Abstract
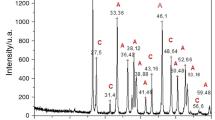
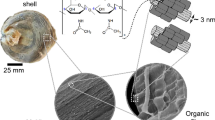
A detailed characterisation of the organic composition of Arctica islandica (Linnaeus, 1767) shells with homogenous microstructure is compared with nacroprismatic shells of Pinctada fucata martensii (Dunker, 1872), Hyriopsis cumingii (Lea, 1852) and Diplodon chilensis patagonicus (d’Orbigny, 1835). Thermogravimetric analysis shows lowest total organic contents of 1.65 wt% for A. islandica shells, while all nacroprismatic shells are higher (3.14–4.13 wt%). Macromolecules extracted from the nacroprismatic shells are dominated by hydrophobic amino acids (~ 54%) in the acid extracts, while EDTA-extracts are moderately rich in aspartate and glutamate (16% in total) and in glycine–alanine (42%). In comparison, A. islandica shells have higher concentrations of proline, glycine and aspartate (ca 40%). Infrared spectroscopy shows some acidic protein bands in A. islandica, which cannot be found in the nacroprismatic shells. Alcian Blue and/or modified silver staining methods reveal many prominent bands. Protein bands at around 10, 14, 17, 21, 26, 31, 40 and 55 kDa are detected in A. islandica shells for the first time, thus may constitute a new set of proteins in mollusc shells. SDS-PAGE exhibits apparent molecular weights from 5 to 63 kDa in nacroprismatic shells. Distinct protein bands at around 17 and 26 kDa in A. islandica shells may correspond to a post-translational modification of proteins; these prominent bands, however, are absent in the nacroprismatic samples. Contrarily, SDS-PAGE of both, homogeneous and nacroprismatic shell microstructures show nonacidic-matrix-proteins.





Similar content being viewed by others
References
Addadi L, Weiner S (2014) Biomineralization: mineral formation by organisms. Phys Scr 89:098003
Addadi L, Joester D, Nudelman F, Weiner S (2006) Mollusk shell formation: a source of new concepts for understanding biomineralization processes. Chem Eur J 12:980–987. doi:10.1002/chem.200500980
Agbaje O, Wirth R, Morales L, Shirai K, Kosnik M, Watanabe T, Jacob D (2017) Architecture of crossed-lamellar bivalve shells: the southern giant clam (Tridacna derasa, Röding, 1798). R Soc Open Sci 4:170622. doi:10.1098/rsos.170622
Arivalagan J, Marie B, Sleight VA, Clark MS, Berland S, Marie A (2016) Shell matrix proteins of the clam, Mya truncata: roles beyond shell formation through proteomic study. Mar Genom 27:69–74. doi:10.1016/j.margen.2016.03.005
Bédouet L, Schuller MJ, Marin F, Milet C, Lopez E, Giraud M (2001) Soluble proteins of the nacre of the giant oyster Pinctada maxima and of the abalone Haliotis tuberculata: extraction and partial analysis of nacre proteins. Comp Biochem Physiol B: Biochem Mol Biol 128:389–400
Bédouet L, Marie A, Dubost L, Péduzzi J, Duplat D, Berland S, Puisségur M, Boulzaguet H, Rousseau M, Milet C (2007) Proteomics analysis of the nacre soluble and insoluble proteins from the oyster Pinctada margaritifera. Mar Biotechnol 9:638–649. doi:10.1007/s10126-007-9017-1
Bevelander G, Nakahara H (1969) An electron microscope study of the formation of the nacreous layer in the shell of certain bivalve molluscs. Calcif Tissue Res 3:84–92. doi:10.1007/BF02058648
Brine CJ, Austin PR (1981) Chitin variability with species and method of preparation. Comp Biochem Physiol B: Biochem Mol Biol 69:283–286
Cárdenas G, Cabrera G, Taboada E, Miranda SP (2004) Chitin characterization by SEM, FTIR, XRD, and 13C cross polarization/mass angle spinning NMR. J Appl Polym Sci 93:1876–1885. doi:10.1002/app.20647
Carter JG (1990) Skeletal biomineralization: patterns, processes and evolutionary trends. Wiley, New York
Carter JG, Aller RC (1975) Calcification in the bivalve periostracum. Lethaia 8:315–320
Cohen SA, Michaud DP (1993) Synthesis of a fluorescent derivatizing reagent, 6 aminoquinolyl-N-hydroxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal Biochem 211:279–287
Dauphin Y (2003) Soluble organic matrices of the calcitic prismatic shell layers of two pteriomorphid bivalves Pinna nobilis and Pinctada margaritifera. J Biol Chem 278:15168–15177. doi:10.1074/jbc.M204375200
Dauphin Y, Denis A (2000) Structure and composition of the aragonitic crossed lamellar layers in six species of Bivalvia and Gastropoda. Comp Biochem Physiol A: Mol Integr Physiol 126:367–377
Farre B, Dauphin Y (2009) Lipids from the nacreous and prismatic layers of two Pteriomorpha Mollusc shells. Comp Biochem Physiol B: Biochem Mol Biol 152:103–109
Giuffre AJ, Hamm LM, Han N, De Yoreo JJ, Dove PM (2013) Polysaccharide chemistry regulates kinetics of calcite nucleation through competition of interfacial energies. Proc Natl Acad Sci 110:9261–9266. doi:10.1073/pnas.1222162110
Gotliv BA, Addadi L, Weiner S (2003) Mollusk shell acidic proteins: in search of individual functions. ChemBioChem 4:522–529. doi:10.1002/cbic.200200548
Hamm LM, Giuffre AJ, Han N, Tao J, Wang D, De Yoreo JJ, Dove PM (2014) Reconciling disparate views of template-directed nucleation through measurement of calcite nucleation kinetics and binding energies. Proc Natl Acad Sci 111:1304–1309. doi:10.1073/pnas.1312369111
Keith J, Stockwell S, Ball D, Remillard K, Kaplan D, Thannhauser T, Sherwood R (1993) Comparative analysis of macromolecules in mollusc shells. Comp Biochem Physiol B: Biochem Mol Biol 105:487–496
Kono M, Hayashi N, Samata T (2000) Molecular mechanism of the nacreous layer formation in Pinctada maxima. Biochem Biophys Res Commun 269:213–218. doi:10.1006/bbrc.2000.2274
Lavall RL, Assis OB, Campana-Filho SP (2007) β-Chitin from the pens of Loligo sp.: extraction and characterization. Bioresour Technol 98:2465–2472
Levi-Kalisman Y, Falini G, Addadi L, Weiner S (2001) Structure of the nacreous organic matrix of a bivalve mollusk shell examined in the hydrated state using cryo-TEM. J Struct Biol 135:8–17
Lombardi SJ, Kaplan DL (1990) The amino acid composition of major ampullate gland silk (dragline) of Nephila clavipes (Araneae, Tetragnathidae). J Arachnol 18:297–306
Lopes A, Hinzmann M, Bobos I, Lopes-Lima M, Gonçalves JF, rgio Ferreira S, Domingues B, Machado J (2014) Functional studies on the shell soluble matrix of Anodonta cygnea (Bivalvia: Unionidae). Nautilus 128:105–113
Ma Y, Qiao L, Feng Q (2012) In-vitro study on calcium carbonate crystal growth mediated by organic matrix extracted from fresh water pearls. Mater Sci Eng, C 32:1963–1970
Ma Y, Berland S, Andrieu JP, Feng Q, Bédouet L (2013) What is the difference in organic matrix of aragonite vs. vaterite polymorph in natural shell and pearl? Study of the pearl forming freshwater bivalve mollusc Hyriopsis cumingii. Mater Sci Eng, C 33:1521–1529
Marie B, Luquet G, Pais De Barros JP, Guichard N, Morel S, Alcaraz G, Bollache L, Marin F (2007) The shell matrix of the freshwater mussel Unio pictorum (Paleoheterodonta, Unionoida). FEBS J 274:2933–2945. doi:10.1111/j.1742-4658.2007.05825.x
Marie B, Zanella-Cléon I, Corneillat M, Becchi M, Alcaraz G, Plasseraud L, Luquet G, Marin F (2011) Nautilin-63, a novel acidic glycoprotein from the shell nacre of Nautilus macromphalus. FEBS J 278:2117–2130. doi:10.1111/j.1742-4658.2011.08129.x
Marin F, Luquet G (2005) Molluscan biomineralization: the proteinaceous shell constituents of Pinna nobilis L. Mater Sci Eng, C 25:105–111
Miyashita T, Takagi R, Okushima M, Nakano S, Miyamoto H, Nishikawa E, Matsushiro A (2000) Complementary DNA cloning and characterization of pearlin, a new class of matrix protein in the nacreous layer of oyster pearls. Mar Biotechnol 2:409–418. doi:10.1007/PL00021687
Morrissey JH (1981) Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem 117:307–310
Natoli A, Wiens M, Schröder HC, Stifanic M, Batel R, Soldati AL, Jacob DE, Müller WE (2010) Bio-vaterite formation by glycoproteins from freshwater pearls. Micron 41:359–366
Pereira-Mouriès L, Almeida MJ, Ribeiro C, Peduzzi J, Barthélemy M, Milet C, Lopez E (2002) Soluble silk-like organic matrix in the nacreous layer of the bivalve Pinctada maxima. Eur J Biochem 269:4994–5003. doi:10.1046/j.1432-1033.2002.03203.x
Philipp EE, Wessels W, Gruber H, Strahl J, Wagner AE, Ernst IM, Rimbach G, Kraemer L, Schreiber S, Abele D (2012) Gene expression and physiological changes of different populations of the long-lived bivalve Arctica islandica under low oxygen conditions. PLoS ONE 7:e44621
Rahman MA, Isa Y, Takemura A, Uehara T (2006) Analysis of proteinaceous components of the organic matrix of endoskeletal sclerites from the alcyonarian Lobophytum crassum. Calcif Tissue Int 78:178–185. doi:10.1007/s00223-005-0253-y
Roeges NP (1994) A guide to the complete interpretation of infrared spectra of organic structures. Wiley, New York
Ropes JW, Jones D, Murawski S, Serchuk F, Jearld A (1984) Documentation of annual growth lines in ocean quahogs, Arctica Islandica LINNE. Fish Bull 82:1–19
Samata T (1990) Ca-binding glycoproteins in molluscan shells with different types of ultrastructure. Veliger 33:190–201
Shirai K, Schöne BR, Miyaji T, Radarmacher P, Krause RA, Tanabe K (2014) Assessment of the mechanism of elemental incorporation into bivalve shells (Arctica islandica) based on elemental distribution at the microstructural scale. Geochim Cosmochim Acta 126:307–320. doi:10.1016/j.gca.2013.10.050
Stankiewicz BA, Mastalerz M, Hof CH, Bierstedt A, Flannery MB, Briggs DE, Evershed RP (1998) Biodegradation of the chitin-protein complex in crustacean cuticle. Org Geochem 28:67–76
Sudo S, Fujikawa T, Nagakura T, Ohkubo T, Sakaguchi K, Tanaka M, Nakashima K, Takahashi T (1997) Structures of mollusc shell framework proteins. Nature 387:563–564
Taylor JD (1973) The structural evolution of the bivalve shell. Palaeontology 16:519–534
Truong HH, Neilson KA, McInerney BV, Khoddami A, Roberts TH, Liu SY, Selle PH (2015) Performance of broiler chickens offered nutritionally-equivalent diets based on two red grain sorghums with quantified kafirin concentrations as intact pellets or re-ground mash following steam-pelleting at 65 or 97 °C conditioning temperatures. Anim Nutr 1:220–228
Wall RS, Gyi TJ (1988) Alcian blue staining of proteoglycans in polyacrylamide gels using the “critical electrolyte concentration” approach. Anal Biochem 175:298–299
Weidman CR, Jones GA (1994) The long-lived mollusc Arctica islandica: A new paleoceanographic tool for the reconstruction of bottom temperatures for the continental shelves of the northern North Atlantic Ocean. J Geophys Res Oceans 99:18305–18314. doi:10.1029/94JC01882
Weiner S, Dove PM (2003) An overview of biomineralization processes and the problem of the vital effect. Rev Mineral Geochem 54:1–29. doi:10.2113/0540001
Weiner S, Traub W (1980) X-ray diffraction study of the insoluble organic matrix of mollusk shells. FEBS Lett 111:311–316
Yan Z, Jing G, Gong N, Li C, Zhou Y, Xie L, Zhang R (2007) N40, a novel nonacidic matrix protein from pearl oyster nacre, facilitates nucleation of aragonite in vitro. Biomacromol 8:3597–3601. doi:10.1021/bm0701494
Zaitoun MA, Lin C (1997) Chelating behavior between metal ions and EDTA in sol–gel matrix. J Phys Chem B 101:1857–1860. doi:10.1021/jp963102d
Zhang C, Li S, Ma Z, Xie L, Zhang R (2006) A novel matrix protein p10 from the nacre of pearl oyster (Pinctada fucata) and its effects on both CaCO3 crystal formation and mineralogenic cells. Mar Biotechnol 8:624–633. doi:10.1007/s10126-006-6037-1
Acknowledgements
Mark Tran and Dr Christopher McRae are well-appreciated for their incessant technical supports. OBAA acknowledges Emily Gibson and Dr Bhumika Shah (Chemistry and Biomolecular Sciences, Macquarie University) for teaching him the basic techniques of gel electrophoresis. The authors are grateful to A. Checa and the anonymous reviewers for their valuable comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded through an Australian Research Council (ARC) Future Fellowship to DEJ. The work was facilitated in part by the Australian Government’s National Collaborative Research Strategy (NCRIS) and its facilities at the Australian Proteome Analysis Facility (APAF).
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of mollusc shells were followed. Recently alive shells: Arctica islandica, Hyriopsis cumingii, Diplodon chilensis patagonicus and Pinctada fucata martensii were provided from the private collection of one of the authors (DEJ).
Additional information
Responsible Editor: A. G. Checa.
Reviewed by Undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Agbaje, O.B.A., Thomas, D.E., Mclnerney, B.V. et al. Organic macromolecules in shells of Arctica islandica: comparison with nacroprismatic bivalve shells. Mar Biol 164, 208 (2017). https://doi.org/10.1007/s00227-017-3238-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-017-3238-2