Abstract
While reaching and grasping are highly prevalent manual actions, neuroimaging studies provide evidence that their neural representations may be shared between different body parts, i.e., effectors. If these actions are guided by effector-independent mechanisms, similar kinematics should be observed when the action is performed by the hand or by a cortically remote and less experienced effector, such as the foot. We tested this hypothesis with two characteristic components of action: the initial ballistic stage of reaching, and the preshaping of the digits during grasping based on object size. We examined if these kinematic features reflect effector-independent mechanisms by asking participants to reach toward and to grasp objects of different widths with their hand and foot. First, during both reaching and grasping, the velocity profile up to peak velocity matched between the hand and the foot, indicating a shared ballistic acceleration phase. Second, maximum grip aperture and time of maximum grip aperture of grasping increased with object size for both effectors, indicating encoding of object size during transport. Differences between the hand and foot were found in the deceleration phase and time of maximum grip aperture, likely due to biomechanical differences and the participants’ inexperience with foot actions. These findings provide evidence for effector-independent visuomotor mechanisms of reaching and grasping that generalize across body parts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A central question in motor control regards the organization of motor cortex in terms of action representation: how the mind and brain form and encode action parameters pertaining to motor planning and execution. Successfully performing actions requires precise control and coordination of each effector (acting body part), i.e., muscle and joint movements of a body part. Consistently, primary motor cortex contains a large-scale, albeit imprecise, somatotopic organization in which each cortical area selectively controls movements of a given body part (Meier et al. 2008; Penfield and Boldrey 1937; Zeharia et al. 2015; Huntley and Jones 1991; Asanuma and Rosen 1972; Strick and Preston 1978). Furthermore, kinematic and muscle synergies (i.e., patterns that reflect covariations among kinematics and muscle activity) during hand movements were found to be encoded by population neural response in primary motor cortex (Gallego et al. 2017, 2018; Leo et al. 2016; Overduin et al. 2015). These findings indicate an organization principle that is specific to effector systems.
Beyond the level of effector-specific action representations, though, there is evidence for more abstract motor representations that generalize across different body parts. In both primates and humans, separable neural pathways were found between reaching and grasping actions with the hand, with reaching engaging dorsomedial frontoparietal areas and grasping involving ventrolateral frontoparietal areas (Connolly et al. 2003; Culham et al. 2006; Kaas, Stepniewska and Gharbawie 2012; Konen et al. 2013; Yttri et al. 2014). Importantly, these pathways are not merely sub-specializations for a somatotopic hand area, as action representations in these frontoparietal areas may not be specific to hand actions. For example, during motor planning (Gallivan, McLean, Smith, and Culham 2011) or execution (Magri et al. 2019), brain areas including premotor cortex and superior parietal lobule encode target location whether individuals reached with their hand or made saccades with the eyes toward it (also see Heed et al. 2011; Heed, Leoné, Toni, and Medendorp 2016; Leoné, Heed, Toni, and Medendorp 2014). Common activation or activity pattern during reaching and grasping actions is also found between the two hands (Gallivan et al. 2013a; Haar et al. 2017; Turella et al. 2020), between the hand and the mouth (Castiello et al. 2000), between the hand and tools (Umiltà et al., 2008; Gallivan et al. 2013b), or between the hand and foot (Heed et al. 2011, 2016; Leoné et al. 2014; Liu et al. 2020). Neurons responding to both hands or hand and eye were also found in posterior parietal cortex in non-human primates (Chang, Dickinson, and Snyder 2008; Diomedi et al. 2020). These findings indicate that some secondary motor areas are organized based on more abstract motor information that are independent of specific effectors. These areas may form action representations for motor plans at abstract levels and later transform them to specific muscle patterns for motor execution (Stelmach and Diggles 1982; Stelmach et al. 1984; Gallego et al. 2022; Wong et al. 2015) in a hierarchical manner (Botvinick 2008; Haar et al. 2017; Grafton and Hamilton 2007; Uithol et al. 2012; Wurm and Lingnau, 2015; Yokoi and Diedrichsen 2019). Moreover, mixed encoding of information at different abstraction levels was found in motor cortex, providing evidence for coexistence of multiple organization principles of the motor network (Graziano and Aflalo 2007; Liu et al. 2020; Ames and Churchland 2019; Diomedi et al. 2020; Zhang et al. 2017).
Importantly, despite accumulating evidence for effector-independent motor representations in the brain, i.e., representation of the motor plan at a level abstract beyond the parameters of any specific effector, it is less clear how these are translated into action behavior. If the brain represents motor programs at abstract levels that generalize across muscle groups and body parts (“motor engram”, Bernstein 1935, 1967), common motor patterns should be observed across different body parts (“motor equivalence”, Stelmach and Diggles 1982; Merton 1972; Lashley 1950). To examine what information may be represented by effector-independent neural substrates, past studies tested whether motor kinematic features are similar between effectors, specifically if hand-like kinematics extend to other body parts. The kinematic profile and features of hand-reaching and grasping actions have been well characterized: Hand-grasping action is commonly characterized by a transport component, where the hand is directed to the target object, and a prehension component by which the hand grasps the object (Jeannerod 1984). By analyzing tangential hand velocity over time, studies have shown that the transport component typically consists of first, an acceleration phase and then, a deceleration phase as the hand approaches the object (Jeannerod 1984, 1986). The deceleration typically prolongs with accuracy demands (e.g., smaller objects), whereas the acceleration remains invariant (Gentilucci et al. 1991; MacKenzie et al. 1987; Maitra et al. 2010; Marteniuk et al. 1990; Jeannerod 1984, 1986; Cooke et al. 1989). Given these findings, past models propose that the acceleration phase reflects a ballistic movement guided by motor planning prior to movement onset based on the spatial location of the target, whereas the later deceleration phase is more strongly affected by online feedback control (MacKenzie et al. 1987; Marteniuk et al. 1990; Woodworth 1899). Many of these properties extend between the two hands as well as to hand-held tools: The velocity profile, especially during time up to peak deceleration, matched between grasping with either hand and with two hands (Tresilian and Stelmach 1997; Grosskopf and Khutz-Buschbeck 2006; Nelson et al. 2018) and between grasping with the hand or a tool (Gentilucci et al. 2004), suggesting an effector-independent ballistic component prior to the final feedback-control phase (MacKenzie et al. 1987; Marteniuk et al. 1990). In addition, percentage deceleration time increased with smaller (i.e., harder to grasp) objects for both grasping with the hand and with a tool (Gentilucci et al., 2004), suggesting a common mechanism by which object size influences the online feedback control stage.
In terms of prehension, during hand transport, the fingers first extend to a maximum grip aperture that is wider than the object, then close as the hand reaches near to the target and grasp (Jeannerod 1984, 1986). Importantly, maximum grip aperture robustly scales with object size (Goodale 1991; Chieffi and Gentilucci 1993; Freud et al. 2016; Freud and Ganel 2015; Westwood et al. 2002; Tang et al. 2016), indicating encoding of object property (i.e., size) already during the transport stage. This feature has also been established regardless of grasping with one hand or with two hands (Tresilian and Stelmach 1997) or whether grasping with the hand or a tool (Gentilucci et al. 2004; Itaguchi and Fukuzawa 2014; but see Maitra et al. 2010), although differed when grasping (to chew) with the mouth (Quinlan and Culham 2015).
Despite ample supportive evidence for motor control extending beyond the hand, the origin and generalizability of effector-independence remains unclear. Studies found shared kinematic features between the two hands and between unimanual and bimanual grasping (Grosskopf and Kuhtz-Buschbeck 2006; Nelson et al. 2018; Tresilian and Stelmach 1997). However, it is unclear if this effector-independence is an apriori trait of action plan and therefore can extend to any body part. Alternatively, it could originate from an action representation specific to one hand, and extend to the other hand via cross-hemispheric connections that mediate bimanual coordination (Brus-Ramer et al. 2009; Cardoso de Oliveira et al. 2001), thus specific for transferring information across the two hands. Yet another alternative is that transfer of action plans and representations originally specific to the hand relies on implementing the motor plan jointly with the hand, and therefore depend on experiece in hand-based coordination (Battaglia-Mayer et al. 2001; Magri et al. 2019). Such effector-independence would allow transfer between hand and hand-held tools, eye and mouth, but would preclude transfer to body parts that are not jointly used with the hands. On both these alternative accounts, effector-independence does not require a cognitive representation that is originally abstract beyond the dominant hand.
However, if action information is represented at an originally effector-independent level regardless of hand-based coordination, similar kinematics should be observed between the hand and any body part: even one that is cortically distant and inexperienced. Although some actions were tested for this more rigorous form of effector indepnednece, such as writing (Rijntjes et al. 1999; Raibert 1977) and tool use (Osiurak et al. 2018), the well-charactrterized ethological actions of reaching and grasping were seldom tested.
To test this hypothesis, we asked participants to perform reaching and grasping actions with either the hand or the foot. Although the foot has the potential to grasp an object with the big toe and second toe, as used by non-human primates, participants had almost no experience with this action. Testing the foot hence allows us to address whether common kinematic features develop as generalization derived from joint experience with the hand (i.e., hand-based interaction) or the existance of an effector-independent motor representation that is generalizable even to an unused effector. We expect such motor plan representations to manifest in effector-independent kinematics in the initial ballistic stages of movement, and the capacity to scale the aperture based on object size. Importantly, given the participants’ inexperience with foot grasping, we anticipated possible effects of difficulty, such as a longer deceleration time (Gentilucci et al. 2004). Furthermore, the hand and foot have different anatomical structures, which affect their movement capacity. The hand can perform opposition between the thumb and other fingers, allowing for precision grasping, whereas the foot is limited (Rolian et al. 2009). In addition, the fingers are more flexible and can move individually, whereas the toes cannot (Dempsey-Jones et al. 2019). Relately, the foot is biomechanically more constrained in how much separation can be made between the big toe and the second toe, whereas the fingers can separate by a larger amount. Given these differences in biomechanical structure and experience, we also expected to observe different kinematics between hand and foot, particularly for action parameters affected by task difficulty. Specifically, the foot may show a longer deceleration phase driven by difficulty in online motor control (Gentilucci et al. 2004), and may form an overall smaller aperture size during grasping, given the shorter digits. However, similarities in initial ballistic stages of movement and the qualitative capacity of scaling maximum aperture size with object size, despite biomechanical and experiential differences, would serve as possible behavioral correlates of previously reported effector-independent neural substrates (Liu et al. 2020; Heed et al. 2011, 2016) and provide additional evidence for effector-independent motor control mechanisms.
Methods
Participants
Fifteen participants (3 male, mean age: 29.8 years, SD = 8.9 years) participated in this study. One participant was excluded due to technical issues with the motion capture system, leading to fourteen participants in the reaching task. Two additional participants were excluded from participation in the grasping task because of an inability to separate the big toe and adjacent toe of the tested (right) foot from its resting position, resulting in twelve participants in the grasping task. All participants were neurotypical, self-reported right-handed adults with normal or corrected-to-normal vision and no history of impaired mobility or neurological disorder. Although we did not assess handedness using questionnaire-based tools, self-categorization shows high consistency with questionnaire-based evaluation specially in right-handed individuals (Chapman and Chapman 1987). Participants provided written informed consent prior to participation and received a monetary reimbursement. The experimental protocol was approved by the Georgetown University Medical Center Institutional Review Board.
Apparatus and stimuli
Participants sat in front of a table on which a target object was positioned at a viewing distance of 30 cm. To ensure that the hand and foot can comfortably lay prone on the table, participants sat on a lower chair for use of the hand and sat atop a separate table for use of the foot. Target objects were Efron blocks (Efron 1969) of different widths (small: 5 mm, medium: 10 mm, large: 15 mm) and heights while matched for surface area (25 cm2), depth, mass, texture and color.
Movement of the hand and the foot was tracked using an Optitrak 13 W motion capture 6-camera system (NaturalPoint, OR, USA). One infrared light-emitting diode (LED) was attached to the side near the tip of the first and second digit of the participant’s right hand and right foot (Fig. 1). This allowed tracking the change in aperture of the digits without interfering with natural movement. The motion capture system tracked the three-dimensional (3D) position of each LED at a sampling rate of 100 Hz and with a positional accuracy of 0.3 mm.
Procedure
Each participant performed a reaching and a grasping task in successive order that was counterbalanced across participants. Each task began with six practice trials with each effector (right hand and right foot).
Reaching
At the beginning of each trial, the participant rested the thumb and the index finger (or the big toe and the second toe on the foot) at home position defined by a cube block with the digits touching each other. Upon an auditory “Go” cue, they were instructed to reach toward and touch the front top edge of the target object with the tip of the fingers/toes at a normal pace and then return to the start block. Only the large object (15 mm width) was used. The object remained at the target position throughout the task. Each effector was tested in one block of 30 trials. The effector (hand and foot) used in the first block of each task was counterbalanced across participants.
Grasping
The procedure is similar to the reaching task except that upon the “Go” cue, the participant reached toward and grasped the width of the target object using either the thumb and index finger on the right hand, or the big toe and second toe on the right foot (digits 1 and 2 in both cases; Fig. 1), as if they intended to pick it up, without actually lifting it. Each effector was tested in two blocks in an ABBA design. An object of each width was tested in 15 trials in randomized order within each block. An experimenter switched the target object after each trial and only the target object was visible to the participant in each trial. Since the fingers and toes may differ in the ability of forming aperture, which in turn could affect grasping kinematics, we additionally measured the maximum possible aperture size by asking the participants to extend their index finger and thumb, and the big toe and second toe, to the maximum possible amount. Maximum possible aperture size from two participants was missing due to technical errors.
Data analysis
3D trajectory data from the motion capture system were preprocessed to extract kinematic information. Average location between the LEDs on the two digits was calculated as a proxy of hand position, based on which we calculated hand and foot velocity. For each trial, movement onset was determined as the point in time when the velocity reached 5% maximum velocity of that trial for at least five consecutive frames (50 ms; as done in Schettino et al. 2003; Ambron et al. 2017; Ganel, Freud, Chajut, and Algom 2012). Since actions were performed by first lifting the hand/foot and then landing on the object, movement offset was identified as the point when the fingers/toes were at the furthest position from the body and the lowest position around the end of the movement (peak y and minimum z values, respectively). Trials were excluded if have missing data points (i.e., LED sensors not captured by the cameras) or if velocity trajectories indicated unsuccessful grasps, characterized by more than one approaches (y-axis peak) towards the object and re-try at grasping the object itself (frequent also in the absence of visual feedback; Karl et al. 2012). Specific kinematic parameters analyzed for each task are described below:
Reaching
As in past literature, we measured movement duration from the onset to the offset, peak velocity, and absolute time to peak velocity, i.e., the length of the acceleration phase (Jeannerod 1984, 1986; Gentilucci et al. 1991; Quinlan and Culham 2015; Tresilian and Stelmach 1997). Given evidence that task difficulty can specifically influence time after peak deceleration (Gentilucci et al. 1991; MacKenzie et al. 1987; Marteniuk et al. 1990), we further broke the deceleration phase into absolute time from peak velocity to peak deceleration and absolute time after peak deceleration, as well as measured peak deceleration. Finally, past studies evaluated whether different velocity profiles belong to the same scalar family of curve, hence have identical shapes, by testing if the relative proportion of acceleration and deceleration phase remains constant (Gentilucci et al. 1991; MacKenzie et al. 1987). We, therefore, calculated percentage time to peak velocity relative to movement duration to evaluate the overall shape of velocity profile.
All statistical analyses were performed in JASP (Version 0.10.2) and SPSS (IBM SPSS Statistics for Windows, Version 27.0). For each dependent variable, a paired t test analysis was performed between hand and foot. Because dependent variables were chosen to test hypotheses regarding specific mechanisms of actions, no multiple-comparisons corrections were performed (Perneger 1998). Since supporting the effector-independence hypothesis relies on null effects, this approach is more conservative in inferring the effector-independent kinematic properties. Post-hoc analyses were Bonferroni corrected. Moreover, on critical null effects that indicate effector-independence, we performed Bayesian statistical tests and reported BF10, i.e., the likelihood of the alternative hypothesis relative to the null hypothesis (Jeffreys 1998; Rouder et al. 2009). BF10 of less than one indicates the alternative hypothesis is no more likely than the null hypothesis, whereas BF10 of less than 1/3 provides support to the null hypothesis over the alternative (Rouder et al. 2009).
Grasping
A 2 (effector: hand, foot) by 3 (object size) repeated-measured ANOVA was performed on each dependent variable. First, we analyzed the same dependent variables as in the reaching task to examine the transport component. In cases where we found an effect of effector in reaching but not grasping or vice versa, we performed a 2 (task: reaching, grasping) by 2 (effector: hand, foot) repeated-measures ANOVA to test the inconsistency between tasks, focusing on the large (15 mm) object that was used in both tasks. A significant task × effector interaction effect would reveal differential effects of effector across tasks, indicating influences of the prehension component on transport.
We then examined the prehension component by analyzing maximum grip aperture (MGA) and absolute time to MGA (Jeannerod 1984, 1986; Freud and Ganel 2015; Ganel et al. 2012). It is possible that some conditions result in both longer movement duration and longer absolute time to MGA (e.g., for larger objects, Gentilucci et al. 1991). We, therefore, also analyzed percentage time to MGA to compare the temporal structure of the manipulation component. In addition, to measure sensitivity to object size, past studies calculated the linear slope between aperture size and object size, with a slope larger than zero signaling sensitive scaling (Goodale 1991; Jeannerod 1986; Freud et al. 2016). Thus, in addition to running an ANOVA on the MGA, we tested whether the slope between MGA and object size is larger than zero for each effector. Finally, to determine how early sensitivity to object size arises in the hand and foot, we calculated the linear slope between aperture size and object size at each frame after resampling all trials to the same length (see below), and at 11 normalized movement timepoints (Freud et al. 2016; from 0 to 100% in 10% steps).
For visualization purposes, we resampled the velocity and aperture profile from each trial to the group average movement duration of each condition, preserving the shape and magnitude of the original time course (Quinlan and Culham 2015). Then we averaged all trials within each condition to show group-level velocity and aperture profile in absolute time (in seconds). We additionally sampled the velocity and aperture size at each normalized timepoint (Freud et al. 2019; Freud and Ganel 2015; Ganel et al. 2012) to plot the velocity profile of the hand and foot at an aligned time scale.
Results
Reaching
Overall 4.9% of trials were excluded, with no difference between hand (M = 5.0%, SE = 2.3%) and foot trials (M = 4.8%, SE = 2.0%; t(13) = 0.09, p = 0.932).
The velocity profiles of hand and foot reaching movements were remarkably similar, both consisting of an acceleration phase followed by deceleration, with visually similar peak velocity and time to peak velocity (Fig. 2). Table 1 summarizes the result of each dependent variable (mean for hand and foot, t test statistics and Bayes factors). First, movement duration was significantly longer for the foot than the hand (t(13) = 2.27, p = 0.041; see detail in Table 1). This effect is driven by a statistically significantly longer time after peak deceleration for the foot vs. hand (t(13) = 2.61, p = 0.021; Table 1), with no difference in absolute time to peak velocity (t(13) = 0.62, p = 0.549, BF10 = 0.32; Table 1, Fig. 2A) or peak velocity to peak deceleration (t(13) = 0.77, p = 0.457, BF10 = 0.35; Table 1). Absolute time to peak velocity offers support to the null hypothesis of similarity between the hand and foot. This finding indicates that the foot and hand differ only during the final feedback-controlled stage (MacKenzie et al. 1987; Marteniuk et al. 1990; Woodworth 1899), but not during the earlier ballistic movement stage.
Comparison of the reaching velocity profile between the hand and the foot. A Velocity profile as a function of absolute time. B Velocity profile over percentage movement time. Vertical lines denote time of peak velocity calculated from raw, unnormalized data. Overall the hand and the foot showed remarkably similar velocity profiles, especially from movement onset to peak velocity. Error bars denote standard errors
Despite difference in absolute movement time, the percentage time to peak velocity of the entire movement duration did not differ between the hand and foot (t(13) = 1.48, p = 0.163, BF10 = 0.66; Table 1, Fig. 2B), suggesting overall consistent reaching movement structure between hand and foot. Finally, there was no difference between the foot and hand in terms of peak deceleration (t(13) = 1.01, p = 0.329, BF10 = 0.42; Table 1), whereas results on peak velocity (t(13) = 2.05, p = 0.062, BF10 = 2; Table 1) were ambiguous.
Overall, reaching movement kinematics was similar between the hand and foot, with the early, ballistic component of the action showing consistency, whereas differences in time course arising only in the later deceleration stage.
Grasping
Overall 12.3% of trials was excluded, with more foot trials (M = 20.0%, SE = 2.4%) excluded than hand trial (M = 4.5%, SE = 2.5%; t(11) = 5.31, p < 0.001), indicating the difficulty of foot grasping.
Transport component
Across all dependent variables tested in the transport component (see Methods), there was no main effect of object size (ps > 0.070) and only one significant interaction between object size and effector on movement duration (see detail below). Therefore, we primarily report the effect of effector on the transport variables, focusing on our main question of whether the foot and hand share similar kinematic properties.
As with the reaching task, both hand and foot showed a bell-shaped velocity profile (Fig. 3). However, the velocity profile of the foot appears to be more right-skewed, i.e., with a longer deceleration phase, than the hand. Statistical analyses support this observation. First, movement duration was longer for the foot than the hand (F(1,11) = 32.85, p < 0.001; Table 2, Fig. 3A). Subsequent analyses revealed that the time to peak velocity was similar between hand and foot (F(1,11) = 0.08, p = 0.785, BF10 = 0.28; Table 2, Fig. 3A), while the foot took a longer time than the hand both from peak velocity to peak deceleration (F(1,11) = 23.70, p < 0.001; Table 2) and from peak deceleration to the end of the movement (F(1,11) = 24.84, p < 0.001; Table 2).
Comparison of the velocity profile between the hand and the foot in the grasping task. A. Velocity profile as a function of absolute time. B Velocity profile over percentage movement time. Vertical lines denote time of peak velocity calculated from raw, unnormalized data, averaged across object sizes given no main effect of object size. The hand and the foot showed remarkably similar velocity profiles in absolute time up to peak velocity, with a longer deceleration phase for the foot. Error bars denote standard errors
As a result of a prolonged deceleration phase for the foot, the proportion of the acceleration phase, i.e., percentage time to peak velocity, was smaller for the foot than the hand (F(1,11) = 37.77, p < 0.001; Table 2, Fig. 3B). Consequently, the shape of the velocity profile of hand and foot was not identical.
As with the reaching task, there was no difference in peak velocity between hand and foot (F(1,11) = 0.57, p = 0.465, BF10 = 0.81; Table 2). However, the foot showed a larger peak deceleration than the hand (F(1,11) = 10.31, p = 0.008; Table 2). The longer deceleration phase and larger peak deceleration may reflect difficulty in grasping with foot so that the movement had to be performed more slowly.
Finally, there was a significant interaction between effector and object size (F(2,22) = 4.88, p = 0.018) on movement duration. Whereas object size did not affect movement duration in the hand condition (p = 0.563), the medium object (M = 1.62 s, SE = 10.74 s) resulted in a longer movement time than the small object (M = 1.54 s, SE = 10.22 s) in the foot condition (p < 0.008, Bonferroni corrected), with no difference between the large object (M = 1.56 s, SE = 11.38 s) and the medium or small object.
Overall, as in reaching, transport of the foot toward the grasped target was again similar to that of the hand for the earlier stages of movement until peak velocity, whereas difference between the hand and foot occurred during the deceleration stage in a manner partly dependent on the intended grasped target size.
For some dependent variables, e.g., percentage time to peak velocity and absolute time from peak velocity to peak deceleration, there was no effect of effector in reaching but an effect in grasping. We performed subsequent post-hoc analyses with task (reaching, grasping) and effector (hand, foot) as independent variables to test these task differences (Table 3; Bonferroni corrected for multiple comparisons). Regarding percentage time to peak velocity, there was an interaction between task and effector (F(1,11) = 9.33, p = 0.011), with no difference between hand and foot in reaching (p = 0.146) but a smaller percentage time to peak velocity for foot vs. hand in grasping (p < 0.001). Regarding absolute time from peak velocity to peak deceleration, there was also an interaction between task and effector (F(1,11) = 12.26, p = 0.005), again with no difference between hand and foot in the reaching task (p > 0.999) but a longer time for the foot than the hand in grasping task (p = 0.033). Overall, velocity profile between hand and foot differed more strongly in grasping than reaching.
In summary, while the velocity profile of hand and foot are highly similar in reaching task, the foot showed a more right-skewed velocity profile than the hand in grasping task that was mainly driven by a prolonged deceleration phase after peak velocity.
Prehension component
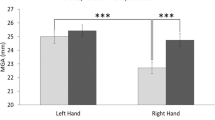
During grasping, the fingers first extend and then close as approaching the object, forming a maximum grip aperture that scales with object size (Chieffi and Gentilucci, 1993; Freud et al. 2016; Freud and Ganel 2015; Westwood et al. 2002). We analyzed maximum grip aperture (MGA) and its scaling with object size (Fig. 4C, Table 4). Regarding MGA, the main effect of effector was significant (F(1,11) = 25.07, p < 0.001; Table 4), with an overall larger MGA for the hand vs. the foot, as expected given the different digit lengths and dexterity. Indeed, the maximum possible aperture size was significantly larger for hand (M = 143.2 mm, SE = 2.00 mm) vs. foot (M = 44.8 mm, SE = 0.98 mm; t(9) = 15.00, p < 0.001), with the foot MGA reaching the maximum aperture limit for all object sizes (Supplementary Figure S1).
Comparison of the prehension kinematics between the hand and the foot. A Changes in aperture size over absolute time for each effector and object size. The dashed vertical lines denote time to maximum grip aperture. B Changes in aperture size over percentage time for each effector and object size. Vertical dashed lines denote percentage time to maximum grip aperture. C Maximum grip aperture for each effector and object size. A line graph is superimposed on the bar graph to visualize the trend. D Slope between aperture size and object size for each effector over absolute and percentage time. The slope for foot began to rise much later than hand
Importantly and as expected, despite these anatomical differences, there was a main effect of object size (F(2,22) = 69.96, p < 0.001; Table 4). Across both hand and foot, MGA increased with object size (Bonferroni corrected ps < 0.001 for all post-hoc pairwise comparisons), consistent with past literature (Chieffi and Gentilucci 1993; Freud et al. 2016). Finally, there was an interaction between effector and object size (F(2,22) = 26.42, p < 0.001) driven by a stronger effect of object size in hand vs. foot. This can be seen in subsequent analyses showing significantly above-zero slope between MGA and object size for both the hand and foot (ps < 0.001, Fig. 4C), indicating sensitivity and oversizing of both effectors with respect to object size, while the slope was larger for the hand (M = 0.81, SE = 0.086) vs. the foot (M = 0.30, SE = 0.067).
We then analyzed time to MGA. As expected based on past research on hand grasping (Jeannerod 1984; Marteniuk et al. 1990; Tresilian and Stelmach 1997), MGA occurred after peak velocity for both foot and hand, with later MGA time for larger objects (Fig. 4A, B). Moreover, MGA occurred later for the foot as compared to the hand. Statistical analyses supported these observations (Table 4). Regarding absolute time to MGA, there was a significant main effect of effector (F(1,11) = 44.29, p < 0.001), with a later time for foot vs. hand (Fig. 4A). The main effect of object size was also significant (F(2,22) = 7.54, p = 0.003), with a later MGA for larger objects across effectors, consistent with past findings for hand grasping (Jeannerod 1984; Marteniuk et al. 1990; Tresilian and Stelmach 1997). Post-hoc comparisons revealed a significant difference between the large and small object (p = 0.028), with no difference between the medium object and the large or small object (ps > 0.06; see Table 4 for descriptive statistics of each object). There was no interaction between effector and object size (F(2,22) = 2.95, p = 0.073). Analyses of percentage time to MGA led to the same conclusions (Table 4, Fig. 4B), with a later percentage MGA time for the foot vs. hand (F(1,11) = 28.03, p < 0.001), a main effect of object size driven by a later time for the large vs. small object (F(2,22) = 7.23, p = 0.004), and no interaction between effector and object size (F(2,22) = 1.10, p = 0.352).
Finally, to examine the time course of sensitivity, we calculated the linear slope of aperture size to object size over time. As shown in Fig. 4D, the slope of the aperture began to increase at a much later timepoint for foot vs. hand in terms of both absolute time and percentage time, indicating that the sensitivity of aperture to object size occurred much later. The same trend can be seen in Fig. 4A, B, where the aperture profile of different object sizes began to show separation early for the hand but rather late for the foot.
Overall, significant differences were found in the timing of sizing sensitivity between the effectors, as well as the differences in aperture size. However, prehension of the foot showed grip aperture properties known to characterize hand prehension, including pre-shaping the digits based on object size and later time of maximum aperture for larger objects.
Discussion
We examined kinematic properties of hand and foot movement during reaching and grasping actions to investigate what aspects of visually guided actions are shared between effectors and may be controlled by effector-independent motor plans. We found similarities in kinematics between foot and hand including (i) same absolute time to peak velocity in both reaching (Table 1) and grasping (Table 2), along with similar velocity profiles (i.e., the proportion of acceleration and deceleration phase) in the reaching task (Fig. 2) (ii) maximum grip aperture scaled with object size (Fig. 4C), and (iii) later time to maximum grip aperture with larger objects (Fig. 4, Table 4). These findings expand on previous literature showing kinematic consistency across the two hands as well as for hand-held tools, thereby showing that at least some effector-independent kinematic properties of ethological actions can extend to a distant and inexperienced effector. Differences between hand and foot were also found, in (i) longer deceleration time for foot vs. hand (Tables 1, 2), (ii) later time to maximum grip aperture for foot vs. hand (Fig. 4A, B, Table 4), (iii) smaller scaling of aperture size with object size for foot vs. hand (Fig. 4C), and (iv) later onset of scaling of aperture size with object size for foot vs. hand (Fig. 4D). These differences could stem from inexperience with foot actions, different biomechanical capacities between the hand and foot, or, alternatively, effector-dependent mechanisms underlying certain properties of these actions. We discuss these findings under the current framework of motor planning and execution.
Similar ballistic movement phase across hand and foot
It has long been proposed that the earlier phase of reaching and grasping actions are primarily controlled by a motor planning mechanism, whereas the later stages are guided by online feedback control mechanisms (Arbib 1981; MacKenzie et al. 1987; Marteniuk et al. 1990; Keele 1968; Dixon and Glover 2009; Glover 2002, 2004). Complete reaching movements can be guided by pre-planned motor commands even when later correction is rendered impossible. For instance, in cases where the target location is changed suddenly after movement onset yet correction of movement trajectory is hindered by posterior parietal lesion or TMS (Desmurget et al. 1999; Gréa et al. 2002), participants could not correct the movement trajectory to the new target position. Importantly, however, they still make smooth movements toward the initial target location, indicating pre-planned movement trajectory regardless of online sensory information.
Under typical circumstances, the motor plan seems to be more strongly reflected in the acceleration phase of transport (Elliott et al. 1999; Gentilucci et al. 1991; Mackenzie et al. 1987; Marteniuk et al. 1990; Brown and Cooke 1981). For instance, in hand reaching/grasping studies that introduce unexpected perturbations (e.g., decrease or increase in resistance) following movement initiation, the hand typically requires longer to complete the movement in the perturbation condition(s); however, acceleration kinematic parameters (e.g., peak velocity, absolute time to peak velocity) tend to not differ (Elliott et al. 1999), suggesting that the acceleration phase is less influenced by online sensory information. With respect to our data, we found that in both reaching and grasping, the acceleration phase of transport shares more similarities (i.e., in absolute time to peak velocity) between hand and foot than the deceleration phase, suggesting that the acceleration phase is less dependent on specific sensorimotor parameters associated with each effector. During the acceleration phase of arm/leg movement, both effectors demonstrated similarity in absolute time to peak velocity and in peak velocity. A matched acceleration phase was also reported between grasping with hand or a tool (Gentilucci et al. 2004; but see Maitra et al. 2010). Taken together, our data posits that (ballistic) motor planning for reaching and grasping actions is effector independent, and the motor command may form at a level of abstraction above that of the generation of effector-specific muscle command, i.e., specificity to the hands.
At neural level, neuroimaging and single-cell recording studies reported common activation or activity patterns in premotor cortex and posterior parietal cortex during motor planning and execution of reaching/pointing and grasping actions across effectors (Gallivan et al. 2013a, b; Heed et al. 2011, 2016; Leoné et al. 2014; Liu et al. 2020; Diomedi et al. 2020; Magri et al. 2019; Chang et al. 2008; Diomedi et al. 2020; Gamberini et al. 2011; Ferraina et al. 1997; Hadjidimitrakis et al. 2011). These areas may serve as neural substrates for shared motor control, i.e., the derivation of the shared kinematic properties observed in our study. Nonetheless, without neuroimaging data, our study does not infer how each kinematic property is instantiated in the brain. Future studies combining neuroimaging and behavioral data are needed to map effector-independent kinematic parameters in the brain.
Past studies found that different parameters of reaching movements are represented independently (e.g., distance, target location, trajectory; Krakauer et al. 2000, 2004; Ghez et al. 2007). In addition, there are asymmetries between the two hands in different aspects of sensorimotor control (Goble and Brown 2007; Goble et al. 2006; Tang and Zhu 2017). Moreover, whereas visuomotor adaptation learned with one hand can be transferred to the non-trained hand, indicating shared action representation (Schulze et al. 2002; Tang et al. 2018; van Mier and Peterson 2006; Kumar et al. 2020), the extent of transfer differs between parameters (Sainburg et al. 2016; Sainburg and Kalakanis 2000; Sainburg and Wang 2002; Tang and Zhu 2017; Kumar et al. 2020). These findings suggest potentially different generalizability of various parameters. Our use of a single target location does not dissociate various parameters involved in motor execution and future studies are needed to investigate the level of effector-independence of different parameters of reaching actions. Furthermore, future characterization of the dynamic changes of joint orientation through time could allow testing other spatiotemporal aspects of the consistency between movements of the effectors.
Overall, although past studies reported shared kinematics between the hand and other effectors, it remained unclear whether such effector-independence is intrinsic or develops from hand-based experience. Specifically, shared kinematics between the dominant hand and the other hand (Grosskopf et al. 2006; Nelson et al. 2018) or a hand-held tool (Gentilucci et al. 2004) could originate from coordination experience between the dominant hand and these alternative effectors (Battaglia-Mayer et al. 2001; Magri et al. 2019). By examining the foot, a novel effector that has distinct biomechanical structures and does not participate in manual coordination, our study provides evidence for an intrinsic effector-independent mechanism that is generalizable to novel and inexperienced effectors. Furthermore, the study of the foot allows controlling for shared low-level motor control. The primary motor cortices of the two hands are connected by rich interhemispheric connectivity which could underlie the motor transfer without relying on higher level task-based more abstract representations (Carson et al. 2004; Perez and Cohen 2008; Ruddy and Carson 2013). As this type of connectivity does not exist between the hand and the foot, the ability to plan actions similarly across them more strongly supports the existence of an abstract, task-level action representation.
Differences in the deceleration phase between the foot and the hand
Despite similarities in the acceleration phase, a longer deceleration was found for the foot as compared with the hand. In the reaching task, the longer deceleration phase for foot was primarily driven by a longer time from peak deceleration to movement offset. During grasping, the foot took an overall longer absolute time after peak velocity, both from peak velocity to peak deceleration and from peak deceleration to the end. The prolonged deceleration phase led to a more right-skewed velocity profile of foot than hand (i.e., shorter percentage time to peak velocity in foot), resulting in different normalized velocity profile shapes. One possibility is that these results reflect differences in neural online control between the effectors, supporting effector-dependent motor control based on somatotopically selective areas (Yttri et al. 2014), as well as a separate representation type for action planning as compared to its control (Glover and Holloway 2004). However, it may also stem from increased difficulty and inexperience of foot action. Past studies reported difference in time after peak deceleration for hand movement between different levels of accuracy demands (Marteniuk et al. 1990; Gentilucci et al. 1991; Mackenzie et al. 1990). This final movement stage requires precise coordination between sensory and motor information, heavily relying on online feedback control (Elliott et al. 1999; Gentilucci et al. 1991; Mackenzie et al. 1987; Marteniuk et al. 1990). Therefore, one possibility is that precise motor control of the foot/leg is intrinsically harder due to its heavier weight, more biomechanical constraints and less flexibility in separating the toes for grasping, leading to longer time after peak deceleration.
An alternative but non-exclusive account is that the participants were simply less experienced in grasping objects with the foot, finding this task more difficult. There is evidence that although grasping with uncommon or novel effectors led to longer grasping time and different aperture profile compared with typical grasping with the thumb and index finger, practice can eliminate these differences (Itaguchi 2020; Bouwsema et al. 2014; Itaguchi and Fukuzawa 2014). Added evidence for the difficulty of foot grasping can be found in the larger number of unsuccessful grasp trials (which were thus excluded from the analyses). On this account, foot and hand actions are controlled by common underlying mechanisms, but it requires experience to efficiently translate the motor program to kinematic patterns.
Similarities and differences in prehension across effectors
The maximum grip aperture scaled with object size for both the foot and the hand. The scaling of maximum grip aperture to object size in grasping has been robustly and widely reported in past studies (Chieffi and Gentilucci 1993; Jeannerod 1984, 1986; Quinlan and Culham, 2015), and has been measured as indicating sensitivity of grasping motor control to object size (Freud et al. 2016; Westwood et al. 2002). MGA is less affected by visual illusion or perceptual deficits, hence reflects visuomotor functions rather than pure perceptual information (Dixon and Glover 2009; Freud et al. 2016; Westwood et al. 2002; Aglioti et al. 1995). As aperture scaling can occur in the absence of visual feedback (Hu et al. 1999), but typically occurs in later stages of movement, i.e., during deceleration, it is considered to be guided by both online control and planning (Glover 2002). Moreover, past studies also found scaling of MGA with object size for unimanual and bimanual grasping (Tresilian and Stelmach 1997), for grasping with a tool (Gentilucci et al. 2004; but see Maitra et al. 2010), and for grasping/biting with the mouth (Castiello 1997; Churchill et al. 1999; Quinlan and Culham 2015), indicating a common mechanism by which the grasping motor control system encodes visual information regarding object size prior to contact. We provide additional evidence that such a motor control mechanism also applies to foot, despite limited experience in grasping with foot.
Although we found scaling of MGA with object size in both effectors, the foot showed a smaller slope (0.30) between MGA and object size than the hand (0.81; well within previously reported ranges; Freud et al. 2016), indicating a lower capacity for scaling the aperture of the foot. Biomechanically, the toes have a more limited movement range than the fingers either due to a shorter length of the toes or less flexible joints, as evident in a larger maximum possible aperture size for the hand as compared with the foot. With these constraints, foot aperture has a ceiling effect to its aperture: its maximal aperture is on average 44.8 mm, whereas the MGA was 43.0 mm, and may only increase by a limited amount with increased object size (Supplementary Figure S1). This, therefore, manifests in an overall smaller maximum grip aperture for foot vs. hand. Similarly, biomechanical constraints and lack of experience may also have resulted in delayed MGA and later-onset sensitivity to object size (Fig. 4D) in foot, where the toes could not separate optimally until the speed of the foot reduced to a certain amount. On these possibilities, the mechanism by which object size is encoded during pre-shaping is shared between hand and foot, but the implementation of such mechanism is limited by practical factors. These possibilities are not mutually exclusive, and can be addressed by future studies by either testing the effect of training on foot grasping, or by testing special populations who are experienced with foot actions (e.g., people born without hands; Striem-Amit et al. 2017; Dempsey-Jones et al. 2019; Liu et al. 2020).
We also note the possibility that difference in the scaling of aperture size to object size between hand and foot may reflect distinct visuomotor representations. Past studies provide evidence for distinct visuomotor representations between typical grasping with the thumb and index finger, and novel grasping with the thumb and little finger (Gonzalez et al. 2008). Therefore, it is possible that perceptual information about object size may not be similarly or efficiently encoded by the visuomotor system during early phase of foot actions (Freud et al. 2016), generating less coordinated reaching and preshaping components. Our data does not allow fully distinguishing what visuomotor representations underlie foot and hand actions, respectively, and future studies can investigate this question by studying how perceptual information is represented during hand and foot actions, e.g., using neuroimaging methods.
Alternative theoretical accounts
While we discuss a framework in which the brain programs the kinematic variables, alternative perspective exists. According to the referent control theory (Feldman 2015, 2019; Latash 2018; Ambike et al. 2016), instead of preprogramming motor output, such as movement trajectory, the brain only specifies the form and timing of referent variables (muscle length threshold that recruits motor neurons) based on task demands, which then elicits movements. Kinematic variables are viewed as emergent properties resulting from the interaction between the body and environmental forces (Feldman 2015, 2019). On this account, effector-independent motor outputs are a natural result underlain by physical laws that any body part obeys given the same task or context. Specifically, moving the arm consists of an initial acceleration phase naturally caused by muscle torque, and a deceleration phase for the limb to stop. Similarly, effector-independent kinematic properties are not prespecified by the brain, but natural results of physical requirements. Under this perspective actions are direct response to the context and task demands. One environmental factor that determines how an object is acted upon is affordance that, on similar theoretical accounts, is directly perceived by the observer (Gibson 1966, 1977, 1979). In our experiment, the size of the target object offers the capacity to be grasped to the hand and the foot (Gibson 1977), hence common scaling of MGA to object size could be observed. However, with additional constraints and less experience of the foot, the foot is less attuned to the affordance of grasping than the hand, leading to differences in kinematic variables, such as the degree of scaling and absolute MGA. Our data cannot discern between these two theoretical frameworks, the computational and ecological accounts (Gibson 1966), in what underlies direct coding of motor kinematic variables; however, it supports the existence of a higher level action response, guided by task/context, which can manifest across body parts, shared among these two theoretical accounts.
Summary
We tested whether the characteristic kinematic profile of hand reaching and grasping may be driven by motor control unique to the hands, or if similar kinematics can be found for the foot, an untrained and cortically remote body part. We found similar velocity profile during the acceleration phase between the hand and foot, likely reflecting common motor plan mechanisms. In addition, maximum grip aperture scaled with object size for both hand and foot, indicating a common grasping control mechanism that takes into account object size during pre-shaping. Together, these findings support effector-independent action plans. In contrast, we found differences in later action stages that reflect online motor control of the action. One remarkable difference between hand and foot lies in longer deceleration phase for foot that may reflect overall higher accuracy demand on the foot. In addition, sensitivity to object size was less elaborate and manifested much later for foot vs. hand. These findings also point to different temporal coupling of transport and manipulation between foot and hand. It is not clear from our data whether these differences reflect distinct neural mechanisms for online motor control, biomechanical constraints in the foot, or lack of experience in foot motor control of participants. Although future studies are required to address the role of experience on some parameters of temporal features of foot grasping, our data provides support for shared kinematics and motor control planning between the hand and the foot. Together with recent imaging studies, this supports the existence of some effector-independent representations of action, that do not rely on shared use and experience.
Data availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aglioti S, DeSouza JF, Goodale MA (1995) Size-contrast illusions deceive the eye but not the hand. Curr Biol 5(6):679–685
Ambike S, Mattos D, Zatsiorsky VM, Latash ML (2016) Synergies in the space of control variables within the equilibrium-point hypothesis. Neuroscience 315:150–161
Ambron E, Schettino LF, Coyle M, Jax S, Coslett HB (2017) When perception trips action! the increase in the perceived size of both hand and target matters in reaching and grasping movements. Acta Physiol (oxf) 180(May):160–168. https://doi.org/10.1016/j.actpsy.2017.09.011
Ames KC, Churchland MM (2019) Motor cortex signals for each arm are mixed across hemispheres and neurons yet partitioned within the population response. Elife 8:e46159
Arbib MA (1981) Perceptual structures and distributed motor con- trol. In: Brooks VB (ed) Handbook of physiology sect 1 part 2, vol 2. William and Wilkins, Baltimore, pp 1449–1480
Asanuma H, Rosen I (1972) Topographical organization of cortical efferent zones projecting to distal forelimb muscles in the monkey. Exp Brain Res 14(3):243–256
Battaglia-Mayer A, Ferraina S, Genovesio A, Marconi B, Squatrito S, Molinari M, Caminiti R (2001) Eye–hand coordination during reaching II an analysis of the relationships between visuomanual signals in parietal cortex and parieto-frontal association projections. Cereb Cortex 11(6):528–544
Bernstein N (1935) The problem of interrelation of co-ordination and localiza- tion. Arch Biol Sci 38 Reproduced In: Whiting HTA (ed) Advances in Psychology, Amsterdam: North-Holland, vol 17. pp 77–119
Bernstein NA (1967) The co-ordination and regulation of movements. Pergamon Press, Oxford
Botvinick MM (2008) Hierarchical models of behavior and prefrontal function. Trends Cogn Sci 12(5):201–208
Bouwsema H, van der Sluis CK, Bongers RM (2014) Changes in performance over time while learning to use a myoelectric prosthesis. J Neuroeng Rehabil 11(1):1–15
Brown SH, Cooke JD (1981) Responses to force perturbations preceding voluntary human arm movements. Brain Res 220(2):350–355
Brus-Ramer M, Carmel JB, Martin JH (2009) Motor cortex bilateral motor representation depends on subcortical and interhemispheric interactions. J Neurosci 29(19):6196–6206
Cardoso de Oliveira S, Gribova A, Donchin O, Bergman H, Vaadia E (2001) Neural interactions between motor cortical hemispheres during bimanual and unimanual arm movements. Eur J Neurosci 14(11):1881–1896
Carson RG, Riek S, Mackey DC, Meichenbaum DP, Willms K, Forner M et al (2004) Excitability changes in human forearm corticospinal projections and spinal reflex pathways during rhythmic voluntary movement of the opposite limb. J Physiol 560:929–940. https://doi.org/10.1113/jphysiol.2004.069088
Castiello U (1997) Arm and mouth coordination during the eating action in humans: a kinematic analysis. Exp Brain Res 115(3):552–556. https://doi.org/10.1007/PL00005726
Castiello U, Bennett KM, Egan GF, Tochon-Danguy HJ, Kritikos A, Dunai J (2000) Human inferior parietal cortex ‘programs’ the action class of grasping. Cogn Syst Res 1(2):89–97
Chang SW, Dickinson AR, Snyder LH (2008) Limb-specific representation for reaching in the posterior parietal cortex. J Neurosci 28(24):6128–6140
Chapman LJ, Chapman JP (1987) The measurement of handedness. Brain Cogn 6(2):175–183
Chieffi S, Gentilucci M (1993) Coordination between the transport and the grasp components during prehension movements. Exp Brain Res 94(3):471–477
Churchill A, Vogt S, Hopkins B (1999) The coordination of two-effector actions: spoon-feeding and intermanual prehension. Br J Psychol 90(2):271–290. https://doi.org/10.1348/000712699161404
Connolly JD, Andersen RA, Goodale MA (2003) FMRI evidence for a parietal reach region in the human brain. Exp Brain Res 153:140–145. https://doi.org/10.1007/s00221-003-1587-1
Cooke JD, Brown SH, Cunningham DA (1989) Kinematics of arm movements in elderly humans. Neurobiol Aging 10(2):159–165
Culham JC, Cavina-Pratesi C, Singhal A (2006) The role of parietal cortex in visuomotor control: what have we learned from neuroimaging? Neuropsychologia 44(13):2668–2684. https://doi.org/10.1016/j.neuropsychologia.2005.11.003
Dempsey-Jones H, Wesselink DB, Friedman J, Makin TR (2019) Organized toe maps in extreme foot users. Cell Rep 28(11):2748–2756
Desmurget M, Epstein CM, Turner RS, Prablanc C, Alexander GE, Grafton ST (1999) Role of the posterior parietal cortex in updating reaching movements to a visual target. Nat Neurosci 2(6):563–567. https://doi.org/10.1038/9219
Diomedi S, Vaccari FE, Filippini M, Fattori P, Galletti C (2020) Mixed selectivity in macaque medial parietal cortex during eye-hand reaching. Iscience 23(10):101616
Dixon P, Glover S (2009) Perseveration and contrast effects in grasping. Neuropsychologia 47(6):1578–1584. https://doi.org/10.1016/j.neuropsychologia.2008.12.032
Efron R. (1969) What is perception? In Proceedings of the Boston Colloquium for the Philosophy of Science 1966/1968. Springer, Dordrecht. (pp 137–173)
Elliott D, Binsted G, Heath M (1999) The control of goal-directed limb movements: Correcting errors in the trajectory. Hum Mov Sci 18(2–3):121–136
Feldman AG (2015) Referent control of action and perception: Challenging Conventional Theories in Behavioral Science. New York: Springer
Feldman AG (2019) Indirect, referent control of motor actions underlies directional tuning of neurons. J Neurophysiol 121(3):823–841
Ferraina S, Johnson PB, Garasto MR, Battaglia-Mayer A, Ercolani L, Bianchi L, Caminiti R (1997) Combination of hand and gaze signals during reaching: activity in parietal area 7m of the monkey. J Neurophysiol 77(2):1034–1038
Freud E, Ganel T (2015) Visual control of action directed toward two-dimensional objects relies on holistic processing of object shape. Psychon Bull Rev 22(5):1377–1382. https://doi.org/10.3758/s13423-015-0803-x
Freud E, Ganel T, Avidan G, Gilaie-Dotan S (2016) Functional dissociation between action and perception of object shape in developmental visual object agnosia. Cortex 76:17–27. https://doi.org/10.1016/j.cortex.2015.12.006
Freud E, Culham JC, Namdar G, Behrmann M (2019) Object complexity modulates the association between action and perception in childhood. J Exp Child Psychol 179:56–72. https://doi.org/10.1016/j.jecp.2018.11.004
Gallego JA, Perich MG, Miller LE, Solla SA (2017) Neural manifolds for the control of movement. Neuron 94:978–984. https://doi.org/10.1016/j.neuron.2017.05.025
Gallego JA, Perich MG, Naufel SN, Ethier C, Solla SA, Miller LE (2018) Cortical population activity within a preserved neural manifold underlies multiple motor behaviors. Nat Commun 9(1):1–13. https://doi.org/10.1038/s41467-018-06560-z
Gallego JA, Makin TR, McDougle SD (2022) Going beyond primary motor cortex to improve brain–computer interfaces. Trends Neurosci 45(3):176–183
Gallivan JP, Adam McLean D, Smith FW, Culham JC (2011) Decoding effector-dependent and effector-independent movement intentions from human parieto-frontal brain activity. J Neurosci 31(47):17149–17168. https://doi.org/10.1523/JNEUROSCI.1058-11.2011
Gallivan JP, McLean DA, Flanagan JR, Culham JC (2013a) Where one hand meets the other: Limb-specific and action-dependent movement plans decoded from preparatory signals in single human frontoparietal brain areas. J Neurosci 33(5):1991–2008. https://doi.org/10.1523/JNEUROSCI.0541-12.2013
Gallivan JP, McLean DA, Valyear KF, Culham JC (2013b) Decoding the neural mechanisms of human tool use. Elife 2:e00425
Gamberini M, Galletti C, Bosco A, Breveglieri R, Fattori P (2011) Is the medial posterior parietal area V6A a single functional area? J Neurosci 31(13):5145–5157
Ganel T, Freud E, Chajut E, Algom D (2012) Accurate visuomotor control below the perceptual threshold of size discrimination. PLoS One 7(4):e36253
Gentilucci M, Castiello U, Corradini ML, Scarpa M, Umiltà C, Rizzolatti G (1991) Influence of different types of grasping on the transport component of prehension movements. Neuropsychologia 29(5):361–378. https://doi.org/10.1016/0028-3932(91)90025-4
Gentilucci M, Roy AC, Stefanini S (2004) Grasping an object naturally or with a tool: are these tasks guided by a common motor representation ? Exp Brain Res. https://doi.org/10.1007/s00221-004-1863-8
Ghez C, Scheidt R, Heijink H (2007) Different learned coordinate frames for planning trajectories and final positions in reaching. J Neurophysiol 98(6):3614–3626
Gibson JJ (1966) The problem of temporal order in stimulation and perception. J Psychol 62(2):141–149. https://doi.org/10.1080/00223980.1966
Gibson JJ (1977) The theory of affordances. In: Shaw R, Brans-ford J (eds) Perceiving, acting, and knowing: toward an ecological psychology. NJ: Erlbaum, Hillsdale, pp 67–82
Gibson JJ. (1979) The ecological approach to visual perception. NJ: Lawrence Eribaum Associates. First published, Hilisdale
Glover S (2002) Visual illusions affect planning but not control. Trends Cogn Sci 6:288–292. https://doi.org/10.1016/S1364-6613(02)01920-4
Glover S (2004) Separate visual representations in the planning and control of action. Behav Brain Sci 27(1):3–24
Goble DJ, Brown SH (2007) Task-dependent asymmetries in the utilization of proprioceptive feedback for goal-directed movement. Exp Brain Res 180(4):693–704
Goble DJ, Lewis CA, Brown SH (2006) Upper limb asymmetries in the utilization of proprioceptive feedback. Exp Brain Res 168(1):307–311
Gonzalez CLR, Ganel T, Whitwell RL, Morrissey B, Goodale MA (2008) Practice makes perfect, but only with the right hand: Sensitivity to perceptual illusions with awkward grasps decreases with practice in the right but not the left hand. Neuropsychologia 46(2):624–631
Goodale MA, Milner AD, Jakobson LS, Carey DP (1991) A neurological dissociation between perceiving objects and grasping them. Nature 349(6305):154–156
Grafton ST, Hamilton AFDC (2007) Evidence for a distributed hierarchy of action representation in the brain. Hum Mov Sci 26(4):590–616
Graziano MS, Aflalo TN (2007) Mapping behavioral repertoire onto the cortex. Neuron 56(2):239–251
Gréa H, Pisella L, Rossetti Y, Desmurget M, Tilikete C, Grafton S, Vighetto A (2002) A lesion of the posterior parietal cortex disrupts on-line adjustments during aiming movements. Neuropsychologia 40(13):2471–2480. https://doi.org/10.1016/S0028-3932(02)00009-X
Grosskopf A, Kuhtz-Buschbeck JP (2006) Grasping with the left and right hand: a kinematic study. Exp Brain Res 168(1–2):230–240. https://doi.org/10.1007/s00221-005-0083-1
Haar S, Dinstein I, Shelef I, Donchin O (2017) Effector-invariant movement encoding in the human motor system. J Neurosci 37(37):9054–9063. https://doi.org/10.1523/JNEUROSCI.1663-17.2017
Hadjidimitrakis K, Breveglieri R, Placenti G, Bosco A, Sabatini SP, Fattori P (2011) Fix your eyes in the space you could reach: neurons in the macaque medial parietal cortex prefer gaze positions in peripersonal space. PLoS One 6(8):e23335
Heed T, Beurze SM, Toni I, Röder B, Medendorp WP (2011) Functional rather than effector-specific organization of human posterior parietal cortex. J Neurosci 31(8):3066–3076. https://doi.org/10.1523/JNEUROSCI.4370-10.2011
Heed T, Leone FTM, Toni I, Medendorp WP (2016) Functional versus effector-specific organization of the human posterior parietal cortex: revisited. J Neurophysiol 116(4):1885–1899. https://doi.org/10.1152/jn.00312.2014
Huntley GW, Jones EG (1991) Relationship of intrinsic connections to forelimb movement representations in monkey motor cortex: a correlative anatomic and physiological study. J Neurophysiol 66(2):390–413
Hu Y, Eagleson R, Goodale MA (1999) The effects of delay on the kinematics of grasping. Exp Brain Res 126(1):109–116
Itaguchi Y (2020) Toward natural grasping with a tool: effects of practice and required accuracy on the kinematics of tool-use grasping. J Neurophysiol 123(5):2024–2036
Itaguchi Y, Fukuzawa K (2014) Hand-use and tool-use in grasping control. Exp Brain Res 232(11):3613–3622
Jeannerod M (1984) The timing of natural prehension movements. J Mot Behav 16(3):235–254. https://doi.org/10.1080/00222895.1984.10735319
Jeannerod M (1986) The formation of finger grip during prehension a cortically mediated visuomotor pattern. Behav Brain Res 19(2):99–116. https://doi.org/10.1016/0166-4328(86)90008-2
Jeffreys H (1998) The theory of probability. Oxford Univ. Press, Oxford
Kaas JH, Stepniewska I, Gharbawie O. (2012) Cortical networks subserving upper limb movements in primates. Eur J of Phys Rehabil Med 48(2) 299–306. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22407009
Karl JM, Sacrey L-AR, Doan JB, Whishaw IQ (2012) Hand shaping using hapsis resembles visually guided hand shaping. Exp Brain Res 219:59–74
Keele SW (1968) Movement control in skilled motor performance. Psychological Bullet 70((6p1)):387
Konen CS, Mruczek REB, Montoya JL, Kastner S (2013) Functional organization of human posterior parietal cortex: grasping- and reaching-related activations relative to topographically organized cortex. J Neurophysiol 109(12):2897–2908. https://doi.org/10.1152/jn.00657.2012
Krakauer JW, Pine ZM, Ghilardi MF, Ghez C (2000) Learning of visuomotor transformations for vectorial planning of reaching trajectories. J Neurosci 20(23):8916–8924
Krakauer JW, Ghilardi MF, Mentis M, Barnes A, Veytsman M, Eidelberg D, Ghez C (2004) Differential cortical and subcortical activations in learning rotations and gains for reaching: a PET study. J Neurophysiol 91(2):924–933
Kumar A, Panthi G, Divakar R, Mutha PK (2020) Mechanistic determinants of effector-independent motor memory encoding. Proc Natl Acad Sci 117(29):17338–17347
Lashley K (1950) In search of the engram Society for experimental biology, physiological mechanisms in animal behavior. Society’s Symposium IV, Academic Press, Oxford, England, pp 454-482
Latash ML (2018) Muscle coactivation: definitions, mechanisms, and functions. J Neurophysiol 120(1):88–104. https://doi.org/10.1152/jn.00084.2018
Leo A, Handjaras G, Bianchi M, Marino H, Gabiccini M, Guidi A, Ricciardi E (2016) A synergy-based hand control is encoded in human motor cortical areas. ELife. https://doi.org/10.7554/eLife.13420 ((FEBRUARY2016))
Leoné FTM, Heed T, Toni I, Pieter Medendorp W (2014) Understanding effector selectivity in human posterior parietal cortex by combining information patterns and activation measures. J Neurosci 34(21):7102–7112. https://doi.org/10.1523/JNEUROSCI.5242-13.2014
Liu Y, Vannuscorps G, Caramazza A, Striem-Amit E (2020) Evidence for an effector-independent action system from people born without hands. Proc Natl Acad Sci 117(45):28433–28441
MacKenzie CL, Marteniuk RG, Dugas C, Liske D, Eickmeier B (1987) Three-dimensional movement trajectories in fitts’ task: implications for control. Quarterly J Exp Psychol Sec A 39(4):629–647. https://doi.org/10.1080/14640748708401806
Magri C, Fabbri S, Caramazza A, Lingnau A (2019) Directional tuning for eye and arm movements in overlapping regions in human posterior parietal cortex. Neuroimage 191:234–242. https://doi.org/10.1016/j.neuroimage.2019.02.029
Maitra KK, Philips K, Rice MS (2010) Grasping naturally versus grasping with a reacher in people without disability: Motor control and muscle activation differences. Am J Occup Ther 64(1):95–104. https://doi.org/10.5014/ajot.64.1.95
Marteniuk RG, Leavitt JL, MacKenzie CL, Athenes S (1990) Functional relationships between grasp and transport components in a prehension task. Hum Mov Sci 9(2):149–176. https://doi.org/10.1016/0167-9457(90)90025-9
Meier JD, Aflalo TN, Kastner S, Graziano MSA (2008) Complex organization of human primary motor cortex: a high-resolution fMRI study. J Neurophysiol 100(4):1800–1812. https://doi.org/10.1152/jn.90531.2008
Merton PA (1972) How we control the contraction of out muscles. Sci Am 226(5):30–37
Nelson EL, Berthier NE, Konidaris GD (2018) Handedness and reach-to-place kinematics in adults: left-handers are not reversed right-handers. J Mot Behav 50(4):381–391
Osiurak F, Lesourd M, Delporte L, Rossetti Y (2018) Tool use and generalized motor programs: we all are natural born poly-dexters. Sci Rep 8(1):1–7
Overduin SA, D’Avella A, Roh J, Carmena JM, Bizzi E (2015) Representation of muscle synergies in the primate brain. J Neurosci 35(37):12615–12624. https://doi.org/10.1523/JNEUROSCI.4302-14.2015
Penfield W, Boldrey E (1937) Somatic motor and sensory representation in man. Brain 60:389–443
Perez MA, Cohen LG (2008) Mechanisms underlying functional changes in the primary motor cortex ipsilateral to an active hand. J Neurosci 28:5631–5640. https://doi.org/10.1523/JNEUROSCI.0093-08.2008
Perneger TV (1998) What’s wrong with bonferroni adjustments. Bmj 316(7139):1236–1238
Quinlan DJ, Culham JC (2015) Direct comparisons of hand and mouth kinematics during grasping, feeding and fork-feeding actions. Front Hum Neurosci 9(OCTOBER):580. https://doi.org/10.3389/fnhum.2015.00580
Raibert MH (1977) Motor control and learning by the state space model (Technical Report AI-TR-439). Cambridge, Artificial Intelligence Laboratory, Massachusett
Rijntjes M, Dettmers C, Büchel C, Kiebel S, Frackowiak RS, Weiller C (1999) A blueprint for movement: functional and anatomical representations in the human motor system. J Neurosci 19(18):8043–8048
Rolian C, Lieberman DE, Hamill J, Scott JW, Werbel W (2009) Walking, running and the evolution of short toes in humans. J Exp Biol 212(5):713–721
Rouder JN, Speckman PL, Sun D, Morey RD, Iverson G (2009) Bayesian t tests for accepting and rejecting the null hypothesis. Psychon Bull Rev 16(2):225–237
Ruddy KL, Carson RG (2013) Neural pathways mediating cross education of motor function. Front Hum Neurosci 7:397
Sainburg RL, Kalakanis D (2000) Differences in control of limb dynamics during dominant and nondominant arm reaching. J Neurophysiol 83(5):2661–2675
Sainburg RL, Wang J (2002) Interlimb transfer of visuomotor rotations: independence of direction and final position information. Exp Brain Res 145(4):437–447
Sainburg RL, Schaefer SY, Yadav V (2016) Lateralized motor control processes determine asymmetry of interlimb transfer. Neuroscience 334:26–38
Schettino LF, Adamovich SV, Poizner H (2003) Effects of object shape and visual feedback on hand configuration during grasping. Exp Brain Res 151(2):158–166
Schulze K, Lüders E, Jäncke L (2002) Intermanual transfer in a simple motor task. Cortex 38:805–815
Stelmach GE, Diggles VA (1982) Motor equivalence and distributed control: evidence for nonspecific muscle commands. Behav Brain Sci 5(4):566–567
Stelmach GE, Mullins PA, Teulings HL (1984) Motor programming and temporal patterns in handwriting. Ann NY Acad Sci 423:144–157
Strick PL, Preston JB (1978) Multiple representation in the primate motor cortex. Brain Res 154(2):366–370
Striem-Amit E, Vannuscorps G, Caramazza A (2017) Sensorimotor-independent development of hands and tools selectivity in the visual cortex. Proc Natl Acad Sci 114(18):4787–4792
Tang R, Zhu H (2017) Programming of left hand exploits task set but that of right hand depends on recent history. Exp Brain Res 235(7):2215–2224
Tang R, Whitwell RL, Goodale MA (2016) Unusual hand postures but not familiar tools show motor equivalence with precision grasping. Cognition 151:28–36
Tang R, Ren S, Enns JT, Whitwell RL (2018) The left hand disrupts subsequent right hand grasping when their actions overlap. Acta Physiol (Oxf) 188:131–138
Tresilian JR, Stelmach GE (1997) Common organization for unimanual and bimanual reach-to-grasp tasks. Exp Brain Res 115(2):283–299
Turella L, Rumiati R, Lingnau A (2020) Hierarchical action encoding within the human brain. Cereb Cortex. https://doi.org/10.1093/cercor/bhz284
Uithol S, van Rooij I, Bekkering H, Haselager P (2012) Hierarchies in action and motor control. J Cogn Neurosci 24(5):1077–1086
Umiltà MA, Intskirveli I, Grammont F, Rochat M, Caruana F, Jezzini A, Rizzolatti G (2008) When pliers become fingers in the monkey motor system. Proc Natl Acad Sci 105(6):2209–2213
van Mier HI, Petersen SE (2006) Intermanual transfer effects in sequential tactuomotor learning: evidence for effector independent coding. Neuropsychologia 44:939–949
Westwood DA, Danckert J, Servos P, Goodale MA (2002) Grasping two-dimensional images and three-dimensional objects in visual-form agnosia. Exp Brain Res 144(2):262–267
Wong AL, Haith AM, Krakauer JW (2015) Motor planning. Neuroscientist 21(4):385–398
Woodworth RS (1899) Accuracy of voluntary movement. Psychological Rev: Monogr Suppl 3(3):i
Wurm MF, Lingnau A (2015) Decoding actions at different levels of abstraction. J Neurosci 35(20):7727–7735
Yokoi A, Diedrichsen J (2019) Neural organization of hierarchical motor sequence representations in the human neocortex. Neuron 103(6):1178–1190
Yttri EA, Wang C, Liu Y, Snyder LH (2014) The parietal reach region is limb specific and not involved in eye-hand coordination. J Neurophysiol 111(3):520–532. https://doi.org/10.1152/jn.00058.2013
Zeharia N, Hertz U, Flash T, Amedi A (2015) New whole-body sensory-motor gradients revealed using phase-locked analysis and verified using multivoxel pattern analysis and functional connectivity. J Neurosci 35(7):2845–2859. https://doi.org/10.1523/JNEUROSCI.4246-14.2015
Zhang CY, Aflalo T, Revechkis B, Rosario ER, Ouellette D, Pouratian N, Andersen RA (2017) Partially mixed selectivity in human posterior parietal association cortex. Neuron 95:697-708.e694
Acknowledgements
We thank Ala Alshomali, Jennifer Kim and Chloë Donovan for their help in carrying out this experiment. This work was supported by the Edwin H. Richard and Elisabeth Richard von Matsch Distinguished Professorship in Neurological Diseases (to E.S.-A.) and by the Vision Science to Applications (VISTA) program funded by the Canada First Research Excellence Fund (CFREF, 2016–2023) (EF).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Communicated by Bill J Yates.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Y., Caracoglia, J., Sen, S. et al. Are reaching and grasping effector-independent? Similarities and differences in reaching and grasping kinematics between the hand and foot. Exp Brain Res 240, 1833–1848 (2022). https://doi.org/10.1007/s00221-022-06359-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-022-06359-x