Abstract
The isotope ratios of zinc (66Zn/64Zn expressed as δ66Zn), a vital nutrient, increasingly demonstrate trophic discrimination among vertebrates, making δ66Zn a valuable dietary proxy for ecological, archaeological, and palaeontological studies. Given the novelty of the methodology, tissue-diet and tissue-tissue zinc isotope fractionation factors remain poorly understood and have so far only been studied in a few terrestrial mammals. Here, we investigate δ66Zn compositions of enameloid, bone, and white muscle of seven artificially-fed pisciculture gilt-head seabreams (Sparus aurata) from offshore Israel, in comparison to the Zn isotope composition of their diet. In addition, we also analysed δ66Zn values in the same tissues of wild-caught S. aurata, bluespotted seabream (Pagrus caeruleostictus) and grey triggerfish (Balistes capriscus) caught off the coast of Israel. We determine a tissue-diet δ66Zn offset for Sparus aurata of − 0.04 ± 0.09 ‰ (2SD) for bone, − 0.29 ± 0.06 ‰ (2SD) for enameloid, and − 0.45 ± 0.07 ‰ (2SD) for white muscle. Wild-caught fish have much higher among-individual δ66Zn variability with values distinct from the pisciculture S. aurata, documenting a much more isotopically heterogeneous diet consumed by the wild individuals. Still, tissue–tissue δ66Zn differences in wild-caught individuals are close to those observed in the pisciculture ones with progressively lower δ66Zn values in the order bone > enameloid > white muscle. Our results demonstrate predictable tissue-diet and tissue-tissue δ66Zn differences among fish hard and soft tissues and can be applied to identify the δ66Zn values of dietary input, thereby informing trophic (palaeo)ecological reconstructions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dietary zinc is a vital nutrient for animal health and has important functions in regulating gene expression and enzyme activity (Cousins 1998; Maret 2011; Maares and Haase 2020). Diets deficient in zinc can lead to high mortality rates, low growth rates, and other deficiency symptoms in fish (Ogino and Yang 1978). As such, zinc is of particular interest as a nutrient, especially for commercially relevant fish species, with research focussing on dietary zinc uptake and requirements (Serra et al. 1996; Isani et al. 2004; Nguyen et al. 2008), and bioaccumulation due to anthropogenic pollution (Clearwater et al. 2002; Reynders et al. 2008; Lozano-Bilbao et al. 2021). To our knowledge, however, zinc isotopes have not yet been used in vertebrate ecotoxicology or fisheries science context. Yet zinc isotope compositions (66Zn/64Zn), reported as δ66Zn value, vary significantly within different tissues of mammals (Balter et al. 2010, 2013; Moynier et al. 2013; Mahan et al. 2018) and as such offer great potential to investigate in a tissue-specific approach Zn anthropogenic contamination, toxicity levels or dietary requirements in fish.
In addition, δ66Zn is increasingly used as a trophic level proxy in ecological, archaeological, and palaeontological studies (Jaouen et al. 2016a, 2016b, 2022; Bourgon et al. 2020, 2021; McCormack et al. 2021, 2022b). Because zinc is supplied to marine fish tissues through the diet, and branchial uptake via gills is negligible (Bury et al. 2003; Ranaldi and Gagnon 2008), zinc isotope ratios in fish tissues, as with mammals, document dietary zinc uptake and trophic levels (McCormack et al. 2022b, 2023). Zinc isotopes, typically measured in the mineral phase of vertebrate osseous tissues (i.e., bioapatite) for ecological purposes, demonstrate progressively lower values with an increase in the animal’s trophic level (Jaouen et al. 2016a; McCormack et al. 2021). As such, zinc isotopes can be applied in a similar manner as the traditional trophic level tracer nitrogen isotopes (δ15N), which in contrast to δ66Zn, generally increases in δ15N values higher up the food chain. Zinc isotopes can be analysed in archaeological or palaeontological remains (mainly teeth, i.e., enamel/enameloid) in the absence of organic matter (i.e., collagen) preservation necessary for traditional δ15N analysis (Bourgon et al. 2020, 2021; Jaouen et al. 2022; McCormack et al. 2022b). Furthermore, zinc isotopes have a great potential to enable more robust and refined trophic analyses of extant and extinct animals when combined with δ15N than possible by analysing δ15N alone (McCormack et al. 2021, 2022a, 2023; Leichliter et al. 2023). Accurately determining trophic levels is imperative for understanding foraging ecology, species interactions and effective management and conservation strategies (Horstmann-Dehn et al. 2012).
Despite the multi-disciplinary applicability of δ66Zn, its variability within and among tissues of an individual is still poorly understood. Only a few studies investigated tissue-diet fractionation factors, so far exclusively limited to terrestrial mammals, namely sheep, mice, and minipigs (Balter et al. 2010, 2013; Moynier et al. 2013; Mahan et al. 2018). Understanding tissue-diet δ66Zn fractionation factors is of importance for ecotoxicology to monitor Zn pollution in vertebrates and species-specific diet-borne Zn toxicity, for fisheries to optimise dietary Zn uptake and bioavailability, and in ecology for investigating dietary shifts within an individual or population (e.g., during ontogeny, migration).
Here we investigate tissue-diet zinc isotope fractionation within pisciculture gilt-head seabreams (Sparus aurata), a common Mediterranean aquaculture species, kept on a controlled pellet diet in cages offshore Central Israel. We compare the δ66Zn values of the pellets to those of enameloid, bone and white muscle from seven S. aurata individuals of similar size and weight. Further, we take advantage of an ongoing marine life monitoring and protection program off the coast of Israel and compare tissue δ66Zn values among wild-caught S. aurata, bluespotted seabream (Pagrus caeruleostictus) and grey triggerfish (Balistes capriscus) individuals. These taxa were chosen as all three species are of commercial relevance in the Mediterranean, live in a coastal habitat and generally feed on similar trophic levels and prey items (Tancioni et al. 2003; Hamida et al. 2009; Taieb et al. 2013; Goldman et al. 2016). Finally, because these wild specimens were caught in the same region in which the pisciculture is located, we can directly compare tissue δ66Zn values and variability among wild and control-fed individuals.
Material and methods
Material
All specimens used in this study were collected legally and ethically, and most are housed in the Osteological Collection, Institute of Geosciences, Johannes Gutenberg-University, Mainz, Germany. We analysed enameloid, jaw bone, and white muscle from seven Sparus aurata individuals, harvested for food consumption, from a pisciculture (Lev-Yam Aquaculture Ltd.) located 3 km offshore of Central Israel (Michmoret). The pisciculture has four 3600-m3 tension leg cages (TLCs, Refamed, Italy) floating approximately 19 m above the seafloor (water depth 40 m) to which they are moored (Korzen et al. 2016). All S. aurata were harvested (June 2019) at body lengths of 250 to 270 mm and weights of 350 to 500 g (Supplementary Data 1). While the sizes of extruded pellets (Zemach Feedmill, Ltd, Israel) provided as diet along the fish life cycle varied, compositionally, the main pellet ingredients do not vary and there is no compositional variation at all for extruded pellets provided to fish > 40 g. The S. aurata were seeded into the open ocean cages at weights of 5 to 10 g were they remained until reaching commercial weights (350–500 g) between 12 and 16 months. Based on studies considering S. aurata tooth replacement (Elgendy et al. 2016; Sisma-Ventura et al. 2018) and feeding studies examining Zn uptake into different fish tissues (Serra et al. 1996; Sun and Jeng 1998), we consider all here analysed tissues of the pisciculture S. aurata to be in equilibrium with the diet.
In addition to the pisciculture S. aurata individuals, we also analysed wild fish caught as part of an ongoing monitoring program (permit number 516192-4399), funded by the Israeli Ministry of Environmental Protection. These include two S. aurata caught in March of 2014 and 2017, one Pagrus caeruleostictus caught in March of 2017, four P. caeruleostictus and three Balistes capriscus caught in March of 2020, two P. caeruleostictus caught in May of 2022 and three P. caeruleostictus caught in December of 2022, all from Haifa Bay, Israel. Details on the fish individuals used in this study (including catalogue numbers) are reported in Supplementary Data 1 with all δ66Zn values.
Tissue preparation
Fish specimens were dissected at the Israel Oceanographic & Limnological Research Institute, Haifa, Israel. White muscle tissue dissected from the dorsal musculature was rinsed with double distilled water, frozen, and lyophilised for 48 h. Freeze-dried samples were homogenised using a mortar and pestle, and then dried at 60 °C. Jaws were soaked in double distilled water for soft tissue removal. Four P. caeruleostictus and three B. capriscus jaws of individuals caught in March of 2020 were soaked in water from the main tap of the Israel Oceanographic & Limnological Research Institute Chemistry department (referred to, from here on, as tap water). In contrast to the jaws soaked in double distilled water, the jaw bones soaked in tap water have taken up Zn from the tap water; thus, these bone values are excluded from data interpretations (Supplementary Discussion 2).
Zinc isotope analysis
The here presented dataset also includes 13 enameloid and two bone δ66Zn values from 13 individuals already presented in McCormack et al. (2022b). Additional enameloid was sampled from different teeth for 11 of these 13 individuals to investigate within individual Zn isotope variability. In addition to the already published 15 δ66Zn values of McCormack et al. (2022b), this study comprises a total of 114 combined enameloid, bone, white muscle, and fish pellet δ66Zn values (Supplementary Data 1).
Prior to sampling tooth enameloid, dentine had been drilled out of the teeth, except in the 2022 caught wild P. caeruleostictus individuals, for which enameloid powder was drilled off the cleaned tooth cap. In both cases, enameloid drilling was done with a rotary tool equipped a diamond-tipped burr. Bone chunks were cut using a diamond-tipped cutting wheel. All enameloid caps, bone pieces, and muscle tissues were cleaned with ultrapure water (Milli-Q water) prior to digestion. The enamel and bone were dissolved in closed perfluoroalkoxy vials with 1 ml 1 M HCl on a hotplate for 1 h at 120 °C, then evaporated and re-dissolved in 1 ml 1.5 M HBr. White muscle tissue and fish pellets were mineralised by microwave digestion (10 min at 100 °C, 10 min at 180 °C in a multiwave PRO microwave, Anton Paar) in 50 ml PTFE-TFM bombs filled with 6 ml concentrated HNO3 at the department of Geography of the Johannes Gutenberg-University Mainz.
All pisciculture enameloid samples were prepared and measured for their Zn isotope compositions at the Max Planck Institute for Evolutionary Anthropology (MPI EVA). One set of bone and muscle (after microwave digestion) was prepared and measured at the MPI EVA, while the other was prepared and measured at the Frankfurt Isotope and Element Research Center (FIERCE) of the Goethe University Frankfurt, together with some of the wild P. caeruleostictus enameloid samples (Supplementary Data 1). Column chromatography and isotope measurements were performed as described in McCormack et al. (2022b). Zinc purification was performed in two steps, following the ion exchange method adapted from Moynier et al. (2006), described in Jaouen et al. (2016a), which included for each batch (13 samples) a chemistry blank and matrix-matched reference standard (NIST SRM 1400, bone ash) to monitor contamination and Zn elution. One ml of AG-1 × 8 resin (100–200 mesh) was placed in 10 ml hydrophobic interaction columns (Macro-Prep® Methyl HIC) cleaned twice with 5 ml 3% HNO3 and 5 ml ultrapure water and then conditioned with 3 ml 1.5 M HBr. Following the sample loading, 2 ml HBr was added to elute the matrix, followed by Zn elution with 5 to 8 ml HNO3. Following the second column step, the solution was evaporated overnight at 100 °C and re-dissolved in 1 ml 2 to 3% HNO3 ready for plasma mass spectrometry.
Zinc isotopes were analysed using a Thermo Fisher Neptune Plus MC-ICP-MS at FIERCE and a Thermo Fisher Neptune MC-ICP-MS at MPI EVA. Instrumental mass bias is corrected by copper doping and standard bracketing after Maréchal et al. (1999). The reference material Zn Alfa Aesar-MPI was used for standard bracketing. All δ66Zn values are expressed relative to the JMC Lyon standard material (mass-dependent Alfa Aesar-MPI offset of + 0.27 ‰ for δ66Zn (Jaouen et al. 2016a; McCoy-West et al. 2018)). Zn concentrations in the respective samples were estimated using a regression equation based on the Zn signal intensity (V) of three solutions with known Zn concentrations (150, 300 and 600 ppb). The δ66Zn measurement uncertainties per analytical session were determined from standard replicate analyses and ranged between 0.03 and 0.05 ‰ (2SD). Samples were typically measured in duplicate with mean analytical repeatability of 0.03 ‰ (2SD, n = 104). Reference material NIST SRM 1400, prepared and analysed alongside the samples for each column chromatography batch, yielded inter-laboratory consistent δ66Zn values of + 0.94 ± 0.06 ‰ (2SD, n = 19) which compares favourably to mean values between + 0.92 to + 0.97 ‰ as reported elsewhere (Bourgon et al. 2020; Jaouen et al. 2020; Mahan et al. 2020; McCormack et al. 2021, 2023). Reference materials and samples show a typical Zn mass-dependent isotopic fractionation, i.e., the absence of interferences, as the δ66Zn vs. δ67Zn and δ66Zn vs. δ68Zn values fall onto lines with slopes close to the theoretic mass fractionation values of 1.5 and 2, respectively (Supplementary Data 1). Column chemistry procedural blanks prepared alongside all samples document no relevant Zn contamination during sample dissolution and column chromatography as procedural blanks have average Zn signal intensities less than 0.1% compared to those from samples and in all cases less than 1.6%.
Results and discussion
Pisciculture Sparus aurata tissue-diet zinc isotope variability
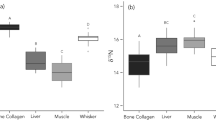
All artificially pellet-fed pisciculture Sparus aurata individuals have homogenous mean δ66Zn values for bone (+ 0.24 ± 0.09 ‰ 2SD, n = 7), enameloid (0.00 ± 0.06 ‰ 2SD, n = 7), and white muscle (− 0.16 ± 0.07 ‰ 2SD, n = 5; Table 1, Fig. 1). Pisciculture S. aurata zinc concentrations have tissue-specific ranges, decreasing from enameloid (545 to 1862 µg/g) to bone (65 to 99 µg/g) to white muscle (26 to 50 µg/g dry wt, Fig. 1b, Supplementary Discussion 1). The intra- and inter-individual tissue-specific δ66Zn standard deviations are within the range of the standard deviation for replicate analysis of NIST SRM 1400 (+ 0.94 ± 0.06 ‰ 2SD, n = 19; Table 1, Fig. 1). The δ66Zn values of the 5 mm extruded pellets (Zemach Feedmill, Ltd, Israel) analysed from a single batch are also very homogenous with a mean of + 0.29 ± 0.02 ‰ (2SD, n = 6, Fig. 1a).
Zinc isotope values (‰ JMC Lyon) in (a) and zinc concentrations (µg/g) in (b) from fish pellets and enameloid, bone, and white muscle of pisciculture Sparus aurata. Mean pisciculture S. aurata δ66Zn values, and Zn concentrations are provided relative to the fish pellets with whiskers indicating 2SD and arrows and numbers depicting mean relative isotopic differences (Δ66Zntissue-diet) and mean relative concentration differences. Maximum measurement uncertainty is given in a) in 2SD. Zinc concentration is plotted on a logarithmic scale
We assume that for the pisciculture Sparus aurata individuals, pellets are the main (and likely only) source of dietary zinc uptake, as these cages are floating too high above the seafloor for S. aurata individuals to reach their common benthic prey sources (Tancioni et al. 2003; Taieb et al. 2013). In addition, the fish were fed twice a day with pellets weighing up to 2% of the total biomass in each cage (Korzen et al. 2016), making it unlikely that any diet other than the pellets could have contributed significantly to the dietary Zn uptake. Therefore, we consider the average S. aurata δ66Zn offsets between pellets and specific tissues (Δ66Zntissue-diet = δ66Zntissue− δ66Zndiet) as tissue-diet discrimination values. Mean tissue-diet δ66Zn discrimination for S. aurata are thus − 0.04 ± 0.09 ‰ (2SD, n = 7) for bone (Δ66Znbone-diet), − 0.29 ± 0.06 ‰ (2SD, n = 7) for enameloid (Δ66Znenameloid-diet), and − 0.45 ± 0.07 ‰ (2SD, n = 5) for white muscle (Δ66Znmuscle-diet) (Fig. 1a, Table 2).
Compared to previous feeding experiments on terrestrial mammals (sheep and mice), tissue-diet fractionation factors for Sparus aurata are notably different and may not be directly comparable (Fig. 2). Mice bone δ66Zn values are higher than dietary values with mean Δ66Znbone-diet values of + 0.47 ± 0.26 ‰ (2SD, n = 16, (Moynier et al. 2013)) and + 0.25 ± 0.12 ‰ (2SD, n = 4, (Balter et al. 2013)). While mice muscle δ66Zn values are also 66Zn depleted compared to their diet, the Δ66Znmuscle-diet values are higher compared to the pisciculture Sparus aurata, with mice Δ66Znmuscle-diet values of − 0.19 ± 0.61 ‰ (2SD, n = 5, (Moynier et al. 2013)) and − 0.14 ± 0.12 ‰ (2SD, n = 3, (Balter et al. 2013)). For sheep, Δ66Znbone-diet values and Δ66Znmuscle-diet values were reported to be similar to each other, + 0.17 ± 0.15 ‰ and + 0.18 ± 0.28 ‰, respectively (2SD, n = 4, (Balter et al. 2010)). The reasons for the observed Δ66Zntissue-diet and Δ66Zntissue-tissue differences among previously reported feeding experiments and S. aurata remain ambiguous (Fig. 2). Besides potential physiological factors, perhaps linked to taxonomy, digestive physiology, and potential differences in Zn bioavailability of respective diets, differences might also relate to tissues not being in equilibrium with the diet. For example, the mice from Moynier et al. (2013) were euthanised at various intervals after birth, whereas the sheep in Balter et al. (2010) are significantly larger animals and were kept on the experimental diet for a limited time period only. Zinc metabolic turnover rates, which are largely unknown, differ among tissues perhaps also due to physiological factors such as body size and growth rate, as is the case for other dietary proxies such as nitrogen and calcium (Skulan and DePaolo 1999; Trueman et al. 2005).
Tissue-diet and tissue-tissue fractionation factors within an individual approximated by the Δ value (i.e., Δ66Zntissue-diet and Δ66Zntissue-tissue) for the pisciculture Sparus aurata, mice from Moynier et al. (2013) (blue) and Balter et al. (2013) (light blue), and sheep from Balter et al. (2010) (yellow). Each point represents a single individual for which tissues and diet plotted on the x-axis could be analysed. The boxes, for n ≥ 5, represent the 25th–75th percentiles (with the median as a horizontal line), and the whiskers show the 10th–90th percentiles. The number of individuals analysed is given by n in grey
Although the here reported δ66Zn tissue-diet fractionation factors differ from those reported for mice and sheep (Balter et al. 2010, 2013; Moynier et al. 2013), the tissue-tissue fractionation factors between bone and muscle of S. aurata and mice are similar. The pisciculture S. aurata δ66Zn muscle–bone fractionation factor (Δ66Znmuscle-bone) is − 0.42 ± 0.04 ‰ (2SD, n = 5, Figs. 1, 2), while mice Δ66Znmuscle-bone are − 0.62 ± 0.65 ‰ (2SD, n = 4, (Moynier et al. 2013)) and − 0.41 ± 0.15 ‰ (2SD, n = 3, (Balter et al. 2013)). The consistently lower δ66Zn values in muscle compared to bone may be tentatively explained by differences in the tissue-specific Zn coordination environment (Balter et al. 2013; Moynier et al. 2013; Mahan et al. 2018). Heavier Zn preferentially binds to ligands with a stronger electronegativity (O > N > S) (Balter et al. 2013; Moynier et al. 2013; Fujii et al. 2014). In bioapatite, Zn is bonded to oxygen atoms of one hydroxyl (OH) and three phosphate groups (PO4) (Tang et al. 2009), accordingly leading to an enrichment of heavy Zn. Conversely, Zn in muscle proteins binds to different amino acids, e.g., histidine in myosin (Ababou et al. 2008), where Zn is bonded to N (Maret 2012), which leads to a depletion of heavy Zn. However, ab initio calculations considering Zn coordination in muscle proteins for different muscle types and taxa, beyond the scope of our paper, would be necessary to quantify these results.
To our knowledge, no enamel(oid)-diet δ66Zn fractionation factors have been previously reported. Yet, the pisciculture S. aurata enameloid-bone δ66Zn offset (Δ66Znenameloid-bone) of − 0.24 ± 0.11 ‰ (2SD, n = 7) is very similar to previously reported mean Δ66Znenamel-bone of − 0.2 ‰ in terrestrial mammals from Koobi Fora, Kenya (Jaouen et al. 2016a), the enamel-bone offset of − 0.18 ‰ in humans (Jaouen et al. 2017) and tooth enameloid-osteodentine offset in divers elasmobranch species of − 0.21 ‰ (McCormack et al. 2022b). Noteworthy, systematic isotopic offsets between enamel and bone/dentine were also observed for bioapatite carbonate–oxygen and –carbon (δ18O and δ13C, (Webb et al. 2014)) and calcium isotopes (δ44/40Ca, (Heuser et al. 2011)) across different species, despite different metabolic functions of these elements. We thus assume that the Δ66Znenamel(oid)-bone offset is related to differences in enamel(oid)–bone mineralisation and maturation and that this offset might be relatively consistent among vertebrates in spite of differences in enamel and enameloid mineralisation (Sasagawa et al. 2009; Kawasaki 2013).
The lower body tissue δ66Zn values in pisciculture Sparus aurata compared to their diet are in good agreement with the decrease in bioapatite δ66Zn values within animals feeding at higher trophic levels (Jaouen et al. 2016a, 2016b; Bourgon et al. 2020, 2021; McCormack et al. 2021, 2022b). Indeed, S. aurata Δ66Znmuscle-diet values of − 0.45 ± 0.07 ‰ (2SD, n = 5) are very much in line with anticipated trophic level fractionation factors within the same tissue (e.g., enameloid) of a predator (or scavenger) and its prey. When assuming that tissue-tissue fractionation factors are constant among taxa and that muscle is the main digested tissue by a consumer, S. aurata Δ66Znmuscle-diet values suggest approximately 0.45 ‰ lower values within the same tissue of a predator compared to its prey. Previous trophic level δ66Zn spacing between predator and prey were reported to be between − 0.32 to − 0.38 ‰ (McCormack et al. 2021), − 0.40 to − 0.50 ‰ (Jaouen et al. 2016a) and − 0.60 ‰ (Bourgon et al. 2020) well within the wider range of the S. aurata Δ66Znmuscle-diet values.
All here examined pisciculture Sparus aurata tissues are 66Zn depleted compared to their diet (Fig. 1). Yet, we cannot exclude the possibility of other tissues being enriched in 66Zn compared to the dietary intake. Notably, Moynier et al. (2013) documented a constant enrichment of 66Zn in faeces and urine of mice relative to their diet. Although this remains to be tested, fish excretions may demonstrate a similar 66Zn enrichment. Besides faecal and urinal, these may even include Zn excretion via the gills, as, even though low seawater zinc concentrations lead to negligible Zn uptake via the gills, the gills are a possible excretion route for excess dietary Zn in fish (Hardy et al. 1987). All or most tissues being 66Zn depleted relative to the diet is in line with the observed lower δ66Zn values higher up the food chain, even though the actual trophic level offset will depend on which tissues (and their respective proportion) are consumed and the differences in Zn concentration and isotope composition in each tissue. For example, the constantly higher δ66Zn values in bone-consuming carnivores such as hyenas compared to non-bone-consuming carnivores may be explained by the combined ingestion of higher δ66Zn bone and lower δ66Zn muscle-derived zinc (Jaouen et al. 2016a, 2022; Bourgon et al. 2020, 2021).
Zinc isotope variability among wild fish
In contrast to the pisciculture individuals, wild-caught fish demonstrate a much larger Zn isotope variability among individuals (Fig. 3). For example, all enameloid δ66Zn values of the pisciculture S. aurata vary between − 0.05 and + 0.10 ‰ (mean of 0.00 ± 0.09 ‰ 2SD, n = 22), while wild Pagrus caeruleostictus enameloid varies between − 0.01 and + 0.44 ‰ (mean of + 0.20 ± 0.27 ‰ 2SD, n = 12). Wild-caught S. aurata individuals also have distinctly higher δ66Zn values than pisciculture ones, with bone values of + 0.66 and + 0.77 ‰ and enameloid values of + 0.30 and + 0.55 ‰ (Fig. 3). Pagrus caeruleostictus have bone δ66Zn value between + 0.35 and + 0.53 ‰ (mean of + 0.42 ± 0.13 ‰ 2SD, n = 6). Balistes capriscus have enameloid δ66Zn values between + 0.11 and + 0.21 ‰ (mean of + 0.18 ± 0.16 ‰ 2SD, n = 6). Mean white muscle values for P. caeruleostictus individuals range between − 0.27 and − 0.04 ‰ (mean of − 0.14 ± 0.22 ‰ 2SD, n = 4) and for B. capriscus between − 0.06 and + 0.03 ‰ (mean of 0.00 ± 0.07 ‰ 2SD, n = 3, Supplementary Data 1, Fig. 3). The larger enameloid intra- and interspecific δ66Zn variability of wild-caught fish compared to the pisciculture individuals likely reflects a different and more variable diet for the former.
Zinc isotope values (‰ JMC Lyon) in enameloid, bone and white muscle of pisciculture Sparus aurata (mean) and wild-caught fish from Haifa Bay. Mean pisciculture S. aurata (n = 7 for enameloid and bone, and 5 for white muscle) and wild fish individual Zn isotope values (n = 2 for enameloid and bone of S. aurata, n = 3 for B. capriscus white muscle and enameloid, n = 4, 6 and 8 for P. caeruleostictus white muscle, bone and enameloid, respectively) are depicted with whiskers indicating 2SD variability. Individuals caught in 2020, depicted by a grey bracket, were treated with tap water and their bones are contaminated by post-mortem Zn uptake. These bone values are thus not depicted here, see Supplementary Discussion 1, 2 and Supplementary Figs. 1, 2, and 3. Maximum measurement uncertainty is given in 2SD
With the exception of the contaminated bone δ66Zn values of the 2020 caught Pagrus caeruleostictus and Balistes capriscus (Supplementary Discussion 2), all wild-caught fish have similar Δ66Zntissue-tissue values as observed in the pisciculture Sparus aurata, with progressively lower δ66Zn values in the order bone > enameloid > white muscle (Fig. 1). For the two wild S. aurata, Δ66Znenameloid-bone values are − 0.22 and − 0.36 ‰. For four P. caeruleostictus, Δ66Znenameloid-bone values range between − 0.25 ‰ and − 0.39 ‰ (mean of − 0.30 ± 0.13 ‰ 2SD, n = 4) and thus close to the mean pisciculture S. aurata Δ66Znenameloid-bone of − 0.24 ± 0.11 ‰ (2SD, n = 7). For the three B. capriscus mean Δ66Znmuscle-enameloid values (− 0.11, − 0.18, − 0.24 ‰) are comparable to the mean pisciculture S. aurata value of − 0.14 ± 0.07‰ (2SD, n = 5). The four P. caeruleostictus caught 2020, however, have generally lower Δ66Znmuscle-enameloid values (− 0.29, − 0.38, − 0.41, − 0.43 ‰). In contrast to the pisciculture S. aurata, the wild fish species may have variable diets over time, both seasonally and through ontogeny (Tancioni et al. 2003; Hamida et al. 2009; Taieb et al. 2013; Goldman et al. 2016). Even though sparid teeth are replaced continuously throughout the animal’s life cycle (Elgendy et al. 2016), perhaps even seasonally (Sisma-Ventura et al. 2018), zinc turnover rates for different tissues are still largely unknown and likely vary. In addition, once Zn is incorporated into enameloid it becomes chemically inert, whereas muscle Zn can still be exchanged. Thus zinc isotope values recorded in muscle may reflect diet consumed at a different time than those recorded in enameloid, perhaps explaining the different Δ66Znmuscle-enameloid for wild P. caeruleostictus individuals. Still, in general, wild fish Δ66Zntissue-tissue values are in agreement with those from control-fed pisciculture S. aurata.
A detailed investigation into differences in diet among wild taxa is not possible with such a limited sample size and we do not observe any correlation for enameloid δ66Zn values with size (weight). All wild-caught individuals come from Haifa Bay, thus potential spatial variability in food web baseline δ66Zn values, as observed elsewhere in the Mediterranean (Chifflet et al. 2022), is most likely negligible among individuals and their δ66Zn variability relates to differences in dietary Zn intake as a result of their trophic ecology. Enameloid δ66Zn values for wild-caught fish range between − 0.01 and + 0.55 ‰ with overlap between species (Fig. 3). This indicates a trophic range among these wild durophageous fish larger than one trophic level when using the pisciculture S. aurata Δ66Znmuscle-diet value of − 0.45 ‰ as an approximate trophic discrimination factor. Applying the Δ66Znenameloid-diet values from the pisciculture S. aurata to all analysed teleost wild fish indicates they have fed on a diet with average compositions between + 0.28 and + 0.84 ‰ (Supplementary Fig. 4). All three species feed on a variety of mainly hard-shelled prey items also depending on seasons, ontogeny and habitat, with bivalves, decapod crustaceans, gastropods, barnacles, echinoderms, annelids, and small teleosts generally considered the most important (Tancioni et al. 2003; Hamida et al. 2009; Taieb et al. 2013; Goldman et al. 2016). In the Levant, contrary to many other populations, smaller teleosts are likely a particularly important prey for P. caeruleostictus (Gilaad et al. 2017).
There is very little information on δ66Zn values for most of the prey species, but δ66Zn values of some filter-feeding bivalves from studies aimed at investigating anthropogenic Zn contamination have a wide range of bivalve soft tissue values from − 0.11 to + 1.43 ‰ worldwide (Shiel et al. 2012, 2013; Petit et al. 2015; Araújo et al. 2017, 2021; Ma et al. 2019; Jeong et al. 2021). A direct comparison with published bivalve soft tissue δ66Zn data is only tentative, as there is no data available from the Eastern Mediterranean, only a few taxa are represented, and reported δ66Zn values can vary significantly across localities in part also as most of these studies, by design, aimed to investigate anthropogenic metal contamination which varies geographically. Nevertheless, our anticipated dietary δ66Zn values are well within the range of values previously reported for filter-feeding bivalves. Our wild fish δ66Zn values are therefore in agreement with a trophic position as secondary to tertiary consumers, feeding on primary consumers (e.g., filter-feeding bivalves) and other secondary consumers (e.g., gastropods, echinoderms, teleosts) (Supplementary Fig. 4).
Because pisciculture and wild fish demonstrate constant and predictable Δ66Zntissue-tissue offsets, and in the case of controlled-fed pisciculture S. aurata also constant Δ66Zntissue-diet values, δ66Zn values of various tissues can be used to investigate marine trophic ecology and potentially identify sources of anthropogenic Zn contamination to marine vertebrates. In addition, because pristine biological δ66Zn values are highly resistant against diagenetic alteration in highly mineralised fish enameloid (McCormack et al. 2022b) and mammalian enamel (Weber et al. 2021), our Δ66Znenameloid-tissue and Δ66Znenameloid-diet values will allow identifying Zn dietary resources and reconstructing the trophic ecology of both extant as well as long-extinct fossil marine vertebrates.
Conclusion
This study compares Eastern Mediterranean pisciculture Sparus aurata bone, enameloid, and white muscle δ66Zn values from seven individuals to the isotope composition of their pellet diet. Supplementing the pisciculture individuals, we also investigate the same tissue δ66Zn values of wild S. aurata, Pagrus caeruleostictus and Balistes capriscus caught in Haifa Bay, close to the location of the pisciculture. Our results show:
-
1.
All pisciculture S. aurata have constant diet-tissue discrimination factors (Δ66Zntissue-diet) of − 0.04 ± 0.09 ‰ (2SD, n = 7) for bone, − 0.29 ± 0.06 ‰ (2SD, n = 7) for enameloid, and − 0.45 ± 0.07 ‰ (2SD, n = 5) for white muscle.
-
2.
For both pisciculture and wild fish, we observe comparable δ66Zn tissue-tissue fractionation with progressively lower δ66Zn values from bone > enameloid > white muscle.
-
3.
Wild-caught fish δ66Zn values are distinct from those of the pisciculture from the same area (i.e., Haifa Bay and Central Israel) and display a larger variability among individuals reflecting an isotopically more heterogonous diet in wild compared to controlled-fed specimen. Thus, tissue δ66Zn values are mainly related to Zn from the diet and not from ambient sea water.
-
4.
The depletion in 66Zn in the here examined tissues compared to the diet is in line with the use of this proxy as a trophic level indicator, with lower δ66Zn values higher up the food chain. Our pisciculture Δ66Znmuscle-diet value of − 0.45 ± 0.07 ‰ (2SD) is close to values previously reported for trophic level spacing for both marine and terrestrial mammals.
In general, our study broadens the knowledge of tissue-diet and tissue-tissue Zn isotope fractionation factors to include the marine realm and non-mammalian vertebrates with multi-disciplinary applicability. Among others, our documented Δ66Zntissue-diet values in both controlled-fed and wild fish species could be used to monitor diet-borne Zn toxicity in marine vertebrates and to estimate individual and population dietary/trophic variability.
Data availability
All relevant data are provided within the paper and its Supporting Information files.
References
Ababou A, Rostkova E, Mistry S, Masurier CL, Gautel M, Pfuhl M (2008) Myosin binding protein C positioned to play a key role in regulation of muscle contraction: structure and interactions of domain C1. J Mol Biol 384:615–630. https://doi.org/10.1016/j.jmb.2008.09.065
Araújo D, Machado W, Weiss D, Mulholland DS, Boaventura GR, Viers J, Garnier J, Dantas EL, Babinski M (2017) A critical examination of the possible application of zinc stable isotope ratios in bivalve mollusks and suspended particulate matter to trace zinc pollution in a tropical estuary. Environ Pollut 226:41–47. https://doi.org/10.1016/j.envpol.2017.04.011
Araújo DF, Ponzevera E, Weiss DJ, Knoery J, Briant N, Yepez S, Bruzac S, Sireau T, Brach-Papa C (2021) Application of Zn isotope compositions in oysters to monitor and quantify anthropogenic Zn bioaccumulation in marine environments over four decades: a “Mussel Watch Program” upgrade. ACS ES&T Water 1:1035–1046. https://doi.org/10.1021/acsestwater.1c00010
Balter V, Zazzo A, Moloney AP, Moynier F, Schmidt O, Monahan FJ, Albarède F (2010) Bodily variability of zinc natural isotope abundances in sheep. Rapid Commun Mass Spectrom 24:605–612. https://doi.org/10.1002/rcm.4425
Balter V, Lamboux A, Zazzo A, Télouk P, Leverrier Y, Marvel J, Moloney AP, Monahan FJ, Schmidt O, Albarède F (2013) Contrasting Cu, Fe, and Zn isotopic patterns in organs and body fluids of mice and sheep, with emphasis on cellular fractionation. Metallomics 5:1470–1482. https://doi.org/10.1039/c3mt00151b
Bourgon N, Jaouen K, Bacon A-M, Jochum KP, Dufour E, Duringer P, Ponche J-L, Joannes-Boyau R, Boesch Q, Antoine P-O, Hullot M, Weis U, Schulz-Kornas E, Trost M, Fiorillo D, Demeter F, Patole-Edoumba E, Shackelford LL, Dunn TE, Zachwieja A, Duangthongchit S, Sayavonkhamdy T, Sichanthongtip P, Sihanam D, Souksavatdy V, Hublin J-J, Tütken T (2020) Zinc isotopes in Late Pleistocene fossil teeth from a Southeast Asian cave setting preserve paleodietary information. Proc Natl Acad Sci 117:4675–4681. https://doi.org/10.1073/pnas.1911744117
Bourgon N, Jaouen K, Bacon AM, Dufour E, McCormack J, Tran NH, Trost M, Fiorillo D, Dunn TE, Zanolli C, Zachwieja A, Duringer P, Ponche JL, Boesch Q, Antoine PO, Westaway KE, Joannes-Boyau R, Suzzoni E, Frangeul S, Crozier F, Aubaile F, Patole-Edoumba E, Luangkhoth T, Souksavatdy V, Boualaphane S, Sayavonkhamdy T, Sichanthongtip P, Sihanam D, Demeter F, Shackelford LL, Hublin JJ, Tütken T (2021) Trophic ecology of a Late Pleistocene early modern human from tropical Southeast Asia inferred from zinc isotopes. J Hum Evol 161:103075. https://doi.org/10.1016/j.jhevol.2021.103075
Bury NR, Walker PA, Glover CN (2003) Nutritive metal uptake in teleost fish. J Exp Biol 206:11–23. https://doi.org/10.1242/jeb.00068
Chifflet S, Briant N, Freydier R, Araújo DF, Quéméneur M, Zouch H, Bellaaj-Zouari A, Carlotti F, Tedetti M (2022) Isotopic compositions of copper and zinc in plankton from the Mediterranean Sea (MERITE-HIPPOCAMPE campaign): tracing trophic transfer and geogenic inputs. Mar Pollut Bull 185:114315. https://doi.org/10.1016/j.marpolbul.2022.114315
Clearwater SJ, Farag AM, Meyer JS (2002) Bioavailability and toxicity of dietborne copper and zinc to fish. Comp Biochem Physiol C: Toxicol Pharmacol 132:269–313. https://doi.org/10.1016/S1532-0456(02)00078-9
Cousins RJ (1998) A role of zinc in the regulation of gene expression. Proc Nutr Soc 57:307–311. https://doi.org/10.1079/PNS19980045
Elgendy SAA, Alsafy MAM, Tanekhy M (2016) Morphological characterization of the oral cavity of the gilthead seabream (Sparus aurata) with emphasis on the teeth-age adaptation. Microsc Res Tech 79:227–236. https://doi.org/10.1002/jemt.22622
Fujii T, Moynier F, Blichert-Toft J, Albarède F (2014) Density functional theory estimation of isotope fractionation of Fe, Ni, Cu, and Zn among species relevant to geochemical and biological environments. Geochim Cosmochim Acta 140:553–576. https://doi.org/10.1016/j.gca.2014.05.051
Gilaad R-L, Galil BS, Diamant A, Goren M (2017) The diet of native and invasive fish species along the eastern Mediterranean coast (Osteichthyes). Zool Middle East 63:325–335. https://doi.org/10.1080/09397140.2017.1375196
Goldman SF, Glasgow DM, Falk MM (2016) Feeding habits of 2 reef-associated fishes, red porgy (Pagrus pagrus) and gray triggerfish (Balistes capriscus), off the southeastern United States. Fish Bull 114:317–329
Hamida NBH, Abdallah OBHH-B, Ghorbel M, Jarboui O, Missaoui H (2009) The feeding habits of the bluespotted seabream, Pagrus caeruleostictus (Valenciennes, 1830), in the Gulf of Gabes (Central Mediterranean). Rev Fish Sci 18:65–72. https://doi.org/10.1080/10641260903318230
Hardy RW, Sullivan CV, Koziol AM (1987) Absorption, body distribution, and excretion of dietary zinc by rainbow trout (Salmo gairdneri). Fish Physiol Biochem 3:133–143. https://doi.org/10.1007/BF02180415
Heuser A, Tütken T, Gussone N, Galer SJG (2011) Calcium isotopes in fossil bones and teeth—diagenetic versus biogenic origin. Geochim Cosmochim Acta 75:3419–3433. https://doi.org/10.1016/j.gca.2011.03.032
Horstmann-Dehn L, Follmann EH, Rosa C, Zelensky G, George C (2012) Stable carbon and nitrogen isotope ratios in muscle and epidermis of arctic whales. Mar Mamm Sci 28:E173–E190. https://doi.org/10.1111/j.1748-7692.2011.00503.x
Isani G, Andreani G, Monari M, Dalla Libera L, Zannoni A, Carpenè E (2004) Zn, Cu, pyruvate kinase and myosin in white muscle of gilthead seabream (Sparus aurata) fed a Zn enriched diet. Basic Appl Myol 14:87–93
Jaouen K, Beasley M, Schoeninger M, Hublin J-J, Richards MP (2016a) Zinc isotope ratios of bones and teeth as new dietary indicators: results from a modern food web (Koobi Fora, Kenya). Sci Rep 6:26281. https://doi.org/10.1038/srep26281
Jaouen K, Szpak P, Richards MP (2016b) Zinc isotope ratios as indicators of diet and trophic level in arctic marine mammals. PLoS ONE 11:e0152299. https://doi.org/10.1371/journal.pone.0152299
Jaouen K, Herrscher E, Balter V (2017) Copper and zinc isotope ratios in human bone and enamel. Am J Phys Anthropol 162:491–500. https://doi.org/10.1002/ajpa.23132
Jaouen K, Trost M, Bourgon N, Colleter R, Le Cabec A, Tütken T, Elias Oliveira R, Pons ML, Méjean P, Steinbrenner S, Chmeleff J, Strauss A (2020) Zinc isotope variations in archeological human teeth (Lapa do Santo, Brazil) reveal dietary transitions in childhood and no contamination from gloves. PLoS ONE 15:e0232379. https://doi.org/10.1371/journal.pone.0232379
Jaouen K, Villalba-Mouco V, Smith GM, Trost M, Leichliter J, Lüdecke T, Méjean P, Mandrou S, Chmeleff J, Guiserix D, Bourgon N, Blasco F, Mendes Cardoso J, Duquenoy C, Moubtahij Z, Salazar Garcia DC, Richards M, Tütken T, Hublin JJ, Utrilla P, Montes L (2022) A Neandertal dietary conundrum: insights provided by tooth enamel Zn isotopes from Gabasa, Spain. Proc Natl Acad Sci USA 119:e2109315119. https://doi.org/10.1073/pnas.2109315119
Jeong H, Ra K, Won J-H (2021) A nationwide survey of trace metals and Zn isotopic signatures in mussels (Mytilus edulis) and oysters (Crassostrea gigas) from the coast of South Korea. Mar Pollut Bull 173:113061. https://doi.org/10.1016/j.marpolbul.2021.113061
Kawasaki K (2013) Odontogenic ameloblast-associated protein (ODAM) and amelotin: major players in hypermineralization of enamel and enameloid. J Oral Biosci 55:85–90. https://doi.org/10.1016/j.job.2013.02.001
Korzen L, Abelson A, Israel A (2016) Growth, protein and carbohydrate contents in Ulva rigida and Gracilaria bursa-pastoris integrated with an offshore fish farm. J Appl Phycol 28:1835–1845. https://doi.org/10.1007/s10811-015-0691-5
Leichliter JN, Lüdecke T, Foreman AD, Bourgon N, Duprey NN, Vonhof H, Souksavatdy V, Bacon A-M, Sigman DM, Tütken T, Martínez-García A (2023) Tooth enamel nitrogen isotope composition records trophic position: a tool for reconstructing food webs. Commun Biol 6:373. https://doi.org/10.1038/s42003-023-04744-y
Lozano-Bilbao E, Domínguez D, González JA, Lorenzo JM, Lozano G, Hardisson A, Rubio C, Weller D-G, Paz S, Gutiérrez ÁJ (2021) Risk assessment and study of trace/heavy metals in three species of fish of commercial interest on the island of El Hierro (Canary Islands, eastern-central Atlantic). J Food Compos Anal 99:103855. https://doi.org/10.1016/j.jfca.2021.103855
Ma L, Li Y, Wang W, Weng N, Evans RD, Wang W-X (2019) Zn isotope fractionation in the Oyster Crassostrea hongkongensis and Implications for contaminant source tracking. Environ Sci Technol 53:6402–6409. https://doi.org/10.1021/acs.est.8b06855
Maares M, Haase H (2020) A guide to human zinc absorption: general overview and recent advances of in vitro intestinal models. Nutrients 12:762
Mahan B, Moynier F, Jørgensen AL, Habekost M, Siebert J (2018) Examining the homeostatic distribution of metals and Zn isotopes in Göttingen minipigs. Metallomics 10:1264–1281. https://doi.org/10.1039/c8mt00179k
Mahan BM, Wu F, Dosseto A, Chung R, Schaefer B, Turner S (2020) SpinChem™: rapid element purification from biological and geological matrices via centrifugation for MC-ICP-MS isotope analyses—a case study with Zn. J Anal at Spectrom 35:863–872. https://doi.org/10.1039/C9JA00361D
Maréchal CN, Télouk P, Albarède F (1999) Precise analysis of copper and zinc isotopic compositions by plasma-source mass spectrometry. Chem Geol 156:251–273. https://doi.org/10.1016/S0009-2541(98)00191-0
Maret W (2011) Metals on the move: zinc ions in cellular regulation and in the coordination dynamics of zinc proteins. Biometals 24:411–418. https://doi.org/10.1007/s10534-010-9406-1
Maret W (2012) New perspectives of zinc coordination environments in proteins. J Inorg Biochem 111:110–116. https://doi.org/10.1016/j.jinorgbio.2011.11.018
McCormack J, Szpak P, Bourgon N, Richards M, Hyland C, Méjean P, Hublin J-J, Jaouen K (2021) Zinc isotopes from archaeological bones provide reliable trophic level information for marine mammals. Commun Biol 4:683. https://doi.org/10.1038/s42003-021-02212-z
McCormack J, Bourgon N, Sinet-Mathiot V, Rezek Z, Smith GM, Hublin J-J, Dabain M, Fewlass H (2022a) Combining collagen extraction with mineral Zn isotope analyses from a single sample for robust palaeoecological investigations. Archaeol Anthropol Sci 14:137. https://doi.org/10.1007/s12520-022-01601-7
McCormack J, Griffiths ML, Kim SL, Shimada K, Karnes M, Maisch H, Pederzani S, Bourgon N, Jaouen K, Becker MA, Jöns N, Sisma-Ventura G, Straube N, Pollerspöck J, Hublin JJ, Eagle RA, Tütken T (2022b) Trophic position of Otodus megalodon and great white sharks through time revealed by zinc isotopes. Nat Commun 13:2980. https://doi.org/10.1038/s41467-022-30528-9
McCormack J, Karnes M, Haulsee D, Fox D, Kim SL (2023) Shark teeth zinc isotope values document intrapopulation foraging differences related to ontogeny and sex. Commun Biol 6:711. https://doi.org/10.1038/s42003-023-05085-6
McCoy-West AJ, Fitton JG, Pons M-L, Inglis EC, Williams HM (2018) The Fe and Zn isotope composition of deep mantle source regions: insights from Baffin Island picrites. Geochim Cosmochim Acta 238:542–562. https://doi.org/10.1016/j.gca.2018.07.021
Moynier F, Albarède F, Herzog GF (2006) Isotopic composition of zinc, copper, and iron in lunar samples. Geochim Cosmochim Acta 70:6103–6117. https://doi.org/10.1016/j.gca.2006.02.030
Moynier F, Fujii T, Shaw AS, Le Borgne M (2013) Heterogeneous distribution of natural zinc isotopes in mice†. Metallomics 5:693–699. https://doi.org/10.1039/c3mt00008g
Nguyen VT, Satoh S, Haga Y, Fushimi H, Kotani T (2008) Effect of zinc and manganese supplementation in Artemia on growth and vertebral deformity in red sea bream (Pagrus major) larvae. Aquaculture 285:184–192. https://doi.org/10.1016/j.aquaculture.2008.08.030
Ogino O, Yang G-Y (1978) Requirement of rainbow trout for dietary zinc. Bull Jpn Soc Sci Fish 44:1015–1018
Petit JCJ, Schäfer J, Coynel A, Blanc G, Chiffoleau J-F, Auger D, Bossy C, Derriennic H, Mikolaczyk M, Dutruch L, Mattielli N (2015) The estuarine geochemical reactivity of Zn isotopes and its relevance for the biomonitoring of anthropogenic Zn and Cd contaminations from metallurgical activities: example of the Gironde fluvial-estuarine system, France. Geochim Cosmochim Acta 170:108–125. https://doi.org/10.1016/j.gca.2015.08.004
Ranaldi MM, Gagnon MM (2008) Zinc incorporation in the otoliths of juvenile pink snapper (Pagrus auratus Forster): the influence of dietary versus waterborne sources. J Exp Mar Biol Ecol 360:56–62. https://doi.org/10.1016/j.jembe.2008.03.013
Reynders H, Bervoets L, Gelders M, De Coen WM, Blust R (2008) Accumulation and effects of metals in caged carp and resident roach along a metal pollution gradient. Sci Total Environ 391:82–95. https://doi.org/10.1016/j.scitotenv.2007.10.056
Sasagawa I, Ishiyama M, Yokosuka H, Mikami M, Uchida T (2009) Tooth enamel and enameloid in actinopterygian fish. Front Mater Sci Chin 3:174
Serra R, Isani G, Cattani O, Carpené E (1996) Effects of different levels of dietary zinc on the gilthead, Sparus aurata during the growing season. Biol Trace Elem Res 51:107–116. https://doi.org/10.1007/BF02790153
Shiel AE, Weis D, Orians KJ (2012) Tracing cadmium, zinc and lead sources in bivalves from the coasts of western Canada and the USA using isotopes. Geochim Cosmochim Acta 76:175–190. https://doi.org/10.1016/j.gca.2011.10.005
Shiel AE, Weis D, Cossa D, Orians KJ (2013) Determining provenance of marine metal pollution in French bivalves using Cd, Zn and Pb isotopes. Geochim Cosmochim Acta 121:155–167. https://doi.org/10.1016/j.gca.2013.07.005
Sisma-Ventura G, Thomas T, Irit Z, Andreas P, Dorit S, Omri L, Ayelet G, Guy B-O (2018) Tooth oxygen isotopes reveal Late Bronze Age origin of Mediterranean fish aquaculture and trade. Sci Rep 8:14086. https://doi.org/10.1038/s41598-018-32468-1
Skulan J, DePaolo DJ (1999) Calcium isotope fractionation between soft and mineralized tissues as a monitor of calcium use in vertebrates. Proc Natl Acad Sci 96:13709–13713. https://doi.org/10.1073/pnas.96.24.13709
Sun L-T, Jeng S-S (1998) Comparative zinc concentrations in tissues of common carp and other aquatic organisms. Zool Stud 37:184–190
Taieb AH, Sley A, Ghorbel M, Jarboui O (1998) Feeding habits of Sparus aurata (Sparidae) from the Gulf of Gabes (central Mediterranean). Cah Biol Mar 54:263–270
Tancioni L, Mariani S, Maccaroni A, Mariani A, Massa F, Scardi M, Cataudella S (2003) Locality-specific variation in the feeding of Sparus aurata L.: evidence from two Mediterranean lagoon systems. Estuar Coast Shelf Sci 57:469–474. https://doi.org/10.1016/S0272-7714(02)00376-1
Tang Y, Chappell HF, Dove MT, Reeder RJ, Lee YJ (2009) Zinc incorporation into hydroxylapatite. Biomaterials 30:2864–2872. https://doi.org/10.1016/j.biomaterials.2009.01.043
Trueman CN, McGill RAR, Guyard PH (2005) The effect of growth rate on tissue-diet isotopic spacing in rapidly growing animals. An experimental study with Atlantic salmon (Salmo salar). Rapid Commun Mass Spectrom 19:3239–3247. https://doi.org/10.1002/rcm.2199
Webb EC, White CD, Longstaffe FJ (2014) Investigating inherent differences in isotopic composition between human bone and enamel bioapatite: implications for reconstructing residential histories. J Archaeol Sci 50:97–107. https://doi.org/10.1016/j.jas.2014.07.001
Weber K, Weber M, Menneken M, Kral AG, Mertz-Kraus R, Geisler T, Vogl J, Tütken T (2021) Diagenetic stability of non-traditional stable isotope systems (Ca, Sr, Mg, Zn) in teeth—an in-vitro alteration experiment of biogenic apatite in isotopically enriched tracer solution. Chem Geol 572:120196. https://doi.org/10.1016/j.chemgeo.2021.120196
Acknowledgements
This research was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) awarded to J.M. (Project Number 505905610), the Rhine-Main Universities (RMU) Initiative Funding for Research awarded to J.M., W.M. and T.T. and the Max Planck Society. FIERCE is financially supported by the Wilhelm and Else Heraeus Foundation and by the Deutsche Forschungsgemeinschaft (DFG: INST 161/921-1 FUGG, INST 161/923-1 FUGG and INST 161/1073-1 FUGG), which is gratefully acknowledged. This is FIERCE contribution No. 149. Funding to T.T. and K.J. by the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Program (grant agreement numbers 681450 and 803676, respectively) is acknowledged. We thank A. Gerdes, A. Schmidt and L. Marko (FIERCE, Goethe University Frankfurt) and S. Steinbrenner and M. Trost (Department of Human Evolution, Max Planck Institute for Evolutionary Anthropology) for technical and analytical support. We also thank K. Grimm (Osteological Collection, Institute of Geosciences, Mainz, Germany) for the curation of sample material. Finally, we thank the two anonymous reviewers who helped improving the first version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) awarded to J.M. (Project Number 505905610), the Rhine-Main Universities (RMU) Initiative Funding for Research awarded to J.M., W.M., and T.T. and the Max Planck Society. Funding to T.T. and K.J. by the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Program (grant agreement numbers 681450 and 803676, respectively) is acknowledged.
Author information
Authors and Affiliations
Contributions
J.M., T.T. and G.S.-V. designed the study. J.M. performed the δ66Zn analysis, J.M., G.S.-V., T.J.G.T. collected and/or prepared the samples for analysis. J.M., K.J., N.B., T.J.G.T., W.M., T.T. interpreted the data and contributed to writing the manuscript. All authors edited the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All specimens used in this study were collected legally and ethically. Analyses on fish farm Sparus aurata were performed on individuals that had been harvested for food consumption. All wild-caught individuals in this study had been caught as part of an ongoing monitoring program (permit number 516192-4399), funded by the Israeli Ministry of Environmental Protection.
Additional information
Responsible Editor: K.Clements.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
McCormack, J., Jaouen, K., Bourgon, N. et al. Zinc isotope composition of enameloid, bone and muscle of gilt-head seabreams (Sparus aurata) raised in pisciculture and their relation to diet. Mar Biol 171, 65 (2024). https://doi.org/10.1007/s00227-023-04383-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04383-1