Abstract
Cystic fibrosis (CF) is one of the most common inherited and life-shortening pulmonary diseases in the Caucasian population. With the widespread introduction of newborn screening and the development of modulator therapy, tremendous advances have been made in recent years both in diagnosis and therapy. Since paediatric CF patients tend to be younger and have lower morbidity, the type of imaging modality that should be used to monitor the disease is often debated. Computed tomography (CT) is sensitive to many pulmonary pathologies, but radiation exposure limits its use, especially in children and adolescents. Conventional pulmonary magnetic resonance imaging (MRI) is a valid alternative to CT and, in most cases, provides sufficient information to guide treatment. Given the expected widespread availability of sequences with ultra-short echo times, there will be even fewer reasons to perform CT for follow-up of patients with CF. This review aims to provide an overview of the process and results of monitoring CF with MRI, particularly for centres not specialising in the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cystic fibrosis (CF) is one of the most common, inherited and life-limiting diseases in the Caucasian population. The mean prevalence in European Union (EU) countries is 0.74/10,000, with an incidence of 1/3,000 to 1/6,000 live births [1, 2]. A gene defect encoding a chloride channel causes this multisystemic disease whereby progressive lung disease is the leading cause of death [3]. While in the past CF often resulted in demise in childhood and adolescence, improvements in therapy and diagnostics have increased the median life expectancy into mid-adulthood [4]. Two factors have significantly altered the management of CF and, thus, survival in recent years: the widespread introduction of newborn screening, resulting in presymptomatic therapy [5] and the development of modulator therapies, allowing treatment of the defective chloride channel [6].
Why image the lungs?
Physical examination, microbiological and pulmonary function tests and laboratory parameters are used to monitor the progression of the disease. Structural lung changes occur in infancy and during the preschool period and may be missed in asymptomatic children [7, 8]. However, high-resolution cross-sectional imaging can detect abnormalities in the lungs more sensitively than lung function diagnostics and, thus, direct therapy at an early stage [8, 9]. It has been shown that in infants and young children, magnetic resonance imaging (MRI) correlates well with the (sensitive) lung clearance index [10]. In addition, imaging can sometimes identify the cause of pulmonary deterioration or even distinguish between fungal and other microbial causes of infection, enabling targeted therapy [11].
What do clinicians need to know?
Ideally, the treating pulmonologist needs precise and comprehensible parameters by which to assess the progression of the disease and from which they can immediately derive clues for patient management. The imaging modality is not the primary objective for the clinician. The radiologist, on the other hand, must be aware of the perspective and requests of the referring physician, even if they have not been explicitly phrased. Before any examination, consider:
a) Are there changes over time that have prognostic or therapeutic relevance for the patient? Multiple scores for each imaging modality are useful for such quantification of longitudinal trends [12,13,14,15].
b) Is there an acute event that requires immediate therapy? Examples include acute respiratory tract infective exacerbations or pulmonary haemorrhage. The radiologic assessment should, irrespective of the score, explicitly evaluate and report this aspect.
The basis for each score is the morphological, pathological alteration of the lung. These appear with different emphasis depending on the imaging modality: bronchial wall thickening, bronchiectasis, consolidations, bullae, mucus plugs and hyperinflation (Figs. 1, 2, 3, 4, 5 and 6) [16, 17]. In addition, functional assessment of regional changes in ventilation and perfusion is also desirable as complementary information.
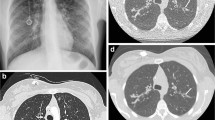
Magnetic resonance images in a 16-year-old girl with bronchiectasis in the upper lobes (arrows). a Coronal respiratory-triggered T2 fast spin echo and self-gated ultra-short echo time sequences with (b) coronal, (c) axial and (d) sagittal multiplanar reconstructions. Please note that air trapping distal to the dilated bronchi is only depicted in the latter
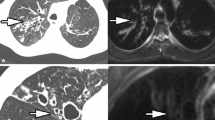
A 16-year-old boy with cystic fibrosis. A T2 fast spin echo magnetic resonance (MR) image (a). The mucus in the bronchiectasis in the basal right upper lobe is hypointense on T2 (arrow). An axial unenhanced computed tomography (CT) image (b). The mucus in the bronchiectasis in the basal right upper lobe is hyperdense on CT (arrow). This combination of MR and CT findings is characteristic of allergic bronchopulmonary aspergillosis and aspergilloma
An example of an unenhanced functional proton magnetic resonance image (MRI) (Fourier decomposition) for the estimation of ventilation by means of phase-resolved functional lung MRI (PREFUL) [16] in a free-breathing 6-year-old boy after severe pneumonia (acquisition time of 60 s). Bullae can be observed in the left lower lobe (basal) and the right upper lobe, which ventilate with delayed filling and emptying of air (ventilation circle with arrows around the image). The automated computation map (within the ventilation circle) reveals healthy lung (green) as well as extensive damage to the lungs with perfusion deficits (red) and ventilation-perfusion mismatch (purple)
Any change in appearance of morphological lesions forms the basis for prophylactic or therapeutic medical treatment and physiotherapy. In infective exacerbations, the imaging modality should ideally provide evidence of bacterial or mycotic aetiology. In the rare case of haemoptysis, angiographic imaging of the bronchial arteries is necessary since the haemorrhage may be treated by interventional occlusion [18].
Why MRI?
Traditionally, imaging of the lungs has been based on conventional projection radiography. Over the past decades, multiple chest radiograph-based scores have been developed to objectify the findings in CF [19, 20]. However, a considerable amount of subjectivity persists in reporting, especially in the evaluation of conventional radiographic images. Many lesions are only visualised as nonspecific surrogate findings (e.g., ring shadows, mottled shadows and soft shadows) [21]. Artificial intelligence-based software has potential to enhance the consistency of radiographic assessment, but still requires further development [22].
Given the limitations of conventional radiography, computed tomography (CT) as a fast, high-resolution, cross-sectional diagnostic technique is, therefore commonly used by many centres for follow-up in CF [23]. In addition, unlike MRI, CT achieves sufficient quality without sedation in children younger than 5 years of age [24].
The benefits of CT notwithstanding, the risk–benefit trade-off of routine CT follow-up needs to be reevaluated [25], even when effective dose is very low [26]. As a result of expanding treatment, the number of relatively healthy children who are regularly followed up has increased. Nowadays, the transition of paediatric patients to adult pulmonology is a regular (if not routine) event. Thus, in the context of the increasing life expectancy of patients with CF, an increase in tumour disease might potentially occur among patients as a late consequence of ionising radiation [27], which should therefore be minimised.
Pulmonary MRI, the radiation-free alternative to radiographs and CT, has the disadvantage of inadequate image quality in about 10% of examinations [28]. However, with simplified respiratory gating and new sequence patterns, as described below, lung MRI examinations are increasingly simpler to perform. This is expected to drastically reduce the number of inadequate MRI lung studies. Today, lung MRI is a viable alternative to chest CT [29, 30], as reflected by the growing body of comprehensive reviews on lung MRI in patients with CF [31, 32].
The basic protocol
Fundamental problems associated with lung imaging using MRI include the low density of protons, rapid signal loss due to T2* decay and, finally, the persistent and ubiquitous periodic motion of the diaphragm, thoracic wall, heart and great vessels. Lung MRI is feasible at both 1.5 T and 3 T [33]. Lower static magnetic field strength is beneficial for lung imaging due to its slower T2* decay [34, 35].
The basis of any pulmonary MRI is a thin-slice T2-weighted sequence [28] (Tables 1 and 2). Typically, this allows hyperintense visualisation of most pathologies: intrabronchial and alveolar mucus plugs, bronchial wall thickening, consolidations and abscesses. Given their excellent visualisation on conventional MRI sequences, these findings are also referred to as MR-plus pathologies. In contrast, smaller bullae without mass effect on surrounding lung structure and interstitial lung lesions such as fibrosis or bronchiectasis cannot reliably be detected with conventional lung sequences unless they are thick-walled or filled with mucus; thus, they are often referred to as MR-minus pathologies [36, 37]. However, this term is losing its relevance due to the more recent developments explained below [38]. T2-weighted fast spin echo sequences (FSE) with respiratory triggering are recommended for optimal quality, with an acquisition time of approximately 3 to 5 min, depending on the patient’s breathing rate. Thin slices of 3–4 mm are recommended to minimize voxel averaging that might prevent visualisation of micronodules or intralobular opacities. Often, substantially faster single-shot T2 spin echo sequences are within a few breath-hold manoeuvres; however, the clarity and hence the diagnostic confidence of these fast techniques for smaller lesions is inferior to respiratory-triggered FSE sequences [39, 40]. Therefore, even in older children, respiratory triggering for T2 imaging might be preferred. The different options for respiratory triggering (respiratory belt, diaphragm scout, phase scout in the liver or trigger systems integrated into the MR table) work comparably well and differ predominantly regarding their convenience of application [41, 42].
While T1 weighting is not mandatory for pulmonary MRI in most other conditions, it should be performed routinely in CF. The reason for this is the increased T1 signal in allergic bronchopulmonary aspergillosis, which along with the low T2 signal is considered characteristic of this disease (Fig. 5) [11]. Usually, T1 weighting is achieved as a 3-D gradient-echo acquisition in free breathing or during a single breath-hold command. Diffusion weighting may emerge as another parameter of disease severity in the future [43], but in routine diagnostics it is reserved for those situations when an abscess is suspected. Tables 1 and 2 provide exemplary protocols for different ages.
For the ambitious
A major advance in lung MRI in recent years has been the development of ultra-short echo time (UTE) sequences (or zero-TE sequences) (Figs. 1 and 3). Extremely short echo times of less than 0.05 ms precede the rapid T2* decay in the lungs, resulting in augmented signal even from healthy lung tissue [38]. At low flip angles, a proton-density weighted impression is obtained. MR-minus pathologies particularly can be delineated more adequately with UTE sequences than with conventional MRI techniques [44]. Self-gated UTE sequences are available in free breathing where even different breathing phases can be reconstructed. In addition, highly optimised UTE sequences that allow imaging of the complete lung in a single breath-hold manoeuvre are possible. High-resolution UTE sequences are often acquired as isovoxel 3-D sequences in free breathing and with a voxel size of less than 1.5 mm, like CT, allow multiplanar reconstruction. Once manufacturers adopt these UTE sequences into their standard portfolio, they will become an integral part of routine CF imaging.
Contrast-enhanced 3-D sequences with high temporal resolution rendered by keyhole imaging techniques are widely available techniques for qualitative assessment of lung perfusion [45]. Through hypoxic pulmonary vasoconstriction, perfusion also yields indirect information about ventilation. However, in cases of haemoptysis, with bronchial artery hypertrophy as a treatable cause of bleeding, CT angiography remains the modality of choice [46]. Whether routine use of contrast-enhanced functional assessments should be recommended outside the research setting remains unclear. Acknowledging that even macrocyclic contrast agents lead to deposition in the paediatric brain [47] and considering that progressively healthier and younger patients will be given an early diagnosis and new therapies in the future, the risk–benefit of routine contrast agent administration must be questioned. Another promising new method is non-contrast perfusion, such as arterial spin labelling [48] and variants of Fourier decomposition [49]. Since the latter provides highly encouraging results [50] and is not far from transitioning into clinical practice, it will be discussed in the next section.
For specialists
The clearest and most elaborate visualisation of ventilation is achieved with hyperpolarised gases such as 3helium and 129xenon. Thereby, a 3-D lung volume is acquired during a single breath-hold manoeuvre after the preceding inhalation of roughly 1 litre of the polarised gas. Though these methods represent ventilation disorders with high sensitivity [51, 52], three drawbacks have hindered their introduction into the clinical arena since their initial description 25 years ago: First, the sourcing and subsequent on-site polarisation of the gases is costly. Second, dedicated MR hardware enhancements are required, since standard high-frequency generators and receiver coils are designed for proton MRI [53]. Finally, handling of gas inhalation followed by immediate scanning requires extensive training. Therefore, this technique is performed only in a few specialised institutions and is not suitable for routine clinical use even in the medium term.
Fourier decomposition for the assessment of perfusion and ventilation is an important and elegant innovation in functional lung MRI and an alternative to both contrast administration and ventilation imaging with hyperpolarised gases (Fig. 6) [54, 55]. This promising technique combines perfusion and ventilation measurement and is based on ordinary proton MRI as opposed to sophisticated methods employing hyperpolarised gases. The requisite for this technique is a fast gradient echo sequence, which is available on every scanner; hence, the need for expensive investment is avoided. Thick coronal slices of about 10 mm are continuously acquired with high temporal resolution at a single slice position in free breathing. For each desired slice position, the acquisition time required for the reconstruction of one virtual ventilation and perfusion cycle is about 60 s. Subsequently, in postprocessing, ventilation and perfusion video clips as well as a quantitative mismatch map are calculated on any computer using dedicated software [56]. To date, this software is not commercially available, which means that the technology is currently only being used in a research context; however, clinical rollout is expected soon.
T1 mapping of the lung as a quantitative parameter has been proposed and explored for CF in the research context. But so far, this method has not found its way into routine clinical practice [57].
How to report?
As a first step, an assessment of whether the quality of the specific MRI examination is sufficient or whether some minor or pronounced diagnostic limitations remain is recommended. This assessment informs the pulmonologist whether the present examination is of sufficient quality to answer all questions. In case of pronounced diagnostic limitations, a short-term repeat MRI examination or CT must be arranged, if of acute relevance. After quality assessment, a free or structured report is prepared regarding acute changes in comparison to the previous examinations focusing on findings that require immediate treatment.
The challenge of MRI reporting arises from the fact that the lesions are often multiple and very unevenly distributed in the lung and that the irregularly confined typical CF lesions are more difficult to measure than, for example, nodules. Further, the T2 signal intensity as a relative value of pathology is not readily quantifiable, although there are early encouraging results in this regard [13, 58, 59]. Attempts have been made to address these challenges with several semiquantitative CF scores for MRI [12,13,14]. To quantify the extent of pulmonary pathology and to reflect disease progression in a single parameter, a semiquantitative score can now be applied. It must be emphasised that CF scores, whether for CT or MRI, have a certain investigator dependence and are not well validated in terms of their predictive power for clinically relevant outcomes [60]. However, there is sufficient evidence that they correlate adequately with other surrogate parameters of disease severity, such as forced expiratory volume in 1 s [12] or the lung clearance index [10].
A simple and feasible score is the Eichinger score [13], which consists of a morphological and an independent functional (MR perfusion) component (Table 3). An extension of this score for mosaic signal intensities has been recently proposed [61]. To estimate this score, the different lobes of the lung (upper lobe, lower lobe, middle lobe and lingula) are considered separately. For each lobe, alterations of the bronchial wall, mucus plugging, abscesses, consolidation and pleural alterations are scored semiquantitatively on a scale from zero to two, depending on whether the lesion is absent in the corresponding lobe or whether it affects less or more than half of the lobe. An improvement in the quantitative assessment of hyperinflation can be expected in the future from the incorporation of ventilation inhomogeneites as shown by contrast-enhanced or unenhanced perfusion, ventilation or UTE imaging. A generic disadvantage of scores is that it is often uncertain what weights should be assigned to the individual components. This might be revolutionized by deep-learning algorithms that can independently determine prognostic criteria. First approaches to the computer-aided diagnosis of CF have already been reported for CT [62, 63]. Adapting these algorithms to MRI is complicated by the more heterogeneous image quality of lung MRI compared with chest CT, but automated quantification seems possible [59, 64].
What to expect for the future?
Ultra-short echo time sequences will become standard additions to conventional CF lung MRI as soon as these sequences are made widely available by manufacturers. In addition, with non-contrast perfusion and ventilation measurements of the lung, these sequences will eliminate the need for contrast administration, which is still standard in some centres due to its sensitivity.
A few fundamental disadvantages of MRI compared to CT will linger on: First, the lengthy acquisition time of MRI sequences usually requires sedation in children up to about 5 years of age. Second, there are no studies on the cost effectiveness of MRI compared to CT. However, it is likely that such a comparison will favor CT even beyond the age of infants. Finally, standardisation of MRI protocols between different manufacturers is much more challenging than for CT. Standardisation of MRI protocols and reporting is strongly advocated for the future [65] and is being advanced by the imaging group of the Clinical Trial Network of the European Cystic Fibrosis Society.
Even today, lung MRI for pulmonary monitoring in CF is performed as a preferred method in many specialised institutions [31, 32]. However, the preference for lung MRI in CF in these institutions has so far been based on individual experience. A robust assessment of advantages and disadvantages regarding sensitivity of MRI compared to CT can be anticipated from a large ongoing multicentre study in France (https://clinicaltrials.gov/ct2/show/NCT03357562).
As a full evaluation of the lung can be achieved in free breathing in about 15 min, pulmonary MRI might soon replace chest CT as a standard in CF imaging in children.
References
Scotet V, L’Hostis C, Férec C (2020) The changing epidemiology of cystic fibrosis: Incidence, survival and impact of the CFTRGene discovery. Genes (Basel) 11:589
Farrell PM (2008) The prevalence of cystic fibrosis in the European Union. J Cyst Fibros 7:450–453
Elborn JS (2016) Cystic fibrosis. Lancet 388:2519–2531
Balfour-Lynn IM, King JA (2020) CFTR modulator therapies – Effect on life expectancy in people with cystic fibrosis. Paediatr Respir Rev 42:3–8
Southern KW, Mérelle MM, Dankert-Roelse JE, Nagelkerke AD (2009) Newborn screening for cystic fibrosis. Cochrane Database Syst Rev 2009:CD001402
Ramsey BW, Davies J, McElvaney NG et al (2011) A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 365:1663–1672
de Jong PA, Nakano Y, Lequin MH et al (2004) Progressive damage on high resolution computed tomography despite stable lung function in cystic fibrosis. Eur Respir J 23:93–97
Stahl M, Steinke E, Graeber SY et al (2021) Magnetic resonance imaging detects progression of lung disease and impact of newborn screening in preschool children with cystic fibrosis. Am J Respir Crit Care Med 204:943–953
Tiddens HAWM (2006) Chest computed tomography scans should be considered as a routine investigation in cystic fibrosis. Paediatr Respir Rev 7:202–208
Stahl M, Wielpütz MO, Graeber SY et al (2016) Comparison of lung clearance index and magnetic resonance imaging for assessment of lung disease in children with cystic fibrosis. Am J Respir Crit Care Med 195:349–359
Dournes G, Berger P, Refait J et al (2017) Allergic bronchopulmonary aspergillosis in cystic fibrosis: MR imaging of airway mucus contrasts as a tool for diagnosis. Radiology 285:261–269
Schaefer JF, Hector A, Schmidt K et al (2018) A semiquantitative MRI-Score can predict loss of lung function in patients with cystic fibrosis: Preliminary results. Eur Radiol 28:74–84
Eichinger M, Optazaite DE, Kopp-Schneider A et al (2012) Morphologic and functional scoring of cystic fibrosis lung disease using MRI. Eur J Radiol 81:1321–1329
Tepper LA, Ciet P, Caudri D et al (2016) Validating chest MRI to detect and monitor cystic fibrosis lung disease in a pediatric cohort. Pediatr Pulmonol 51:34–41
Helbich TH, Heinz-Peer G, Eichler I et al (1999) Cystic fibrosis: CT assessment of lung involvement in children and adults. Radiology 213:537–544
Glandorf J, Klimeš F, Voskrebenzev A et al (2020) Comparison of phase-resolved functional lung (PREFUL) MRI derived perfusion and ventilation parameters at 1.5T and 3T in healthy volunteers. PLoS One 15:e0244638
Hansell DM, Bankier AA, MacMahon H et al (2008) Fleischner Society: Glossary of terms for thoracic imaging. Radiology 246:697–722
Ittrich H, Bockhorn M, Klose H, Simon M (2017) The diagnosis and treatment of hemoptysis. Dtsch Arztebl Int 114:371–381
Terheggen-Lagro S, Truijens N, Van Poppel N et al (2003) Correlation of six different cystic fibrosis chest radiograph scoring systems with clinical parameters. Pediatr Pulmonol 35:441–445
Chrispin AR, Norman AP (1974) The systematic evaluation of the chest radiograph in cystic fibrosis. Pediatr Radiol 2:101–105
Davis SD, Fordham LA, Brody AS et al (2007) Computed tomography reflects lower airway inflammation and tracks changes in early cystic fibrosis. Am J Respir Crit Care Med 175:943–950
Zucker EJ, Barnes ZA, Lungren MP et al (2020) Deep learning to automate Brasfield chest radiographic scoring for cystic fibrosis. J Cyst Fibros 19:131–138
Smyth AR, Bell SC, Bojcin S et al (2014) European Cystic Fibrosis Society Standards of Care: Best Practice guidelines. J Cyst Fibros 13(Suppl 1):S23–S42
Kino A, Zucker EJ, Honkanen A et al (2019) Ultrafast pediatric chest computed tomography: comparison of free-breathing vs. breath-hold imaging with and without anesthesia in young children. Pediatr Radiol 49:301–307
Joyce S, Carey BW, Moore N et al (2021) Computed tomography in cystic fibrosis lung disease: a focus on radiation exposure. Pediatr Radiol 51:544–553
Moloney F, Kavanagh RG, Ronan NJ et al (2021) Ultra-low-dose thoracic CT with model-based iterative reconstruction (MBIR) in cystic fibrosis patients undergoing treatment with cystic fibrosis transmembrane conductance regulators (CFTR). Clin Radiol 76:393.e9-393.e17
Miglioretti DL, Johnson E, Williams A et al (2013) The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr 167:700–707
Hirsch FW, Sorge I, Vogel-Claussen J et al (2020) The current status and further prospects for lung magnetic resonance imaging in pediatric radiology. Pediatr Radiol 50:734–749
Renz DM, Scholz O, Böttcher J et al (2015) Comparison between magnetic resonance imaging and computed tomography of the lung in patients with cystic fibrosis with regard to clinical, laboratory, and pulmonary functional parameters. Investig Radiol 50:733–742
Hatabu H, Ohno Y, Gefter WB et al (2020) Expanding applications of pulmonary MRI in the clinical evaluation of lung disorders: Fleischner Society Position Paper. Radiology 297:286–301
Dournes G, Walkup LL, Benlala I et al (2021) The clinical use of lung MRI in cystic fibrosis. Chest 159:2205–2217
Woods JC, Wild JM, Wielpütz MO et al (2020) Current state of the art MRI for the longitudinal assessment of cystic fibrosis. J Magn Reson Imaging 52:1306–1320
Biederer J, Beer M, Hirsch W et al (2012) MRI of the lung (2/3). Why... when … how? Insights Imaging 3:355–371
Campbell-Washburn AE, Malayeri AA, Jones EC et al (2021) T2-weighted lung imaging using a 0.55-T MRI system. Radiol Cardiothorac Imaging 3:e200611
Chassagnon G, Martin C, Ben Hassen W et al (2019) High-resolution lung MRI with ultrashort-TE: 1.5 or 3 Tesla? Magn Reson Imaging 61:97–103
Dournes G, Menut F, Macey J et al (2016) Lung morphology assessment of cystic fibrosis using MRI with ultra-short echo time at submillimeter spatial resolution. Eur Radiol 26:3811–3820
Wielpütz M, Kauczor H-U (2012) MRI of the lung: state of the art. Diagn Interv Radiol 18:344–353
Wielpütz MO, Triphan SMF, Ohno Y et al (2019) Outracing lung signal decay – potential of ultrashort echo time MRI. RöFo 191:415–423
Cieszanowski A, Lisowska A, Dabrowska M et al (2016) MR imaging of pulmonary nodules: detection rate and accuracy of size estimation in comparison to computed tomography. PLoS ONE 11:e0156272
Hirsch W, Sorge I, Krohmer S et al (2008) MRI of the lungs in children. Eur J Radiol 68:278–288
Morita S, Ueno E, Suzuki K, Machida H, Fujimura M, Kojima S, Hirata M, Ohnishi T, Imura C (2008) Navigator-triggered prospective acquisition correction (PACE) technique vs. conventional respiratory-triggered technique for free-breathing 3D MRCP: an initial prospective comparative study using healthy volunteers. J Magn Reson Imaging 28:673–677
Santelli C, Nezafat R, Goddu B, Manning WJ, Smink J, Kozerke S, Peters DC (2011) Respiratory bellows revisited for motion compensation: preliminary experience for cardiovascular MR. Magn Reson Med 65:1097–1102
Ciet P, Serra G, Andrinopoulou ER et al (2016) Diffusion weighted imaging in cystic fibrosis disease: beyond morphological imaging. Eur Radiol 26:3830–3839
Gräfe D, Anders R, Prenzel F et al (2021) Pediatric MR lung imaging with 3D ultrashort-TE in free breathing: are we past the conventional T2 sequence? Pediatr Pulmonol 56:3899–3907
Wielpütz MO, Puderbach M, Kopp-Schneider A et al (2014) Magnetic resonance imaging detects changes in structure and perfusion, and response to therapy in early cystic fibrosis lung disease. Am J Respir Crit Care Med 189:956–965
Monroe EJ, Pierce DB, Ingraham CR et al (2018) An interventionalist’s guide to hemoptysis in cystic fibrosis. Radiographics 38:624–641
Blumfield E, Swenson DW, Iyer RS, Stanescu AL (2019) Gadolinium-based contrast agents — review of recent literature on magnetic resonance imaging signal intensity changes and tissue deposits, with emphasis on pediatric patients. Pediatr Radiol 49:448–457
Donnola SB, Dasenbrook EC, Weaver D et al (2017) Preliminary comparison of normalized T1 and non-contrast perfusion MRI assessments of regional lung disease in cystic fibrosis patients. J Cyst Fibros 16:283–290
Fischer A, Weick S, Ritter CO et al (2014) SElf-gated Non-Contrast-Enhanced FUnctional Lung imaging (SENCEFUL) using a quasi-random fast low-angle shot (FLASH) sequence and proton MRI. NMR Biomed 27:907–917
Behrendt L, Smith LJ, Voskrebenzev A et al (2022) A dual center and dual vendor comparison study of automated perfusion-weighted phase-resolved functional lung magnetic resonance imaging with dynamic contrast-enhanced magnetic resonance imaging in patients with cystic fibrosis. Pulm Circ 12:e12054
Thomen RP, Walkup LL, Roach DJ et al (2017) Hyperpolarized 129Xe for investigation of mild cystic fibrosis lung disease in pediatric patients. J Cyst Fibros 16:275–282
Bannier E, Cieslar K, Mosbah K et al (2010) Hyperpolarized 3 He MR for sensitive imaging of ventilation function and treatment efficiency in young cystic fibrosis patients with normal lung function. Radiology 255:225–232
Roos JE, McAdams HP, Kaushik SS, Driehuys B (2015) Hyperpolarized gas MR imaging: technique and applications. Magn Reson Imaging Clin N Am 23:217–229
Couch MJ, Munidasa S, Rayment JH et al (2021) Comparison of functional free-breathing pulmonary 1H and hyperpolarized 129Xe magnetic resonance imaging in pediatric cystic fibrosis. Acad Radiol 28:e209–e218
Bauman G, Puderbach M, Heimann T et al (2013) Validation of Fourier decomposition MRI with dynamic contrast-enhanced MRI using visual and automated scoring of pulmonary perfusion in young cystic fibrosis patients. Eur J Radiol 82:2371–2377
Behrendt L, Voskrebenzev A, Klimeš F et al (2020) Validation of automated perfusion-weighted phase-resolved functional lung (PREFUL)-MRI in patients with pulmonary diseases. J Magn Reson Imaging 52:103–114
Neemuchwala F, Ghadimi Mahani M, Pang Y et al (2020) Lung T1 mapping magnetic resonance imaging in the assessment of pulmonary disease in children with cystic fibrosis: a pilot study. Pediatr Radiol 50:923–934
Puderbach M, Eichinger M, Haeselbarth J et al (2007) Assessment of morphological MRI for pulmonary changes in cystic fibrosis (CF) patients. Investig Radiol 42:715–724
Benlala I, Hocke F, Macey J et al (2020) Quantification of MRI T2-weighted high signal volume in cystic fibrosis: a pilot study. Radiology 294:186–196
Calder AD, Bush A, Brody AS, Owens CM (2014) Scoring of chest CT in children with cystic fibrosis: state of the art. Pediatr Radiol 44:1496–1506
Leutz-Schmidt P, Stahl M, Sommerburg O et al (2018) Non-contrast enhanced magnetic resonance imaging detects mosaic signal intensity in early cystic fibrosis lung disease. Eur J Radiol 101:178–183
Konietzke P, Weinheimer O, Wielpütz MO et al (2018) Validation of automated lobe segmentation on paired inspiratory-expiratory chest CT in 8–14 year-old children with cystic fibrosis. PLoS ONE 13:e0194557
Chassagnon G, Martin C, Burgel PR et al (2018) An automated computed tomography score for the cystic fibrosis lung. Eur Radiol 28:5111–5120
Benlala I, Point S, Leung C et al (2020) Volumetric quantification of lung MR signal intensities using ultrashort TE as an automated score in cystic fibrosis. Eur Radiol 30:5479–5488
Ciet P, Bertolo S, Ros M et al (2022) State-of-the-art review of lung imaging in cystic fibrosis with recommendations for pulmonologists and radiologists from the “iMAging managEment of cySTic fibROsis” (MAESTRO) consortium. Eur Respir Rev 31:210173
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gräfe, D., Prenzel, F. & Hirsch, F.W. Chest magnetic resonance imaging in cystic fibrosis: technique and clinical benefits. Pediatr Radiol 53, 640–648 (2023). https://doi.org/10.1007/s00247-022-05539-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-022-05539-9