Abstract
Collagen extraction from bones or dentine, commonly used for radiocarbon (14C) dating and stable carbon and nitrogen isotope (δ13C and δ15N) analyses, involves the dissolution of the bioapatite of skeletal elements. This fraction is typically disposed of during pretreatment. Here, we test the possibility of utilising this dissolved mineral solution for analysis of the bioapatite zinc isotope composition (δ66Zn). Bioapatite δ66Zn is a novel trophic level indicator similar to collagen δ15N but with isotopic fractionation independent from nitrogen, thus providing additional dietary information. We tested ways to minimise Zn contamination of the dissolved mineral phase during collagen extraction. We then used archaeological bone samples from Ain Difla (Jordan) and Ranis (Germany) to compare δ66Zn values of dissolved bioapatite following our collagen extraction protocol with δ66Zn values from the same sample material dissolved in a metal-free cleanroom. Our results demonstrate that with only minor adjustments to minimise Zn contamination, the dissolved mineral solution from collagen extraction protocols commonly employed for 14C dating and (palaeo)dietary analysis can be used for additional δ66Zn analyses even when collagen extraction does not take place in a cleanroom. Our protocol allows us to gain an additional dietary proxy to complement δ15N trophic level interpretations and perform more robust (palaeo)ecological investigations without further destructive sampling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For decades, stable isotope analysis of carbon and nitrogen (δ13C and δ15N) from bulk bone or dentine collagen has been one of the most powerful tools in archaeology and ecology to reconstruct the diet of an organism. Because of the relatively large biological fractionation, δ15N values can be used as a trophic level indicator with an average of 3 to 5‰ higher values with increasing trophic level (e.g. Hobson and Welch 1992; Vander Zanden and Rasmussen 2001; Bocherens and Drucker 2003). In contrast to nitrogen, δ13C values behave much more conservatively and are commonly used as a tracer of the primary production source(s) of a food web (e.g. Sponheimer et al. 2006; De la Vega et al. 2019).
The application of this approach relies on the assumption of a predictable isotopic fractionation factor between diet and consumer tissue; i.e. metabolic processes governing isotopic fractionation within an organism are mostly constant (Warinner and Tuross 2010). However, some physiological factors may also influence diet-tissue δ15N fractionation in consumers, including nutritional stress (Hobson et al. 1993; Fuller et al. 2005) and growth rate (Warinner and Tuross 2010). Other than physiological factors, environmental factors may cause differences among consumer bulk δ13C and δ15N values. The carbon and nitrogen isotope values of primary producers (food web baseline) can vary significantly, and these differences are passed along to higher trophic level consumers. Isotopic baseline variability can compromise (palaeo)ecological dietary interpretations when comparing animals from different times and/or regions, consumers that integrate multiple food webs, or when investigating highly mobile animals (Casey and Post 2011).
Including additional geochemical dietary proxies, independent from δ13C and δ15N values, can help verify traditional stable isotope results and provide otherwise inaccessible dietary information, ultimately leading to more robust (palaeo)ecological interpretations (e.g. McCormack et al. 2021). In the past decade, non-traditional metal isotopes such as calcium, magnesium, and zinc (Zn) from the skeletal mineral phase (bioapatite) have shown potential as (palaeo)dietary and trophic level proxies in the marine (Martin et al. 2015; Jaouen et al. 2016a) and terrestrial systems (Heuser et al. 2011; Martin et al. 2020; Jaouen et al., 2016b). Zinc 66Zn/64Zn ratios (expressed as the δ66Zn value) show decreasing δ66Zn values in carnivores’ bioapatite relative to herbivores (Jaouen et al. 2016b; Bourgon et al. 2020). Muscles and most organs appear typically 66Zn depleted compared to the diet of an animal and its bulk body δ66Zn composition (Moynier et al. 2013; Balter et al. 2013; Mahan et al. 2018), resulting in lower δ66Zn values as the trophic level increases. This trophic level effect has been demonstrated for tooth enamel/enameloid (Bourgon et al. 2020, 2021; McCormack et al. 2022), tooth dentine (McCormack et al. 2022), and bone (McCormack et al. 2021), with an offset of bone/dentine values of approximately + 0.2‰ compared to enamel/enameloid within an individual (Jaouen et al., 2016b; McCormack et al. 2022).
Combining independent dietary proxies (e.g. δ15N with δ66Zn) can help avoid misinterpretation of physiological or environmental controls on the isotopic composition as dietary. In addition, bioapatite δ66Zn values show potential for identifying specific feeding behaviours, such as the consumption of bone and omnivory (Jaouen et al., 2016b; Bourgon et al. 2020, 2021). At least for marine mammal bone, δ66Zn values within a species appear more homogenous geographically than δ13C and δ15N values, indicating a potentially lower isotopic variability at the base of the marine food web (McCormack et al. 2021). The inclusion of zinc isotope analysis may thus allow for a more direct comparability of marine species trophic ecology (and consumers thereof) between spatially and temporally distinct locations than traditional δ13C and δ15N isotope analysis alone.
Here, we investigate a method to routinely combine collagen extraction used for bulk δ13C and δ15N analyses and 14C dating with δ66Zn analyses on the same sampled material, thereby avoiding resampling of precious archaeological skeletal material. Many laboratories routinely use diluted hydrochloric acid (HCl) to dissolve the mineral portion of the bone as the first step in the collagen extraction and purification process, which is then discarded (e.g. Longin 1971; Brock et al. 2010; Sealy et al. 2014; Talamo et al. 2021). Here, we demonstrate that the acidic solution can be used for subsequent δ66Zn analysis following the bone mineral’s dissolution. Using the acidic solution allows the combination of traditional δ13C and δ15N (and 14C dating) with δ66Zn analyses, allowing more comprehensive (palaeo)ecological and dietary reconstructions through a multi-proxy approach. One of the challenges lies in potential Zn contamination introduced to the mineral solution, as collagen extraction is usually not performed in metal-free cleanrooms with acid-precleaned equipment. Therefore, we systematically tested potential Zn contamination introduced to the HCl solution during a standard collagen extraction protocol (e.g. Fewlass et al. 2019; Talamo et al. 2021), as well as methods to reduce Zn contamination without substantially modifying the collagen extraction protocol or contaminating the collagen for subsequent analyses.
We then compared δ66Zn values from bone fragments dissolved in two ways: (1) dissolution during collagen extraction carried out in a standard collagen extraction laboratory for 14C dating and (2) dissolution within a metal-free Pico Trace clean laboratory (standard for Zn purification and analyses). Our bone samples come from two archaeological sites with drastically different levels of bone preservation. Bones from the Ain Difla rockshelter in Jordan are fragmentary and mostly unidentifiable (based on morphology), coming from a depositional context indicating the likelihood of very low levels of collagen preservation. In contrast, the environmental context of the cave of Ranis-Ilsenhöhle in Germany is likely to demonstrate exceptionally well-preserved collagen. Thus, bones from these sites should represent the two extreme conditions regarding collagen preservation in archaeology and are ideal for testing our method.
Material and methods
All isotopic analyses were carried out in the Department of Human Evolution at the Max Planck Institute for Evolutionary Anthropology (MPI-EVA), Leipzig, Germany.
Material
Ranis-Ilsenhöhle, Germany
Ranis-Ilsenhöhle is a cave located in Thuringia, Germany (50°39′45.3″N, 11°33′53.5″E), containing archaeological deposits spanning the Middle Palaeolithic to the recent Holocene (Hülle 1977). New excavations were carried out between 2016 and 2022 by the Department of Human Evolution at the MPI-EVA and the Landesamt für Denkmalpflege und Archäologie Thüringen (Weimar, Germany). The bones from layers 7–9 included in this study were excavated in 2020, and all appeared well preserved. They were pretreated as part of a 14C dating program to reconstruct the site’s chronology and were analysed through collagen peptide mass fingerprinting, also known as zooarchaeology by mass spectrometry (ZooMS), to identify the taxon.
Ain Difla, Jordan
Ain Difla in Wadi Hasa, Jordan (30°54′13.14″N, 35°48′43.70″E), is a rock shelter containing Middle Palaeolithic and arguably Initial Upper Paleolithic deposits (Clark et al. 1997; Rezek 2020). The bones in this study (Supplementary Data 1) were excavated in the upper part of the archaeological sequence at the site during the excavation undertaken in 2019 by the Department of Human Evolution (MPI-EVA). This upper part (divided provisionally into layers 1–7 during the 2019 field season) corresponds to arbitrary levels 1–5 of Clark’s excavations (Clark et al. 1997). Bone remains are rare at the site and those excavated were fragmented, bleached, and highly weathered, making them difficult to identify to species level based on their morphology. We selected bones for collagen extraction and 14C dating to reconstruct the chronology of the corresponding deposits. We also sampled them for taxonomic identification through ZooMS.
Collagen extraction laboratory Zn contamination tests
As collagen extraction is usually not performed in metal-free cleanrooms, a series of blank tests, i.e. without actual sample material, were performed to identify any potential non-sample related Zn contamination sources when applying our collagen extraction protocol carried out in a standard laboratory. Blank tests were carried out in a laminar flow hood and performed using the same equipment and steps used for the demineralisation of archaeological samples following our typical collagen extraction protocol (Fewlass et al., 2019; Talamo et al. 2021). Briefly, demineralisation involves ~ 5 ml 0.5 M HCl added into borosilicate glass tubes containing the bone sample using a borosilicate glass beaker and subsequent removal of HCl with glass Pasteur pipettes when demineralisation is complete (see also “Collagen extraction bone dissolution”). For the blank tests, we assessed each step of the demineralisation protocol for potential Zn contamination and collected the ~ 5 ml 0.5 M HCl solution in precleaned perfluoroalkoxy alkane (PFA) vials (Table 1).
The same steps were then repeated with additional HCl precleaning of the glass beakers and tubes and personnel wearing vinyl gloves rather than nitrile gloves, as commonly used in our laboratory. Such practice is a crucial consideration as vinyl gloves have been shown to have a lower Zn contamination potential than nitrile gloves (Jaouen et al. 2020). Hydrochloric acid glass cleaning steps involved filling beakers and tubes with 2 M high-purity HCl for 24 h and subsequently rinsing them with ultrapure water to remove any Zn. In a final procedural blank test, we repeated the same steps with HCl-precleaned glass beakers and tubes and wore vinyl gloves while using suprapure HCl instead of the laboratory-grade HCl, which is generally used for collagen extraction (Table 1).
To test the contamination potential of the glass Pasteur pipettes and a possible glass pipette cleaning protocol, we pipetted approximately 2 ml suprapure HCl from HCl-precleaned glass tubes into PFA vials, reusing the glass pipette three times. This procedure was repeated for three glass pipettes.
All blank solutions were subsequently taken to our clean laboratory, evaporated, and redissolved in 1 ml 3% nitric acid (HNO3). Zinc concentrations were measured using a Thermo Fisher Neptune multicollector–inductively coupled plasma–mass spectrometer (MC-ICP-MS). Zn contamination was estimated by comparing the procedural blanks’ Zn signal intensity (V) to a solution with a known Zn concentration (300 ng/ml).
Bone sampling and dissolution
Ten bone samples from Ain Difla and nine bone samples from Ranis were sampled using a diamond-tipped cutting wheel, ultrasonicated in ultrapure water (Milli-Q water, TOC < 5 ppb) between samples. Bone surfaces were mechanically abraded (cleaned) with a sandblaster to remove exogenous material. For each bone, between 350 and 680 mg of bone was sampled for the collagen extraction protocol, and ∼30–50 mg was sampled for the clean laboratory dissolution protocol (Table 2). All ∼30–50 mg bone samples were subsequently cleaned by ultrasonication in Milli-Q water for 5 min and dried in a drying chamber at 50 °C (standard practice for Zn isotope analyses). Samples for the collagen extraction protocol were weighed into glass tubes, which had been previously heated at 500 °C for 8 h to remove organics and cleaned in 2 M high-purity HCl for 24 h before rinsing with ultrapure water to remove Zn contaminants. Sampling and subsequent work were carried out using vinyl gloves. A cave bear long bone (R-EVA 2907) from Austria dating to > 50,000 BP was sampled and prepared with each batch for both dissolution protocols as it is used as a ‘blank’ bone during all 14C pretreatments to monitor lab-based contaminants.
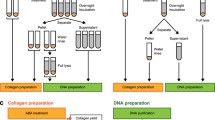
Collagen extraction bone dissolution
Collagen extraction was carried out using the protocol described in Fewlass et al. (2019) for stable isotopic analysis and 14C dating. All glassware is routinely cleaned and heated at 500 °C for 8 h to remove organics. For the initial demineralisation stage, ∼5 ml 0.5 M HCl was added to each sample tube using a glass beaker (additionally precleaned in 2 M HCl at room temperature). Samples were kept in the refrigerator (4 °C) during demineralisation, with daily checks for CO2 effervescence and softness (using glass pipettes). HCl was renewed once or twice per week. To estimate the potential of Zn contamination, procedural blanks were prepared alongside the samples for the demineralisation stage of the collagen extraction. The dissolved mineral and blank solution (HCl) were collected in acid-cleaned PFA vials. The vials were previously weighed to calculate the volume of the mineral solution collected during the collagen extraction protocol. All PFA vials used in this study were precleaned in our clean laboratory in a series of steps, including 3 M HNO3, ultrapure water, and 6 M HCl, each step heated to 80 °C for 8 h.
After demineralisation, the PFA vials containing the HCl solutions were transferred to a metal-free Pico Trace clean laboratory for preparation for Zn isotope analyses. Based on the final solution volume, corrected for the density of 1.01 g/ml 0.5 M HCl, and the initial sample weight, a 30 to 40 mg pretreatment equivalent (between 1 and 2 ml) of the solution was extracted and evaporated. The residue was then dissolved in 1 ml 1.5 M hydrobromic acid (HBr) and placed in an ultrasonic bath for 30 min for the ensuing column chromatography Zn purification.
Following the demineralisation stage, collagen extraction of samples proceeded without further modifications. Samples were treated with 0.1 M NaOH for 30 min to remove humic acid contamination and treated again with 0.5 M HCl (15 min) to remove atmospheric CO2. Samples were gelatinised in weakly acidic water (HCl pH3) at 75 °C for 20 h, and then filtered to remove > 80 µm particles (Eeze-Filter™ from Elkay Laboratory Products (UK) Ltd.). Gelatin extracts were then ultrafiltered to concentrate the large molecular weight (> 30 kDa) fraction (Sartorius “VivaspinTurbo” ultrafilters with 30 kDa molecular weight cut-off), precleaned to remove carbon contaminants according to Bronk Ramsey et al. (2004). Extracts were freeze-dried (48 h) and weighed to determine the collagen yield, expressed as a percentage of the initial dry bone weight.
Clean laboratory bone dissolution
Bone samples were dissolved in a metal-free cleanroom in closed PFA vials with 1 ml 1 M HCl on a hotplate for 3 h at 120 °C and then evaporated at 120 °C. The residue was then dissolved in 1 ml 1.5 M HBr and placed in an ultrasonic bath for 30 min.
Column chromatography and zinc isotope measurements
The column chromatography steps for quantitative recovery of sample Zn were the same for all samples regardless of the dissolution methods used. Each column chromatography batch included one clean laboratory chemistry blank and one reference standard (NIST SRM 1400). Zn purification was performed in two steps, following the modified ion exchange method adapted from Moynier et al. (2006), first described in Jaouen et al. (2006b). One millilitre of AG-1 × 8 resin (100–200 mesh) was placed in 10 ml hydrophobic interaction columns (Macro-Prep® Methyl HIC). Resin cleaning involved 5 ml 3% HNO3 followed by 5 ml ultrapure water, repeated twice. The resin was then conditioned with 3 ml 1.5 M HBr. After loading the sample, 2 ml 1.5 M HBr was added for matrix residue elution, followed by Zn elution with 5 ml 3% HNO3. Following the second column step, the solution was evaporated at 105 °C and the residue was redissolved in 1 ml 3% HNO3.
Zn isotope ratios and Zn concentrations were measured using a Thermo Fisher Neptune MC-ICP-MS at the Max Planck Institute for Evolutionary Anthropology (Leipzig, Germany). Instrumental mass fractionation was corrected by Cu doping following the protocol of Maréchal et al. (1999) and Toutain et al. (2008). The Alfa Aesar zinc plasma standard solution was used for standard bracketing. All δ66Zn values are expressed relative to the JMC-Lyon isotope standard. Zn concentrations were estimated following a protocol adapted from one used for Sr by Copeland et al. (2008) by applying a regression equation based on the Zn signal intensity (V) of three solutions with known Zn concentrations (150, 300, and 600 ng/ml). The δ66Zn measurement uncertainty was estimated from standard replicate analyses and was better than ± 0.04 ‰ (2 SD). All samples were measured at least twice with a mean sample reproducibility of ± 0.01‰ (2 SD). NIST SRM 1400 was analysed alongside the samples with δ66Zn values, as reported elsewhere (Jaouen et al., 2016b, 2020; Bourgon et al. 2020; McCormack et al. 2021). SRM reference material and samples show a normal Zn mass-dependent isotope fractionation, i.e. the absence of isobaric interferences, whereby the δ66Zn versus δ67Zn and δ66Zn versus δ68Zn values fall onto lines with slopes close to the theoretic mass fractionation values of 1.5 and 2, respectively.
Carbon and nitrogen isotope analysis
Where sufficient collagen was extracted (e.g. Ranis bone samples), ~ 0.5 mg collagen was weighed into a tin cup and analysed with a Thermo Finnigan Flash Elemental Analyzer (EA) coupled to a Thermo Delta Plus XP isotope ratio mass spectrometer to obtain elemental (C%, N%, C:N) and stable isotopic data (δ13C, δ15N). Carbon and nitrogen stable isotope values were two-point scale normalised to the VPDB (Vienna PeeDee Belemnite) and AIR (atmospheric N2) scale respectively, using IAEA-CH-6 (sucrose, δ13C = − 10.449 ± 0.033 ‰), IAEA-CH-7 (polyethylene, δ13C = − 32.151 ± 0.050 ‰), IAEA-N-1 (ammonium sulphate, δ15N = 0.43 ± 0.2 ‰), and IAEA-N-2 (ammonium sulphate, δ15N = 20.41 ± 0.2 ‰). An in-house methionine standard was used as a quality control, which gave average values of δ13C = -28.05 ± 0.05 ‰ (1 SD) and δ15N = − 6.41 ± 0.05 ‰ (1 SD), as well as an in-house collagen standard which gave values of δ13C = − 19.82 ± 0.3 ‰ (1 SD) and δ15N = 4.89 ± 0.1 ‰ (1 SD). Samples were measured in duplicate, and variation between samples fell within ± 0.2 ‰ instrumental error.
Zooarchaeology by mass spectrometry (ZooMS)
Zooarchaeology by mass spectrometry (collagen type I peptide mass fingerprinting) analyses followed protocols detailed elsewhere (Buckley et al. 2009; Van Doorn et al. 2011; Welker et al. 2016). In short, nine bone specimens from Ranis-Ilsenhöhle were minimally destructively sampled using a diamond-tipped cutting wheel. Soluble collagen from the bone sample was extracted using a semi-destructive soluble collagen extraction technique, through incubation in 100 µl of 50 mM ammonium bicarbonate (NH3CO3, AmBic) buffer at 65 °C for 1 h. Subsequently, in order to improve and verify the AmBic taxonomic identity, all bone samples were demineralised in 120 µl of 0.6 M HCl at 4 °C for 21 h. After being rinsed thrice with AmBic, bone samples were incubated for 1 h in 100 µl of AmBic solution at 65 °C. Then, 50 µl of the resulting supernatant was digested with trypsin (0.5 µg/µL, Promega) overnight at 37 °C, acidified using 1 µl of 10% trifluoroacetic acid (TFA) and cleaned on C18 ZipTips (Thermo Scientific). Eluted peptides were subsequently spotted in triplicate on a MALDI Bruker plate with the addition of α-cyano-4-hydroxycinnamic acid (CHCA, Sigma). MALDI-TOF MS analysis was conducted at the IZI Fraunhofer in Leipzig, and spectra were identified through peptide marker mass identification in comparison to a database containing peptide markers masses for all medium to large-sized mammalian genera in existence in Europe during the Pleistocene (Welker et al. 2016).
To assess any potential contamination by non-endogenous peptides, we performed a set of blank extractions alongside the rest of the samples to exclude any potential protein contamination introduced during laboratory extraction. The MALDI-TOF MS spectra show no collagenous peptides, demonstrating that the taxonomic identification does not derive from laboratory contamination.
All Ain Difla bone samples were analysed through ZooMS, following protocols described above, but had insufficient collagen preserved to provide identifiable spectra, thus preventing any taxonomic assignment.
Results
The Zn blank tests performed in the collagen extraction laboratory showed Zn concentrations between 5 and 110 ng/ml using glass equipment (500 °C, 8 h) that was not precleaned with HCl (Table 1). Blank tests performed with HCl-precleaned glass equipment and handled with vinyl instead of nitrile gloves led to Zn concentrations between 3 and 19 ng/ml for the blanks. Blanks produced with HCl-precleaned glass equipment, using vinyl gloves and suprapure HCl, had the lowest Zn contaminations, with Zn concentrations between < 1 and 18 ng/ml (Table 1). Importantly, glass pipettes were not additionally cleaned with HCl to remove Zn for either of these blank tests (only heated at 500 °C for 8 h to remove organics). Our results indicate that the glass pipettes can induce a minor source of Zn contamination with Zn concentrations between 5 and 19 ng/ml (Table 1). However, a subsequent test, which involved pipetting approximately 2 ml suprapure HCl from HCl-precleaned glass tubes into PFA vials, reusing the glass pipette thrice, did not document any noteworthy Zn contamination (< 0.5 ng/ml, Supplementary Table 1).
Blanks collected alongside the pretreatment of the bone samples using HCl-precleaned glass equipment and personnel wearing vinyl gloves had Zn concentrations between 1 and 8 ng/ml for the Ain Difla samples and 31 ng/ml for the Ranis samples. In comparison, the final mineral solution collected after Zn purification from the collagen extraction protocol yielded Zn concentrations between 1565 and 3112 ng/ml for Ain Difla and 8760 to 19,884 ng/ml for Ranis bones. The highest Zn concentration within the blanks of each collagen extraction procedure represents less than 1% of the amount of Zn present in the final dissolved bone mineral solution. No noteworthy amount of Zn was added during the subsequent column chemistry, as all clean laboratory chemistry blanks had Zn concentrations < 1 ng/ml.
The unsystematic difference in δ66Zn values between a sample prepared following the collagen extraction protocol and the same sample dissolved under cleanroom conditions (Δ66Zn) is on average 0.02‰ and thereby within measurement uncertainty (± 0.04 ‰, 2 SD). Only two samples from Ain Difla have Δ66Zn values exceeding 0.04‰ with values of 0.07‰ (Fig. 1, Table 2). The average difference in bone Zn concentration between both dissolution protocols is 2.2 µg/g for the Ain Difla and 55.6 µg/g for Ranis samples (Table 2). The cave bear bone (R-EVA 2907) that was prepared with both the Ranis and Ain Difla samples for both dissolution protocols (n = 4) has a mean δ66Zn value of 1.38 ± 0.05‰ with a Zn concentration of 79.4 ± 3.6 µg/g (Supplementary Table 2). For each bone, the two samples taken for collagen extraction and cleanroom dissolution have homogenous Zn isotope values and concentrations, even though cleaning of the samples by ultrasonication in Milli-Q water was only carried out for samples taken for cleanroom dissolution. Given the reproducibility of the Zn values between bone samples, we conclude that no Zn contamination was introduced from the diamond-tipped cutting wheel during sampling.
The δ66Zn values of bones from Ranis range between + 0.31 and + 0.59‰ with Zn concentrations between 369 and 789 µg/g. The bone fragments from Ain Difla have significantly higher δ66Zn values between + 1.21 and + 1.56 ‰ and lower Zn concentrations between 49 and 102 µg/g. We do not observe a significant correlation between δ66Zn values and Zn concentrations, expressed as 1/[Zn], for Ain Difla (R2 = 0.08, p = 0.42) or Ranis (R2 = 0.20, p = 0.23), when the average of both dissolution methods is used to compare δ66Zn values with Zn concentrations (Fig. 2).
Zinc isotope values from bone samples from Ain Difla (a) and Ranis (b) plotted against Zn concentration expressed as 1/Zn concentration (1/[Zn]). We observe no statistically significant relationship between bone δ66Zn values and Zn concentration. Mean values of both dissolution protocols were used here
Generally, stable isotope and 14C dating laboratories require a minimum collagen yield of ∼1% to proceed with further analysis (van Klinken 1999). The Ain Difla bone collagen preservation was too low for further analyses (isotopic, 14C or ZooMS). All Ranis samples had collagen yields, elemental (wt% C, wt% N, C:Natomic) compositions, and isotope ranges characteristic of collagen that is uncontaminated and unaltered by the burial environment (Supplementary Data 1; DeNiro 1985; Ambrose 1990; van Klinken 1999). Carbon isotope values were between − 21.6 and − 18.1‰ and nitrogen values between + 1.7 and + 6‰ (Supplementary Data 1). The spectra obtained from Ranis-Ilsenhöhle bone specimens through ZooMS analysis have been taxonomically identified as Rangifer tarandus (5 bone specimens), Ursidae (3 bone specimens), and Equidae (1 bone specimen) (Supplementary Data 1). Peptide marker series can be similar for some closely related species, which is the case for Ursidae and Equidae. Ursidae includes species from the genera Ursus, most likely U. spelaeus or U. arctos, and Equidae includes species from the genera Equus, most likely E. ferus.
Discussion
We observe no systematic difference in δ66Zn values, whether the bone samples were dissolved during collagen extraction without clean laboratory conditions or dissolved within a metal-free cleanroom (Fig. 1). The minimal variability in δ66Zn values and Zn concentrations between both dissolution methods in some samples is likely related to analytical uncertainty and/or intra-bone Zn heterogeneity linked to the differences in sample sizes used for both protocols. Significant Zn contamination to the dissolved mineral solution during collagen extraction can be excluded. Indeed, Zn contamination, monitored with procedural blanks during the collagen extraction, demonstrates that a potential Zn contamination during our collagen extraction was likely less than 1% of the Zn present in the final dissolved mineral solution. However, Zn contamination remains a potential issue. It needs to be closely monitored through procedural blanks collected along with the dissolved mineral phase during collagen extraction and ideally in a series of blank tests before sample analysis for each laboratory adopting the protocol.
Hydrochloric acid precleaning of all glass equipment can eliminate a potential Zn contamination source, reducing total Zn contamination (Table 1). Zinc contamination introduced by atmospheric deposition was not observed in our collagen laboratory, as tubes left unclosed in the flow hood for 24 h did not yield a higher Zn contamination than closed tubes (Table 1). In contrast, tubes closed with a plastic lid indicate a higher Zn contamination than observed for blanks with no lid or blanks closed with aluminium foil. Still, even though plastic lids may act as a potential Zn contamination source, we did not observe them having any impact on our δ66Zn values from bones samples, despite using plastic lids during collagen extraction.
Our results demonstrate that δ66Zn bone mineral analyses can be coupled to collagen extraction procedures with only minor adjustments to minimise Zn contamination. This combination of protocols is possible due to the relatively high concentration of Zn in bioapatite, permitting Zn contamination to be higher than under cleanroom conditions without necessarily affecting the final sample δ66Zn value. Zinc contamination might pose a greater issue when using smaller sample sizes for collagen extraction. Still, our results show that sampling only 10 mg of bone for collagen extraction would still result in mean Zn concentrations for the dissolved mineral solution in 1 ml 3% HNO3, after column chromatography Zn purification, of approximately 727 and 6303 ng/ml for Ain Difla and Ranis bones, respectively. When applying all steps to minimise Zn contamination presented here, including the use of vinyl gloves during all preparation steps, HCl precleaning of all glass equipment used and the use of high purity HCl for bioapatite demineralisation, Zn contamination can be kept to negligible levels (< 10 ng/ml) even for small sample sizes (Table 1).
The collagen laboratory demineralisation protocol applied here involves bone mineral dissolution taking place over days to weeks at low temperatures (Fewlass et al., 2019; Talamo et al. 2021) but some laboratories use faster demineralisation protocols involving powdered samples. If the necessary steps are taken to avoid Zn contamination during demineralisation, our protocol could be easily adapted to these methods. Indeed, this type of demineralisation is very similar to the demineralisation protocols commonly applied for Zn analysis on both bone and enamel samples (Jaouen et al. 2016a, b; Bourgon et al. 2020; McCormack et al. 2021). However, special care should be taken to avoid Zn contamination during bone or dentine powder grinding.
Coupling δ66Zn with collagen extraction protocols provides an additional nitrogen-independent dietary and trophic level indicator, enabling more robust palaeoecological interpretations by (in)validation of results, as a baseline control, and by providing additional dietary/physiological information, depending on the sample material. The homogeneity of same sample δ66Zn values independent of the here applied demineralisation method within both Ranis and Ain Difla bones implies that our protocol is applicable across the range of collagen preservation in archaeological skeletal remains. Although it remains to be tested, there is also the potential of using aliquots of the dissolved bioapatite as demineralised herein to also analyse other dietary tracers likely less impacted by contamination issues, such as calcium isotopes, together with δ66Zn. Another benefit of this method is that collecting the dissolved mineral solution for further δ66Zn analyses is not limited to laboratories with the necessary infrastructure to perform clean laboratory Zn purification and δ66Zn MC-ICP-MS analyses. When the dissolved mineral solution is collected in precleaned closed PFA vials, it can be safely stored and transported to other laboratories for further processing and analyses if necessary.
Stable isotope values of both inorganic and organic components of vertebrate skeletal remains are less likely to be influenced by diagenetic alteration in more recent skeletal material compared to the Palaeolithic material examined herein; i.e. the likelihood of alteration increases with age, but the level of preservation will also strongly depend on the burial environment (van Klinken 1999; Collins et al. 2002; Clementz 2012). However, collecting the dissolved mineral solution for δ66Zn analyses may provide a dietary proxy even when collagen extraction fails to provide bulk δ13C and δ15N data due to poor organic preservation. For example, the bone fragments from Ain Difla are not sufficiently preserved to allow taxonomic identification or collagen extraction for additional analyses, yet the δ66Zn bone values and Zn concentrations are within the range of modern herbivore bones from Kenya (Jaouen et al., 2016b). Nevertheless, care must be taken when interpreting δ66Zn values in the absence of collagen. The loss of collagen can potentially impact bone mineral δ66Zn values as the increase in porosity following the loss of the organic matrix can lead to diagenetic modification via bioapatite recrystallisation, contamination, or secondary mineral precipitation (Hedges 2002; Trueman et al. 2004). A significant modification of original bone mineral elemental values might result in a correlation between the elemental concentration, expressed as 1/concentration and the isotopic composition of the element in question (Reynard and Balter 2014). The lack of a correlation between δ66Zn and Zn concentration for bones from both Ain Difla and Ranis suggests that soil Zn addition and/or diagenetic bone Zn exchange is unlikely to have completely modified the samples’ δ66Zn values (Fig. 2).
There are apparent differences in the Zn concentration and isotope composition between Ain Difla and Ranis, which could be related to differences in diagenetic modification between the bones of these sites and/or environmental differences in local Zn baselines. With our current dataset, we cannot unambiguously exclude either possibility. Zinc concentration in bones can vary depending on the dietary Zn uptake, as demonstrated by the large variability in recent and archaeological bone and dentine Zn concentrations observed in, e.g., marine Arctic remains and terrestrial remains from Kenya and Idaho (24 to 1025 µg/g, Kohn et al. 2013; Jaouen et al. 2016a, b; McCormack et al. 2021). It is noteworthy that we observe a correlation between bone δ66Zn values and bulk collagen δ13C values at Ranis, suggesting a dietary influence on both proxies and further indicating preservation of bone δ66Zn values (Fig. 3e). Still, without clearly identifiable carnivore remains analysed in conjunction with herbivore remains and an observed trophic offset between them, we cannot exclude a possible diagenetic alteration of the bones δ66Zn values for either site. We thus recommend careful evaluation of potential Zn diagenetic alteration (e.g. Kohn and Cerling 2002) when using older fossil bone or dentine material for palaeoecological investigations.
Nitrogen, zinc, and carbon isotope values from bone samples from Ranis. a, b, and c respectively show δ15N, δ66Zn, and δ13C bone values arranged according to their taxonomy (x-axis). d shows δ66Zn plotted against δ15N; e shows δ66Zn plotted against δ13C; f shows δ15N plotted against δ13C. Note, that the only statistically relevant relationship for d–f is given between δ66Zn and δ13C (i.e. p < 0.05). The boxes in a–c (for n > 5) represent the 25th–75th percentiles, with the mean as a smaller box and whiskers showing the 10th–90th percentiles. Squares represent Equidae, circles Rangifer and triangles Ursidae
Conclusions
We demonstrate that bone collagen extraction protocols for 14C dating and δ13C and δ15N analyses can be routinely coupled with δ66Zn analyses of the dissolved mineral solution without the necessity of resampling precious archaeological or palaeontological skeletal material. Zinc concentration in bioapatite is typically high, allowing the collection of the dissolved mineral solution from collagen extraction protocols for further δ66Zn analyses with only minor and easily implemented adjustments to established protocols to minimise Zn contamination. We observe no difference in bone δ66Zn values between bones dissolved following our collagen extraction protocol, with further collagen processing, in a standard collagen extraction laboratory and the same material dissolved in a metal-free clean laboratory. Instead of discarding the dissolved mineral solution after collagen extraction, the solution can be retained and used for δ66Zn analysis to gain an additional dietary and trophic level indicator that will allow more robust (palaeo)ecological interpretations. Additionally, collecting the demineralisation solution may provide δ66Zn as a dietary proxy even if collagen preservation is insufficient for traditional δ13C and δ15N analyses. This approach can also be implemented in dating/isotopic laboratories without cleanroom and MC-ICP-MS facilities, as the solution can be safely stored and transported to laboratories with the necessary setup for further Zn purification and isotope analysis.
Obtaining necessary samples from archaeological material for interpretations of the palaeoenvironment, chronology, and genetic makeup is understandably associated with certain unease due to the destructive nature of many sampling methods. Accordingly, any method that allows the utilisation of a by-product that is usually and regularly disposed of from ongoing analyses (in our case, the mineral solution of bioapatite) for additional or supplementary knowledge should be especially adopted. Apart from the obvious benefits for conservation of archaeological specimens undergoing sampling, such a method of salvaging samples and products of their analysis increases the value of existing archaeological collections and, at the same time, lowers the quantity of new archaeological material necessary to excavate. Equally important, such methods could prove invaluable for archaeological sciences to operate as a scientific practice (Kosso 2011; Smith 2017) because they supplement and add to insights gained by other analyses (in this case, traditional dietary δ13C and δ15N analyses), which can then be used for (in)validation and falsification-control of results. For these reasons, we think that such an approach and the development of such methods should warrant a particular research emphasis in archaeological sciences in general.
Data availability
All data are available in the main text or the supplementary materials.
References
Ambrose SH (1990) Preparation and characterization of bone and tooth collagen for isotopic analysis. J Archaeol Sci 17(4):431–451. https://doi.org/10.1016/0305-4403(90)90007-R
Balter V, Lamboux A, Zazzo A, Télouk P, Leverrier Y, Marvel J, Moloney AP, Monahan FJ, Schmidt O, Albarède F (2013) Contrasting Cu, Fe, and Zn isotopic patterns in organs and body fluids of mice and sheep, with emphasis on cellular fractionation. Metallomics 5(11):1470–1482. https://doi.org/10.1039/c3mt00151b
Bocherens H, Drucker D (2003) Trophic level isotopic enrichment of carbon and nitrogen in bone collagen: case studies from recent and ancient terrestrial ecosystems. Int J Osteoarchaeol 13(1–2):46–53. https://doi.org/10.1002/oa.662
Bourgon N, Jaouen K, Bacon A-M, Jochum KP, Dufour E, Duringer P, Ponche J-L, Joannes-Boyau R, Boesch Q, Antoine P-O, Hullot M, Weis U, Schulz-Kornas E, Trost M, Fiorillo D, Demeter F, Patole-Edoumba E, Shackelford LL, Dunn TE, Zachwieja A, Duangthongchit S, Sayavonkhamdy T, Sichanthongtip P, Sihanam D, Souksavatdy V, Hublin J-J, Tütken T (2020) Zinc isotopes in Late Pleistocene fossil teeth from a Southeast Asian cave setting preserve paleodietary information. Proc Natl Acad Sci 117(9):4675–4681. https://doi.org/10.1073/pnas.1911744117
Bourgon N, Jaouen K, Bacon A-M, Dufour E, McCormack J, Tran N-H, Trost M, Fiorillo D, Dunn TE, Zanolli C, Zachwieja A, Duringer P, Ponche J-L, Boesch Q, Antoine P-O, Westaway KE, Joannes-Boyau R, Suzzoni E, Frangeul S, Crozier F, Aubaile F, Patole-Edoumba E, Luangkhoth T, Souksavatdy V, Boualaphane S, Sayavonkhamdy T, Sichanthongtip P, Sihanam DF, Schackelford, LL, Hublin, J-J, Tütken, T (2021) Diet of a Late Pleistocene early modern human from Southeast Asia inferred from Zn and C isotopes. J Hum Evol 161:103075. https://doi.org/10.1016/j.jhevol.2021.103075
Brock F, Higham T, Ditchfield P, Bronk Ramsey C (2010) Current pretreatment methods for AMS radiocarbon dating at the oxford radiocarbon accelerator unit (ORAU). Radiocarbon 52(1):103–112. https://doi.org/10.1017/S0033822200045069
Bronk Ramsey C, Higham T, Bowles A, Hedges R (2004) Improvements to the pretreatment of bone at Oxford. Radiocarbon 46(1):155–164. https://doi.org/10.1017/S0033822200039473
Buckley M, Collins M, Thomas-Oates J, Wilson JC (2009) Species identification by analysis of bone collagen using matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 23(23):3843–3854. https://doi.org/10.1002/rcm.4316
Casey MM, Post DM (2011) The problem of isotopic baseline: reconstructing the diet and trophic position of fossil animals. Earth Sci Rev 106(1–2):131–148. https://doi.org/10.1016/j.earscirev.2011.02.001
Clark G, Schulderein J, Donaldson M, Schwarcz H, Rink W, Fish S (1997) Chronostratigraphic contexts of Middle Palaeolithic horizons at the Ain Difla Rockshelter (WHS 634), west-central Jordan. In: Gebel H-G, Kafafi Z, Rollefson GO (eds) The Prehistory of Jordan II: Perspectives From 1997. Ex Oriente, Berlin, pp 77–100
Clementz MT (2012) New insight from old bones: stable isotope analysis of fossil mammals. J Mammal 93(2):368–380. https://doi.org/10.1644/11-MAMM-S-179.1
Collins MJ, Nielsen–Marsh CM, Hiller J, Smith CI, Roberts JP, Prigodich RV, Weiss TJ, Csapò J, Millard AR, Turner-Walker G (2002) The survival of organic matter in bone: a review. Archaeometry 44(3):383–394
Copeland SR, Sponheimer M, le Roux PJ, Grimes V, Lee-Thorp JA, de Ruiter DJ, Richards MP (2008) Strontium isotope ratios (87Sr/86Sr) of tooth enamel: a comparison of solution and laser ablation multicollector inductively coupled plasma mass spectrometry methods. Rapid Commun Mass Spectrom 22(20):3187–3194. https://doi.org/10.1002/rcm.3717
De la Vega C, Jeffreys RM, Tuerena R, Ganeshram R, Mahaffey C (2019) Temporal and spatial trends in marine carbon isotopes in the Arctic Ocean and implications for food web studies. Glob Change Biol 25(12):4116–4130. https://doi.org/10.1111/gcb.14832
DeNiro MJ (1985) Postmortem preservation and alteration of in vivo bone collagen isotope ratios in relation to palaeodietary reconstruction. Nature 317(6040):806–809. https://doi.org/10.1038/317806a0
Fewlass H, Tuna T, Fagault Y, Hublin J-J, Kromer B, Bard E, Talamo S (2019) Pretreatment and gaseous radiocarbon dating of 40–100 mg archaeological bone. Sci Rep 9(5342). https://doi.org/10.1038/s41598-019-41557-8
Fuller BT, Fuller JL, Sage NE, Harris DA, O’Connell TC, Hedges RE (2005) Nitrogen balance and δ15N: why you’re not what you eat during nutritional stress. Rapid Commun Mass Spectrom 19(18):2497–2506. https://doi.org/10.1002/rcm.2090
Hedges RE (2002) Bone diagenesis: an overview of processes. Archaeometry 44(3):319–328. https://doi.org/10.1111/1475-4754.00064
Heuser A, Tütken T, Gussone N, Galer SJ (2011) Calcium isotopes in fossil bones and teeth—diagenetic versus biogenic origin. Geochim Cosmochim Acta 75(12):3419–3433. https://doi.org/10.1016/j.gca.2011.03.032
Hobson KA, Welch HE (1992) Determination of trophic relationships within a high Arctic marine food web using δ13C and δ15N analysis. Mar Ecol Prog Ser 84(1):9–18
Hobson KA, Alisauskas RT, Clark RG (1993) Stable-nitrogen isotope enrichment in avian tissues due to fasting and nutritional stress: implications for isotopic analyses of diet. The Condor 95(2):388–394. https://doi.org/10.2307/1369361
Hülle W (1977) Die Ilsenhöhle unter Burg Ranis, Thüringen. Gustav Fischer Verlag, Stuttgart
Jaouen K, Szpak P, Richards MP (2016a) Zinc isotope ratios as indicators of diet and trophic level in Arctic marine mammals. PLoS ONE 11:e0152299. https://doi.org/10.1371/journal.pone.0152299
Jaouen K, Beasley M, Schoeninger M, Hublin J-J, & Richards MP (2016b) Zinc isotope ratios of bones and teeth as new dietary indicators: results from a modern food web (Koobi Fora, Kenya). Sci Rep 6(26281). https://doi.org/10.1038/srep26281
Jaouen K, Trost M, Bourgon N, Colleter R, Le Cabec A, Tütken T, Oliveira RE, Pons ML, Méjean P, Steinbrenner S, Chmeleff J, Strauss A (2020) Zinc isotope variations in archeological human teeth (Lapa do Santo, Brazil) reveal dietary transitions in childhood and no contamination from gloves. PLoS ONE 15:e0232379. https://doi.org/10.1371/journal.pone.0232379
Kohn MJ, Cerling TE (2002) Stable isotope compositions of biological apatite. Rev Mineral Geochem 48(1):455–488. https://doi.org/10.2138/rmg.2002.48.12
Kohn MJ, Morris J, Olin P (2013) Trace element concentrations in teeth – a modern Idaho baseline with implications for archeometry, forensics, and palaeontology. J Archaeol Sci 40(4):1689–1699. https://doi.org/10.1016/j.jas.2012.11.012
Kosso P (2011) A summary of scientific method. Springer, New York
Longin R (1971) New method of collagen extraction for radiocarbon dating. Nature 230(5291):241–242. https://doi.org/10.1038/230241a0
Mahan B, Moynier F, Jørgensen AL, Habekost M, Siebert J (2018) Examining the homeostatic distribution of metals and Zn isotopes in Göttingen minipigs. Metallomics 10(9):1264–1281. https://doi.org/10.1039/c8mt00179k
Maréchal CN, Télouk P, Albarède F (1999) Precise analysis of copper and zinc isotopic compositions by plasma-source mass spectrometry. Chem Geol 156(1–4):251–273. https://doi.org/10.1016/S0009-2541(98)00191-0
Martin JE, Tacail T, Adnet S, Girard C, Balter V (2015) Calcium isotopes reveal the trophic position of extant and fossil elasmobranchs. Chem Geol 415:118–125. https://doi.org/10.1016/j.chemgeo.2015.09.011
Martin JE, Tacail T, Braga J, Cerling TE, Balter V (2020) Calcium isotopic ecology of Turkana Basin hominins. Nat Commun 11:3587. https://doi.org/10.1038/s41467-020-17427-7
McCormack J, Griffiths ML, Kim SL, Shimada K, Karnes M, Maisch H, Pederzani S, Bourgon N, Jaouen K, Becker MA, Jöns N, Sisma-Ventura G, Straube N, Pollerspöck J, Hublin J-J, Eagle RA, Tütken T (2022) Trophic position of Otodus megalodon and great white sharks through time revealed by zinc isotopes. Nat Commun 13:2980. https://doi.org/10.1038/s41467-022-30528-9
McCormack J, Szpak P, Bourgon N, Richards M, Hyland C, Méjean P, Hublin J-J, Jaouen K (2021) Zinc isotopes from archaeological bones provide reliable trophic level information for marine mammals. Commun Biol 4(683). https://doi.org/10.1038/s42003-021-02212-z
Moynier F, Albarède F, Herzog GF (2006) Isotopic composition of zinc, copper, and iron in lunar samples. Geochim Cosmochim Acta 70(24):6103–6117. https://doi.org/10.1016/j.gca.2006.02.030
Moynier F, Fujii T, Shaw AS, Le Borgne M (2013) Heterogeneous distribution of natural zinc isotopes in mice. Metallomics 5(6):693–699. https://doi.org/10.1039/c3mt00008g
Reynard B, Balter V (2014) Trace elements and their isotopes in bones and teeth: diet, environments, diagenesis, and dating of archeological and paleontological samples. Palaeogeogr Palaeoclimatol Palaeoecol 416:4–16. https://doi.org/10.1016/j.palaeo.2014.07.038
Rezek Z (2020) Ain Difla. Archaeology in Jordan 2:34–35
Sealy J, Johnson M, Richards M, Nehlich O (2014) Comparison of two methods of extracting bone collagen for stable carbon and nitrogen isotope analysis: comparing whole bone demineralization with gelatinization and ultrafiltration. J Archaeol Sci 47:64–69. https://doi.org/10.1016/j.jas.2014.04.011
Smith ME (2017) Social science and archaeological enquiry. Antiquity 91(356):520–528. https://doi.org/10.15184/aqy.2017.19
Sponheimer M, Passey BH, De Ruiter DJ, Guatelli-Steinberg D, Cerling TE, Lee-Thorp JA (2006) Isotopic evidence for dietary variability in the early hominin Paranthropus robustus. Science 314(5801):980–982. https://doi.org/10.1126/science.1133827
Talamo S, Fewlass H, Maria R, Jaouen K (2021) “Here we go again”: the inspection of collagen extraction protocols for 14C dating and palaeodietary analysis. Sci Technol Archaeol Res 7(1):62–77. https://doi.org/10.1080/20548923.2021.1944479
Toutain JP, Sonke J, Munoz M, Nonell A, Polvé M, Viers J, Freydier R, Sortino F, Joron J-L, Sumarti S (2008) Evidence for Zn isotopic fractionation at Merapi volcano. Chem Geol 253(1–2):74–82. https://doi.org/10.1016/j.chemgeo.2008.04.007
Trueman CN, Behrensmeyer AK, Tuross N, Weiner S (2004) Mineralogical and compositional changes in bones exposed on soil surfaces in Amboseli National Park, Kenya: diagenetic mechanisms and the role of sediment pore fluids. J Archaeol Sci 31(6):721–739. https://doi.org/10.1016/j.jas.2003.11.003
van Klinken GJ (1999) Bone collagen quality indicators for palaeodietary and radiocarbon measurements. J Archaeol Sci 26(6):687–695. https://doi.org/10.1006/jasc.1998.0385
van Doorn NL, Hollund H, Collins MJ (2011) A novel and non-destructive approach for ZooMS analysis: ammonium bicarbonate buffer extraction. Archaeol Anthropol Sci 3(3):281. https://doi.org/10.1007/s12520-011-0067-y
Vander Zanden MJ, Rasmussen JB (2001) Variation in δ15N and δ13C trophic fractionation: implications for aquatic food web studies. Limnol Oceanogr 46(8):2061–2066. https://doi.org/10.4319/lo.2001.46.8.2061
Warinner C, Tuross N (2010) Brief communication: tissue isotopic enrichment associated with growth depression in a pig: Implications for archaeology and ecology. Am J Phys Anthropol 141(3):486–493. https://doi.org/10.1002/ajpa.21222
Welker F, Hajdinjak M, Talamo S, Jaouen K, Dannemann M, David F, Julien M, Meyer M, Kelso J, Barnes I, Brace S, Kamminga P, Fischer R, Kessler BM, Stewart JR, Pääbo S, Collins MJ, Hublin J-J (2016) Palaeoproteomic evidence identifies archaic hominins associated with the Châtelperronian at the Grotte du Renne. Proc Natl Acad Sci 113(40):11162–11167. https://doi.org/10.1073/pnas.1605834113
Acknowledgements
We thank Manuel Trost for laboratory support and Sven Steinbrenner for technical assistance with the EA-IRMS. We are grateful for the productive and fruitful discussions with Klervia Jaouen. We thank Marcel Weiss and Tim Schüler for access to the Ranis material. We also thank the Department of Antiquities of Jordan for the excavation permission, logistical support in the field, and the permission to loan and export bone samples. We thank the Fraunhofer Institute for Cell Therapy and Immunology (Leipzig, Germany), Stefan Kalkhof, and Johannes Schmidt for access to the MALDI-TOF MS instrument.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was funded by the Max Planck Society. GMS received funding from the European Union’s Horizon Europe research and innovation programme under the Marie Skłodowska-Curie grant agreement No 101027850.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
McCormack, J., Bourgon, N., Sinet-Mathiot, V. et al. Combining collagen extraction with mineral Zn isotope analyses from a single sample for robust palaeoecological investigations. Archaeol Anthropol Sci 14, 137 (2022). https://doi.org/10.1007/s12520-022-01601-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12520-022-01601-7