clinical signs. The clinical score of fecal consistency after the viral challenge for each day in each group is presented in Table 1. In the control group, all the piglets (3/3) started to show pasty to semi-fluid feces from 2 days post-challenge (DPC) and persisted clinical symptoms throughout the study. In the IM PEDV S-LTB group, only one piglet (A3) started showing pasty feces at 2 DPC. Another two piglets showed normal feces at 2 DPC. However, all piglets exhibited typical clinical signs (scores 2 and 3) at 3 DPC and persisted clinical symptoms throughout the study.
Fecal viral shedding in the IM PEDV S-LTB and control groups
The mean values of fecal viral shedding in each group after the oral challenge with the virulent PEDV-PT strain passage 5 is illustrated in Fig. 6. In the control group, piglets started shedding virus in feces from 1 DPC, reached the plateau at 2 DPC, and persisted the fecal viral shedding for at least 3 days (mean values ranging from 6.94 to 7.62 log10 GE). Compared to the control group, piglets in the IM PEDV S-LTB group showed a delay of 1 day in fecal viral shedding, which started from 2 DPC and persisted till the end of the study, with relatively lower fecal viral load. The mean value of the fecal PEDV viral load was 4.34 log10 GE at 2 DPC which reached the plateau of 6.11 to 6.44 log10 GE during 3–5 DPC. Additionally, piglet A1 had much lower fecal viral shedding, ranging from 2.81 to 4.42 log10 GE, than those of the other two piglets (data not shown).
The change in the fecal viral load after challenge with virulent PEDV-PT strain passage 5. Five-week-old conventional piglets were intramuscularly immunized with PBS (control group) or 50 μg of recombinant spike protein mixed with 10 μg of B subunit of Escherichia coli heat-labile enterotoxin (LTB) (IM PEDV S-LTB group) three times with 2-week intervals and were orally challenged with PEDV-PT-P5 at 11 weeks old. The fecal viral loads are expressed as mean values of the logarithm (base 10) of genomic equivalents ± standard error of mean (SEM). The threshold illustrates the limitation of detection of the SYBR Green-based real-time PCR. The black and dash lines demonstrate the results of IM PEDV S-LTB and control groups, respectively
Discussion
In the present study, a recombinant S glycoprotein, with temperature-dependent trimeric structure and mammalian posttranslational modifications to mimic immunogenicity, biological activity, and protein folding of S protein of the PEDV virion, was successfully generated by adding T4 bacteriophage foldon sequence of fibritin trimerization domain at C-terminal of the PEDV-PT S ectodomain. The recombinant S protein also exhibited immunoreactivity with PEDV hyperimmune serum, suggestive of the similarity of the S structure to the natural virus. Using the trimeric S protein combined with LTB immunization strategy, the trimeric PEDV S-LTB immunized group generated systemic PEDV S-specific IgG and neutralizing antibody in blood but a low level of fecal PEDV-specific IgA and limited protection against challenge of PEDV-PT strain. Our results suggest that the recombinant PEDV trimeric S glycoprotein could be a potential subunit vaccine candidate against PEDV, but IM immunization of the trimeric S glycoprotein with LTB as the adjuvant provided insufficient protection. The development of a vaccine regimen for inducing mucosal immunity is an important task for generating a successful subunit vaccine against PEDVs.
Intramuscular administration is the most predominant vaccine delivery method in pig farms. Recently, there have been several studies focusing on generation of different regions of S proteins, such as the COE domain, N-terminal domain (NTD), S1 domain, or the full-length monomeric S protein, via variable expression systems, such as E. coli, yeast, or HEK cells (Ge et al. 2012; Makadiya et al. 2016; Oh et al. 2014; Piao et al. 2016). By IM administration, these S proteins in combination with commercial adjuvants were not able to completely protect pigs against the challenge with virulent PEDV strains in different-aged pig models (Makadiya et al. 2016; Oh et al. 2014). Generation of protective immunity at intestinal mucosal surface is important to assist the host defense against PEDV.
To modify the strategy for PEDV subunit vaccine development, IM vaccination using trimeric S glycoprotein to mimic natural conformational structure of PEDV S glycoprotein for eliciting conformational neutralizing antibodies, along with the previous reported mucosal immune adjuvant, LTB, for inducing both humoral and mucosal immunity, was performed and evaluated in 5-week-old conventional piglets in the present study. LTB has been widely used as a potent mucosal adjuvant by oral, intranasal, or IM immunization routes to induce systemic and mucosal immunities in mice and pigs (Haan et al. 2001; Marchioro et al. 2014; Weltzin et al. 2000). Unfortunately, despite the high titers of plasma IgG and neutralizing antibody, the trimeric S glycoprotein combined with LTB was unable to elicit sufficient viral specific fecal IgA and protection against PEDV in piglets. For most viruses, specific IgA antibodies are generally accepted playing a key role in clearing the virus from mucosal sites during primary and secondary infections (Macpherson et al. 2001). IgA antibody in mucosal secretions has also been shown to protect against viral challenge in a dose-dependent manner in experimental animals (Johnson et al. 1986; Michalek et al. 1978). The low titer of fecal PEDV S-specific IgA detected in the IM PEDV-LTB group in the present study proposed a possibility, as supported by earlier studies mentioned above that IgA antibody if not the only, is the major factor responsible for the failure of the regimen of trimeric S ectodomain protein combined with LTB against PEDV in piglets. In order to effectively stimulate mucosal immunity against the disease, new regimens of potent mucosal adjuvants should be investigated in further studies.
In this study, 5-week-old postweaning piglets were used to evaluate the efficacy of the primitive vaccine regimen of the trimeric S ectodomain protein in combination with LTB. In the field, PEDV majorly causes severe enteric disease and high mortality rate in neonatal piglets. Therefore, vaccine strategies should focus on passive lactogenic immunity which could immediately provide protection to neonatal piglets (Langel et al. 2016). In sows, the colostrum is composed of large amount of IgG, which transudates from sow serum, transports across neonatal enterocytes and enters the neonatal bloodstream to provide systemic protection; the milk after the first 48 h becomes IgA-predominant, which is secreted by plasmablasts, traffics to mammary glands, and persists in the lumen of neonatal intestine to provide local protection (Klobasa et al. 1987; Langel et al. 2016). Therefore, to develop a subunit vaccine which could elicit both systemic IgG and local IgA in sow is important. In the present study, robust systemic IgG has been successfully induced in trimeric PEDV S protein immunized pigs. Further studies need to be done to evaluate if the systemic IgG could be secreted in colostrum for early protection of neonatal piglets against PEDV.
A full-length ectodomain trimeric S glycoprotein protein exhibiting folding and post-translational modifications similar to the native PEDV S protein, has been generated. The protein exhibiting mammalian glycosylation could be used for further structural, immunological, and biological analyses to provide a rational basis for understanding the accessibility to neutralizing antibodies and may pave the way for guiding future design of immunogens. The immunity generated through the IM vaccination strategy was systemic and robust but insufficient to protect from the disease. Our study demonstrates that systemic PEDV-specific IgG or neutralizing antibody could not be used as an indicator to evaluate the efficacy of the PEDV subunit vaccines. Development of a vaccine regimen for inducing both systemic and mucosal immunity will be an important task for generating a successful PEDV subunit vaccine.
References
Chang Y-C, Kao C-F, Chang C-Y, Jeng C-R, Tsai P-S, Pang V, Chiou H-Y, Peng J-Y, Cheng I-C, Chang H-W (2017) Evaluation and comparison of the pathogenicity and host immune responses induced by a G2b Taiwan porcine epidemic diarrhea virus (strain Pintung 52) and its highly cell-culture passaged strain in conventional 5-week-old pigs. Viruses 9(5):121
Chiou HY, Huang YL, Deng MC, Chang CY, Jeng CR, Tsai PS, Yang C, Pang VF, Chang HW (2017) Phylogenetic analysis of the spike (S) gene of the new variants of porcine epidemic diarrhoea virus in Taiwan. Transbound Emerg Dis 64(1):157–166. https://doi.org/10.1111/tbed.12357
Cruz DJM, Kim C-J, Shin H-J (2008) The GPRLQPY motif located at the carboxy-terminal of the spike protein induces antibodies that neutralize porcine epidemic diarrhea virus. Virus Res 132(1–2):192–196. https://doi.org/10.1016/j.virusres.2007.10.015
Deng F, Ye G, Liu Q, Navid MT, Zhong X, Li Y, Wan C, Xiao S, He Q, Fu ZF, Peng G (2016) Identification and comparison of receptor binding characteristics of the spike protein of two porcine epidemic diarrhea virus strains. Viruses 8(3):55. https://doi.org/10.3390/v8030055
Ge JW, Liu DQ, Li YJ (2012) Construction of recombinant lactobacilli expressing the core neutralizing epitope (COE) of porcine epidemic diarrhea virus and a fusion protein consisting of COE and Escherichia coli heat-labile enterotoxin B, and comparison of the immune responses by orogastric immunization. Can J Microbiol 58(11):1258–1267. https://doi.org/10.1139/w2012-098
Haan L, Verweij WR, Holtrop M, Brands R, van Scharrenburg GJ, Palache AM, Agsteribbe E, Wilschut J (2001) Nasal or intramuscular immunization of mice with influenza subunit antigen and the B subunit of Escherichia coli heat-labile toxin induces IgA- or IgG-mediated protective mucosal immunity. Vaccine 19(20–22):2898–2907
Johnson PR, Feldman S, Thompson JM, Mahoney JD, Wright PF (1986) Immunity to influenza A virus infection in young children: a comparison of natural infection, live cold-adapted vaccine, and inactivated vaccine. J Infect Dis 154(1):121–127
Jung K, Wang Q, Scheuer KA, Lu Z, Zhang Y, Saif LJ (2014) Pathology of US porcine epidemic diarrhea virus strain PC21A in gnotobiotic pigs. Emerg Infect Dis 20(4):662–665
Jung K, Annamalai T, Lu Z, Saif LJ (2015) Comparative pathogenesis of US porcine epidemic diarrhea virus (PEDV) strain PC21A in conventional 9-day-old nursing piglets vs. 26-day-old weaned pigs. Vet Microbiol 178(1–2):31–40. https://doi.org/10.1016/j.vetmic.2015.04.022
Klobasa F, Werhahn E, Butler JE (1987) Composition of sow milk during lactation. J Anim Sci 64(5):1458–1466
Langel SN, Paim FC, Lager KM, Vlasova AN, Saif LJ (2016) Lactogenic immunity and vaccines for porcine epidemic diarrhea virus (PEDV): historical and current concepts. Virus Res 226:93–107. https://doi.org/10.1016/j.virusres.2016.05.016
Lee SH, Yang DK, Kim HH, Cho IS (2018) Efficacy of inactivated variant porcine epidemic diarrhea virus vaccines in growing pigs. Clin Exp Vaccine Res 7(1):61–69. https://doi.org/10.7774/cevr.2018.7.1.61
Li W, Li H, Liu Y, Pan Y, Deng F, Song Y, Tang X, He Q (2012) New variants of porcine epidemic diarrhea virus, China, 2011. Emerg Infect Dis 18(8):1350–1353. https://doi.org/10.3201/eid1808.120002
Lin C-M, Annamalai T, Liu X, Gao X, Lu Z, El-Tholoth M, Hu H, Saif LJ, Wang Q (2015) Experimental infection of a US spike-insertion deletion porcine epidemic diarrhea virus in conventional nursing piglets and cross-protection to the original US PEDV infection. Vet Res 46(1):134. https://doi.org/10.1186/s13567-015-0278-9
Macpherson AJ, Hunziker L, McCoy K, Lamarre A (2001) IgA responses in the intestinal mucosa against pathogenic and non-pathogenic microorganisms. Microbes Infect 3(12):1021–1035
Makadiya N, Brownlie R, van den Hurk J, Berube N, Allan B, Gerdts V, Zakhartchouk A (2016) S1 domain of the porcine epidemic diarrhea virus spike protein as a vaccine antigen. Virol J 13:57. https://doi.org/10.1186/s12985-016-0512-8
Marchioro SB, Fisch A, Gomes CK, Jorge S, Galli V, Haesebrouck F, Maes D, Dellagostin O, Conceicao FR (2014) Local and systemic immune responses induced by a recombinant chimeric protein containing Mycoplasma hyopneumoniae antigens fused to the B subunit of Escherichia coli heat-labile enterotoxin LTB. Vet Microbiol 173(1–2):166–171. https://doi.org/10.1016/j.vetmic.2014.07.009
Michalek SM, McGhee JR, Babb JL (1978) Effective immunity to dental caries: dose-dependent studies of secretory immunity by oral administration of Streptococcus mutans to rats. Infect Immun 19(1):217–224
Oh J, Lee KW, Choi HW, Lee C (2014) Immunogenicity and protective efficacy of recombinant S1 domain of the porcine epidemic diarrhea virus spike protein. Arch Virol 159(11):2977–2987. https://doi.org/10.1007/s00705-014-2163-7
Park SJ, Kim HK, Song DS, Moon HJ, Park BK (2011) Molecular characterization and phylogenetic analysis of porcine epidemic diarrhea virus (PEDV) field isolates in Korea. Arch Virol 156(4):577–585. https://doi.org/10.1007/s00705-010-0892-9
Piao DC, Lee YS, Bok JD, Cho CS, Hong ZS, Kang SK, Choi YJ (2016) Production of soluble truncated spike protein of porcine epidemic diarrhea virus from inclusion bodies of Escherichia coli through refolding. Protein Expr Purif 126:77–83. https://doi.org/10.1016/j.pep.2016.05.018
Song D, Park B (2012) Porcine epidemic diarrhoea virus: a comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes 44(2):167–175. https://doi.org/10.1007/s11262-012-0713-1
Subramaniam S, Yugo DM, Heffron CL, Rogers AJ, Sooryanarain H, LeRoith T, Overend C, Cao D, Meng XJ (2018) Vaccination of sows with a dendritic cell-targeted porcine epidemic diarrhea virus S1 protein-based candidate vaccine reduced viral shedding but exacerbated gross pathological lesions in suckling neonatal piglets. J Gen Virol 99(2):230–239. https://doi.org/10.1099/jgv.0.001001
Sun D, Feng L, Shi H, Chen J, Liu S, Chen H, Wang Y (2006) Spike protein region (aa 636789) of porcine epidemic diarrhea virus is essential for induction of neutralizing antibodies. Acta Virol 51(3):149–156
Sun D, Feng L, Shi H, Chen J, Cui X, Chen H, Liu S, Tong Y, Wang Y, Tong G (2008) Identification of two novel B cell epitopes on porcine epidemic diarrhea virus spike protein. Vet Microbiol 131(1–2):73–81. https://doi.org/10.1016/j.vetmic.2008.02.022
Tao Y, Strelkov SV, Mesyanzhinov VV, Rossmann MG (1997) Structure of bacteriophage T4 fibritin: a segmented coiled coil and the role of the C-terminal domain. Structure 5(6):789–798
Temeeyasen G, Srijangwad A, Tripipat T, Tipsombatboon P, Piriyapongsa J, Phoolcharoen W, Chuanasa T, Tantituvanont A, Nilubol D (2014) Genetic diversity of ORF3 and spike genes of porcine epidemic diarrhea virus in Thailand. Infect Genet Evol 21:205–213. https://doi.org/10.1016/j.meegid.2013.11.001
Wang JY, Song WT, Li Y, Chen WJ, Yang D, Zhong GC, Zhou HZ, Ren CY, Yu HT, Ling H (2011) Improved expression of secretory and trimeric proteins in mammalian cells via the introduction of a new trimer motif and a mutant of the tPA signal sequence. Appl Microbiol Biotechnol 91(3):731–740. https://doi.org/10.1007/s00253-011-3297-0
Weltzin R, Guy B, Thomas WD Jr, Giannasca PJ, Monath TP (2000) Parenteral adjuvant activities of Escherichia coli heat-labile toxin and its B subunit for immunization of mice against gastric Helicobacter pylori infection. Infect Immun 68(5):2775–2782
Zhou YJ, Wu YL, Zhu JP, Tong W, Yu H, Jiang YF, Tong GZ (2012) Complete genome sequence of a virulent porcine epidemic diarrhea virus strain. J Virol 86(24):13862. https://doi.org/10.1128/JVI.02635-12
Acknowledgments
This work was supported by the Ministry of Science and Technology, Taiwan, R.O.C. (MOST 103-2321-B-002-077-MY3 and 105-2622-B-002-014-CC2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The animal experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of National Taiwan University (Taiwan, Republic of China, NTU105-EL-00087).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Chang, YC., Chang, CY., Tsai, PS. et al. Efficacy of heat-labile enterotoxin B subunit-adjuvanted parenteral porcine epidemic diarrhea virus trimeric spike subunit vaccine in piglets. Appl Microbiol Biotechnol 102, 7499–7507 (2018). https://doi.org/10.1007/s00253-018-9110-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9110-6