Abstract
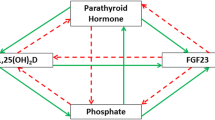
Phosphorus, a 5A element with atomic weight of 31, comprises just over 0.6% of the composition by weight of plants and animals. Three isotopes are available for studying phosphorus metabolism and kinetics. 31P is stable, whereas the radioactive isotope 33P has a half-life of 25 days and 32P has a half-life of 14 days. Phosphate ester and phosphoanhydride are common chemical linkages and phosphorus is a key element in organic molecules involved in a wide variety of essential cellular functions. These include biochemical energy transfer via adenosine triphosphate (ATP), maintenance of genetic information with nucleotides DNA and RNA, intracellular signaling via cyclic adenosine monophosphate (cAMP), and membrane structural integrity via glycerophospholipids. However, this review focuses on the metabolism of inorganic phosphorus (Pi) acting as a weak acid. Phosphoric acid has all three hydrogens attached to oxygen and is a weak diprotic acid. It has 3 pKa values: pH 2.2, pH 7.2, and pH 12.7. At physiological pH of 7.4, Pi exists as both H2PO4(−) and HPO4(2−) and acts as an extracellular fluid (ECF) buffer. Pi is the form transported across tissue compartments and cells. Measurement of Pi in biological fluids is based on its reaction with ammonium molybdate which does not measure organic phosphorus. In humans, 80% of the body phosphorus is present in the form of calcium phosphate crystals (apatite) that confer hardness to bone and teeth, and function as the major phosphorus reservoir (Fig. 1). The remainder is present in soft tissues and ECF. Dietary phosphorus, comprising both inorganic and organic forms, is digested in the upper gastrointestinal tract. Absorbed Pi is transported to and from bone, skeletal muscle and soft tissues, and kidney at rates determined by ECF Pi concentration, rate of blood flow, and activity of cell Pi transporters (Fig. 2). During growth, there is net accretion of phosphorus, and with aging, net loss of phosphorus occurs. The bone phosphorus reservoir is depleted and repleted by overall phosphorus requirement. Skeletal muscle is rich in phosphorus used in essential biochemical energy transfer. Kidney is the main regulator of ECF Pi concentration by virtue of having a tubular maximum reabsorptive capacity for Pi (TmPi) that is under close endocrine control. It is also the main excretory pathway for Pi surplus which is passed in urine. Transcellular and paracellular Pi transports are performed by a number of transport mechanisms widely distributed in tissues, and particularly important in gut, bone, and kidney. Pi transporters are regulated by a hormonal axis comprising fibroblast growth factor 23 (FGF23), parathyroid hormone (PTH), and 1,25 dihydroxy vitamin D (1,25D). Pi and calcium (Ca) metabolism are intimately interrelated, and clinically neither can be considered in isolation. Diseases of Pi metabolism affect bone as osteomalacia/rickets, soft tissues as ectopic mineralization, skeletal muscle as myopathy, and kidney as nephrocalcinosis and urinary stone formation.

Content of phosphorus in human adult: skeleton, soft tissue, and extracellular fluid (grams, log scale). Corresponding data for calcium are shown for comparison

Phosphate (Pi) transport to and from tissue compartments in mg/24 h. At a dietary phosphorus of 1400 mg, 1120 mg is absorbed in upper intestine to the ECF, 210 mg returned to intestine by endogenous secretion, resulting in 910 mg net Pi absorption and 490 mg fecal excretion. At bone, 180 mg is deposited by bone formation and 180 mg return to the ECF by bone resorption. At kidney, 5040 mg is filtered at the glomerulus and 4130 mg return to the ECF by tubular reabsorption with 910 mg excreted in the urine. In soft tissue, Pi is exchanged between ECF and cells











Similar content being viewed by others
References
Marshall RW (1976) Plasma Fractions. In: Nordin BEC (ed) Calcium, Phosphate, and Magnesium Metabolism. Churchill Livingstone, Edinburgh, pp 162–185
Kruse K, Kracht U, Gopfert G (1982) Renal threshold phosphate concentration (TmPO4/GFR). Arch Dis Child 57:217–223
Whitfield JB, Martin NG (1984) The effects of inheritance on constituents of plasma: a twin study on some biochemical variables. Ann Clin Biochem 21(Pt 3):176–183
Hunter DJ, Lange M, Snieder H, MacGregor AJ, Swaminathan R, Thakker RV, Spector TD (2002) Genetic contribution to renal function and electrolyte balance: a twin study. Clin Sci (Lond) 103:259–265
Portale AA, Halloran BP, Morris RC Jr (1987) Dietary intake of phosphorus modulates the circadian rhythm in serum concentration of phosphorus. Implications for the renal production of 1,25-dihydroxyvitamin D. J Clin Invest 80:1147–1154
Martin KJ, Bell G, Pickthorn K, Huang S, Vick A, Hodsman P, Peacock M (2014) Velcalcetide (AMG 416), a novel peptide agonist of the calcium-sensing receptor, reduces serum parathyroid hormone and FGF23 levels in healthy male subjects. Nephrol Dial Transplant 29:385–392
Shiber JR, Mattu A (2002) Serum phosphate abnormalities in the emergency department. J Emerg Med 23:395–400
Subramanian R, Khardori R (2000) Severe hypophosphatemia: Pathophysiologic implications, clinical presentations, and treatment. Medicine 79:1–8
Block GA, Ix JH, Ketteler M, Martin KJ, Thadhani RI, Tonelli M, Wolf M, Juppner H, Hruska K, Wheeler DC (2013) Phosphate homeostasis in CKD: report of a scientific symposium sponsored by the national kidney foundation. Am J Kidney Dis 62:457–473
Block GA, Hulbert-Shearon TE, Levin NW, Port FK (1998) Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 31:607–617
Calvo MS, Uribarri J (2013) Contributions to total phosphorus intake: all sources considered. Semin Dial 26:54–61
Vorland CJ, Martin BR, Weaver CM, Peacock M, Gallant KMH (2018) Phosphorus balance in adolescent girls and the effect of supplemental dietary calcium. JBMR Plus 2:103–108
Medicine IO (ed) (1997) DRI dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. The National Academies Press, Washington, DC, pp 1–413
Yates AA, Schlicker SA, Suitor CW (1998) Dietary Reference Intakes: the new basis for recommendations for calcium and related nutrients, B vitamins, and choline. J Am Diet Assoc 98:699–706
Fulgoni VL 3rd, Keast DR, Bailey RL, Dwyer J (2011) Foods, fortificants, and supplements: where do americans get their nutrients? J Nutr 141:1847–1854
Calvo MS, Uribarri J (2013) Public health impact of dietary phosphorus excess on bone and cardiovascular health in the general population. Am J Clin Nutr 98:6–15
Schlemmer U, Frolich W, Prieto RM, Grases F (2009) Phytate in foods and significance for humans: food sources, intake, processing, bioavailability, protective role and analysis. Mol Nutr Food Res 53(Suppl 2):S330–375
Lotz M, Zisman E, Bartter FC (1968) Evidence for a phosphorus-depletion syndrome in man. N Engl J Med 278:409–415
Kazama JJ (2009) Oral phosphate binders: history and prospects. Bone 45(Suppl 1):S8–12
Wilkinson R (1976) Absorption of calcium, phosphorus and magnesium. In: Nordin BEC (ed) Calcium phosphate and magnesium metabolism. Churchill Livingstone, Edinburgh, pp 36–112
Fleet JC, Peacock M (2014) Physiology of vitamin D, calcium, and phosphate absorption. In: Morris AH, Anderson P, Nordin BEC (eds) The physiological basis of metabolic bone disease. CRC Press, Boca Raton, pp 13–40
Sabbagh Y, Giral H, Caldas Y, Levi M, Schiavi SC (2011) Intestinal phosphate transport. Adv Chronic Kidney Dis 18:85–90
Stokman L, Nossent EJ, Grunberg K, Meijboom L, Yakicier MC, Voorhoeve E, Houweling AC (2016) A case of pulmonary alveolar microlithiasis associated with a homozygous 195 kb deletion encompassing the entire SLC34A2 gene. Clin Case Rep 4:412–415
Candeal E, Caldas YA, Guillen N, Levi M, Sorribas V (2017) Intestinal phosphate absorption is mediated by multiple transport systems in rats. Am J Physiol Gastrointest Liver Physiol 312:G355–G366
Labonte ED, Carreras CW, Leadbetter MR, Kozuka K, Kohler J, Koo-McCoy S, He L, Dy E, Black D, Zhong Z, Langsetmo I, Spencer AG, Bell N, Deshpande D, Navre M, Lewis JG, Jacobs JW, Charmot D (2015) Gastrointestinal inhibition of sodium-hydrogen exchanger 3 reduces phosphorus absorption and protects against vascular calcification in CKD. J Am Soc Nephrol 26:1138–1149
King AJ, Siegel M, He Y, Nie B, Wang J, Koo-McCoy S, Minassian NA, Jafri Q, Pan D, Kohler J, Kumaraswamy P, Kozuka K, Lewis JG, Dragoli D, Rosenbaum DP, O'Neill D, Plain A, Greasley PJ, Jonsson-Rylander AC, Karlsson D, Behrendt M, Stromstedt M, Ryden-Bergsten T, Knopfel T, Pastor Arroyo EM, Hernando N, Marks J, Donowitz M, Wagner CA, Alexander RT, Caldwell JS (2018) Inhibition of sodium/hydrogen exchanger 3 in the gastrointestinal tract by tenapanor reduces paracellular phosphate permeability. Sci Transl Med 10:eaam6474
Block GA, Rosenbaum DP, Leonsson-Zachrisson M, Astrand M, Johansson S, Knutsson M, Langkilde AM, Chertow GM (2017) Effect of tenapanor on serum phosphate in patients receiving hemodialysis. J Am Soc Nephrol 28:1933–1942
Walker GS, Peacock M, Marshall DH, Giles GR, Davison AM (1980) Factors influencing the intestinal absorption of calcium and phosphorus following renal transplantation. Nephron 26:225–229
Segawa H, Kaneko I, Yamanaka S, Ito M, Kuwahata M, Inoue Y, Kato S, Miyamoto K (2004) Intestinal Na-P(i) cotransporter adaptation to dietary P(i) content in vitamin D receptor null mice. Am J Physiol Renal Physiol 287:F39–47
Capuano P, Radanovic T, Wagner CA, Bacic D, Kato S, Uchiyama Y, St-Arnoud R, Murer H, Biber J (2005) Intestinal and renal adaptation to a low-Pi diet of type II NaPi cotransporters in vitamin D receptor- and 1alphaOHase-deficient mice. Am J Physiol Cell Physiol 288:C429–434
Fox J, Care AD (1978) Effect of low calcium and low phosphorus diets on the intestinal absorption of phosphate in intact and parathyroidectomized pigs. J Endocrinol 77:225–231
Danisi G, Caverzasio J, Trechsel U, Bonjour JP, Straub RW (1990) Phosphate transport adaptation in rat jejunum and plasma level of 1,25-dihydroxyvitamin D3. Scand J Gastroenterol 25:210–215
Zhang X, Imel EA, Ruppe MD, Weber TJ, Klausner MA, Ito T, Vergeire M, Humphrey J, Glorieux FH, Portale AA, Insogna K, Carpenter TO, Peacock M (2016) Pharmacokinetics and pharmacodynamics of a human monoclonal anti-FGF23 antibody (KRN23) in the first multiple ascending-dose trial treating adults with X-linked hypophosphatemia. J Clin Pharmacol 56:176–185
Armstrong WD (1955) Radioisotope studies of the physiology of calcified tissues. Minn Med 38:618–622
Dias RS, Kebreab E, Vitti DM, Roque AP, Bueno IC, France J (2006) A revised model for studying phosphorus and calcium kinetics in growing sheep. J Anim Sci 84:2787–2794
Koide M, Kobayashi Y, Yamashita T, Uehara S, Nakamura M, Hiraoka BY, Ozaki Y, Iimura T, Yasuda H, Takahashi N, Udagawa N (2017) Bone formation is coupled to resorption via suppression of sclerostin expression by osteoclasts. J Bone Miner Res 32:2074–2086
Langdahl B, Ferrari S, Dempster DW (2016) Bone modeling and remodeling: potential as therapeutic targets for the treatment of osteoporosis. Ther Adv Musculoskelet Dis 8:225–235
Belanger LF (1969) Osteocytic osteolysis. Calcif Tissue Res 4:1–12
Wysolmerski JJ (2012) Osteocytic osteolysis: time for a second look. BoneKEy Rep 229:1–7
Wittig NK, Birkbak ME, Bach-Gansmo FL, Pacureanu A, Wendelboe MH, Bruel A, Thomsen JS, Birkedal H (2019) No Signature of osteocytic osteolysis in cortical bone from lactating NMRI Mice. Calcif Tissue Int 105:308–315
Minisola S, Peacock M, Fukumoto S, Cipriani C, Pepe J, Tella SH, Collins MT (2017) Tumour-induced osteomalacia. Nat Rev Dis Primers 3:17044
Imel EA, Peacock M (2010) X-linked hypophosphatemia: understanding and management. Drugs Fut 35:755–763
Peacock M (1993) Osteomalacia and rickets. In: Nordin BEC, Need AG, Morris HA (eds) Metabolic bone and stone disease. Churchill Livingstone, London, pp 83–118
Wang L, Nancollas GH, Henneman ZJ, Klein E, Weiner S (2006) Nanosized particles in bone and dissolution insensitivity of bone mineral. Biointerphases 1:106–111
Cowan CM, Zhang X, James AW, Kim TM, Sun N, Wu B, Ting K, Soo C (2012) NELL-1 increases pre-osteoblast mineralization using both phosphate transporter Pit1 and Pit2. Biochem Biophys Res Commun 422:351–357
Zoidis E, Ghirlanda-Keller C, Gosteli-Peter M, Zapf J, Schmid C (2004) Regulation of phosphate (Pi) transport and NaPi-III transporter (Pit-1) mRNA in rat osteoblasts. J Endocrinol 181:531–540
Albano G, Moor M, Dolder S, Siegrist M, Wagner CA, Biber J, Hernando N, Hofstetter W, Bonny O, Fuster DG (2015) Sodium-dependent phosphate transporters in osteoclast differentiation and function. PLoS ONE 10:e0125104
Suzuki A, Ghayor C, Guicheux J, Magne D, Quillard S, Kakita A, Ono Y, Miura Y, Oiso Y, Itoh M, Caverzasio J (2006) Enhanced expression of the inorganic phosphate transporter Pit-1 is involved in BMP-2-induced matrix mineralization in osteoblast-like cells. J Bone Miner Res 21:674–683
Bottini M, Mebarek S, Anderson KL, Strzelecka-Kiliszek A, Bozycki L, Simao AMS, Bolean M, Ciancaglini P, Pikula JB, Pikula S, Magne D, Volkmann N, Hanein D, Millan JL, Buchet R (2018) Matrix vesicles from chondrocytes and osteoblasts: Their biogenesis, properties, functions and biomimetic models. Biochim Biophys Acta Gen Subj 1862:532–546
Bolean M, Simao AMS, Barioni MB, Favarin BZ, Sebinelli HG, Veschi EA, Janku TAB, Bottini M, Hoylaerts MF, Itri R, Millan JL, Ciancaglini P (2017) Biophysical aspects of biomineralization. Biophys Rev 9:747–760
Dillon S, Staines KA, Millan JL, Farquharson C (2019) How To Build a Bone: PHOSPHO1, Biomineralization, and Beyond. JBMR Plus 3:e10202
McKee MD, Hoac B, Addison WN, Barros NM, Millan JL, Chaussain C (2000) Extracellular matrix mineralization in periodontal tissues: Noncollagenous matrix proteins, enzymes, and relationship to hypophosphatasia and X-linked hypophosphatemia. Periodontol 2013(63):102–122
Millan JL (2013) The Role of Phosphatases in the Inititiation of Skeletal Mineralization. Calcif Tiss Int 93:299–306
Hatch NE, Franceschi RT (2009) Osteoblast differentiation stage-specific expression of the pyrophosphate-generating enzyme PC-1. Cells Tissues Org 189:65–69
Foster BL, Ao M, Salmon CR, Chavez MB, Kolli TN, Tran AB, Chu EY, Kantovitz KR, Yadav M, Narisawa S, Millan JL, Nociti FH Jr, Somerman MJ (2018) Osteopontin regulates dentin and alveolar bone development and mineralization. Bone 107:196–207
Qin C, Baba O, Butler WT (2004) Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med 15:126–136
Kelly TL, Wilson KE, Heymsfield SB (2009) Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS ONE 4:e7038
Kemp GJ, Meyerspeer M, Moser E (2007) Absolute quantification of phosphorus metabolite concentrations in human muscle in vivo by 31P MRS: a quantitative review. NMR Biomed 20:555–565
Bevington A, Mundy KI, Yates AJ, Kanis JA, Russell RG, Taylor DJ, Rajagopalan B, Radda GK (1986) A study of intracellular orthophosphate concentration in human muscle and erythrocytes by 31P nuclear magnetic resonance spectroscopy and selective chemical assay. Clin Sci (Lond) 71:729–735
Kavanaugh MP, Kabat D (1996) Identification and characterization of a widely expressed phosphate transporter/retrovirus receptor family. Kidney Int 49:959–963
Pesta DH, Tsirigotis DN, Befroy DE, Caballero D, Jurczak MJ, Rahimi Y, Cline GW, Dufour S, Birkenfeld AL, Rothman DL, Carpenter TO, Insogna K, Petersen KF, Bergwitz C, Shulman GI (2016) Hypophosphatemia promotes lower rates of muscle ATP synthesis. FASEB J 30:3378–3387
Wallimann T, Tokarska-Schlattner M, Schlattner U (2011) The creatine kinase system and pleiotropic effects of creatine. Amino Acids 40:1271–1296
Peacock M (2018) Hypoparathyroidism and the Kidney. Endocrinol Metab Clin North Am 47:839–853
Virkki LV, Biber J, Murer H, Forster IC (2007) Phosphate transporters: a tale of two solute carrier families. Am J Physiol Renal Physiol 293:F643–654
Biber J, Hernando N, Forster I (2013) Phosphate transporters and their function. Annu Rev Physiol 75:535–550
Forster IC (2019) The molecular mechanism of SLC34 proteins: insights from two decades of transport assays and structure-function studies. Pflugers Arch 471:15–42
Forster IC, Hernando N, Biber J, Murer H (2013) Phosphate transporters of the SLC20 and SLC34 families. Mol Aspects Med 34:386–395
Thomas L, Xue J, Murali SK, Fenton RA, Dominguez Rieg JA, Rieg T (2019) Pharmacological Npt2a inhibition causes phosphaturia and reduces plasma phosphate in mice with normal and reduced kidney function. J Am Soc Nephrol 30:2128–2139
Ansermet C, Moor MB, Centeno G, Auberson M, Hu DZ, Baron R, Nikolaeva S, Haenzi B, Katanaeva N, Gautschi I, Katanaev V, Rotman S, Koesters R, Schild L, Pradervand S, Bonny O, Firsov D (2016) Renal fanconi syndrome and hypophosphatemic rickets in the absence of xenotropic and polytropic retroviral receptor in the nephron. J Am Soc Nephrol 28:1073–1078
Bijvoet OL, Morgan DB, Fourman P (1969) The assessment of phosphate reabsorption. Clin Chim Acta 26:15–24
Walton RJ, Bijvoet OL (1975) Nomogram for derivation of renal threshold phosphate concentration. Lancet 2:309–310
Burnett SM, Gunawardene SC, Bringhurst FR, Juppner H, Lee H, Finkelstein JS (2006) Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res 21:1187–1196
Ferrari SL, Bonjour JP, Rizzoli R (2005) Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab 90:1519–1524
Lau AH, Kuk JM, Franson KL (1998) Phosphate-binding capacities of calcium and aluminum formulations. Int J Artif Organs 21:19–22
Robertson WG, Peacock M, Heyburn PJ, Marshall DH, Clark PB (1978) Risk factors in calcium stone disease of the urinary tract. Br J Urol 50:449–454
Hodgkinson A, Peacock M, Nicholson M (1969) Quantitative analysis of calcium-containing urinary calculi. Invest Urol 6:549–561
Coe FL, Evan AP, Worcester EM, Lingeman JE (2010) Three pathways for human kidney stone formation. Urol Res 38:147–160
Lederer E (2014) Regulation of serum phosphate. J Physiol 592:3985–3995
Blau JE, Collins MT (2015) The PTH-Vitamin D-FGF23 axis. Rev Endocr Metab Disord 16:165–174
Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T (2004) FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19:429–435
Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE (2006) Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 38:1310–1315
Feng JQ, Ye L, Schiavi S (2009) Do osteocytes contribute to phosphate homeostasis? Curr Opin Nephrol Hypertens 18:285–291
Ito N, Fukumoto S, Takeuchi Y, Takeda S, Suzuki H, Yamashita T, Fujita T (2007) Effect of acute changes of serum phosphate on fibroblast growth factor (FGF)23 levels in humans. J Bone Miner Metab 25:419–422
Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T (2006) Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444:770–774
Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro M (2006) Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281:6120–6123
Baum M, Schiavi S, Dwarakanath V, Quigley R (2005) Effect of fibroblast growth factor-23 on phosphate transport in proximal tubules. Kidney Int 68:1148–1153
Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T (2004) Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113:561–568
Bergwitz C, Juppner H (2011) Phosphate sensing. Adv Chronic Kidney Dis 18:132–144
Sabbagh Y (2013) Phosphate as a sensor and signaling molecule. Clin Nephrol 79:57–65
Takashi Y, Kosako H, Sawatsubashi S, Kinoshita Y, Ito N, Tsoumpra MK, Nangaku M, Abe M, Matsuhisa M, Kato S, Matsumoto T, Fukumoto S (2019) Activation of unliganded FGF receptor by extracellular phosphate potentiates proteolytic protection of FGF23 by its O-glycosylation. Proc Natl Acad Sci USA 116:11418–11427
Bon N, Frangi G, Sourice S, Guicheux J, Beck-Cormier S, Beck L (2018) Phosphate-dependent FGF23 secretion is modulated by PiT2/Slc20a2. Mol Metab 11:197–204
Glosse P, Feger M, Mutig K, Chen H, Hirche F, Hasan AA, Gaballa MMS, Hocher B, Lang F, Foller M (2018) AMP-activated kinase is a regulator of fibroblast growth factor 23 production. Kidney Int 94:491–501
Imel EA, Peacock M, Gray AK, Padgett LR, Hui SL, Econs MJ (2011) Iron Modifies Plasma FGF23 Differently in Autosomal Dominant Hypophosphatemic Rickets and Healthy Humans. J Clin Endocrinol Metab 96:3541–3549
Wolf M, Koch TA, Bregman DB (2013) Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res 28:1793–1803
Clinkenbeard EL, Farrow EG, Summers LJ, Cass TA, Roberts JL, Bayt CA, Lahm T, Albrecht M, Allen MR, Peacock M, White KE (2014) Neonatal iron deficiency causes abnormal phosphate metabolism by elevating FGF23 in normal and ADHR mice. J Bone Miner Res 29:361–369
White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ (2001) Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int 60:2079–2086
Wolf M, White KE (2014) Coupling fibroblast growth factor 23 production and cleavage: iron deficiency, rickets, and kidney disease. Curr Opin Nephrol Hypertens 23:411–419
Estepa JC, Aguilera-Tejero E, Lopez I, Almaden Y, Rodriguez M, Felsenfeld AJ (1999) Effect of phosphate on parathyroid hormone secretion in vivo. J Bone Miner Res 14:1848–1854
Krajisnik T, Bjorklund P, Marsell R, Ljunggren O, Akerstrom G, Jonsson KB, Westin G, Larsson TE (2007) Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol 195:125–131
Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J (2007) The parathyroid is a target organ for FGF23 in rats. J Clin Invest 117:4003–4008
Chertow BS, Baylink DJ, Wergedal JE, Su MH, Norman AW (1975) Decrease in serum immunoreactive parathyroid hormone in rats and in parathyroid hormone secretion in vitro by 1,25-dihydroxycholecalciferol. J Clin Invest 56:668–678
Jaaskelainen T, Huhtakangas J, Maenpaa PH (2005) Negative regulation of human parathyroid hormone gene promoter by vitamin D3 through nuclear factor Y. Biochem Biophys Res Commun 328:831–837
Kolek OI, Hines ER, Jones MD, LeSueur LK, Lipko MA, Kiela PR, Collins JF, Haussler MR, Ghishan FK (2005) 1alpha,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol 289:G1036–G1042
Rhee Y, Bivi N, Farrow E, Lezcano V, Plotkin LI, White KE, Bellido T (2011) Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone 49:636–643
Peacock M (2010) Calcium metabolism in health and disease. Clin J Am Soc Nephrol 5(Suppl 1):S23–30
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Munro Peacock declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peacock, M. Phosphate Metabolism in Health and Disease. Calcif Tissue Int 108, 3–15 (2021). https://doi.org/10.1007/s00223-020-00686-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-020-00686-3