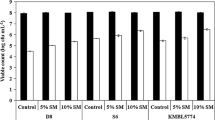
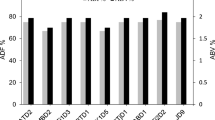
Abstract
In this study, Debaryomyces hansenii Y-3 with good freeze–thaw tolerance and Saccharomyces cerevisiae H-1 with strong fermentation ability were used to obtain a mixed starter culture (MSC), which can be used for frozen dough production. Angel yeast A-1 and a single-strain S. cerevisiae H-1 were used as references. Sensory evaluation and electronic nose combined with headspace solid-phase micro-extraction gas chromatography–mass spectrometry (HS–SPME–GC–MS) were used to analyze the taste and flavor of fermented steamed bread samples. The results showed that more kinds of volatile flavor compounds were detected in steamed bread samples fermented by MSC than in the reference group. Among all frozen dough steamed bread samples, only MSC-fermented samples could detect compounds, such as 2-methyl-1-propanol, 3-methyl-1-butanol, 1-hexanol, and 1-pentanol. Moreover, the content of ethyl octanoate, phenethyl alcohol, and 3-methyl-1-butanol was the highest in this sample. Meanwhile, whole-genome sequencing analysis was performed on D. hansenii Y-3 and S. cerevisiae H-1. Based on KEGG pathway localization, four main pathways are involved in yeast freeze–thaw tolerance. Through genome comparison analysis, there were significant differences in the related coding genes involved in the synthesis of trehalose, glycerol, proline, and arginine that affect their freeze–thaw resistance in the genomes of the two yeast strains. Therefore, it can be speculated that these significant differences are the reason for their large differences in freeze–thaw resistance.






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Akbarian M, Dehkordi MSM, Ghasemkhani N et al (2015) Hydrocolloids and cryoprotectant used in frozen dough and effect of freezing on yeast survival and dough structure: a review. Int J Life Sci 9:1–7
Arguelles JC (2000) Physiological roles of trehalose in bacteria and yeasts: a comparative analysis. Arch Microbiol 174:217–224
Birch AN, Petersen MA, Hansen AS et al (2013) Influence of commercial baker’s yeasts on bread aroma profiles. Food Res Int 52:160–166
Birch AN, Petersen MA, Hansen AS (2014) Aroma of wheat bread crumb. Cereal Chem 91:105–114
Cai X, Seitl I, Mu WM et al (2018) Biotechnical production of trehalose through the trehalose synthase pathway: current status and future prospects. Appl Microbiol Biotechnol 102:2965–2976
Chen QF, Haddad GG (2004) Role of trehalose phosphate synthase and trehalose during hypoxia: from flies to mammals. J Exp Biol 207:3125–3129
Cordente AG, Schmidt S, Beltran G et al (2019) Harnessing yeast metabolism of aromatic amino acids for fermented beverage bioflavouring and bioproduction. Appl Microbiol Biotechnol 103:4325–4336
Dalvi-Isfahan M, Jha PK, Tavakoli J et al (2019) Review on identification, underlying mechanisms and evaluation of freezing damage. J Food Eng 255:50–60
Eleutherio E, Panek A, De Mesquita JF et al (2015) Revisiting yeast trehalose metabolism. Curr Genet 61:263–274
Gancedo C, Flores CL (2004) The importance of a functional trehalose biosynthetic pathway for the life of yeasts and fungi. FEMS Yeast Res 4:351–359
Geertman J-MA, van Dijken JP, Pronk JT (2006) Engineering NADH metabolism in Saccharomyces cerevisiae: formate as an electron donor for glycerol production by anaerobic, glucose-limited chemostat cultures. FEMS Yeast Res 6:1193–1203
Giannou V, Tzia C (2008) Cryoprotective role of exogenous trehalose in frozen dough products. Food Bioproc Tech 1:276–284
Hazelwood LA, Daran JM, Maris A et al (2008) The ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism. Appl Environ Microbiol 74:2259–2266
Hu W, Yang X, Ji Y et al (2021) Effect of starter cultures mixed with different autochthonous lactic acid bacteria on microbial, metabolome and sensory properties of Chinese northeast sauerkraut. Food Res Int 148:110605
Huang S, Miskelly D (2016) In: Rob S, Karen M, Jason M (ed) Frozen steamed products and frozen doughs. Nikki Levy, UK
Huang YY, Jia XZ, Yu JJ et al (2021) Effect of different lactic acid bacteria on nitrite degradation, volatile profiles, and sensory quality in Chinese traditional paocai. LWT Food Sci Technol 147:111597
Juzhong T, Jie X (2020) Applications of electronic nose (e-nose) and electronic tongue (e-tongue) in food quality-related properties determination: a review. Artif Intell Agric 4:104–115
Kim Y, Huang W, Zhu H et al (2009) Spontaneous sourdough processing of Chinese Northern-style steamed breads and their volatile compounds. Food Chem 114:685–692
Klein M, Swinnen S, Thevelein JM et al (2016) Glycerol metabolism and transport in yeast and fungi: established knowledge and ambiguities. Environ Microbiol 19:878–893
Loutfi A, Coradeschi S, Mani GK et al (2015) Electronic noses for food quality: a review. J Food Eng 144:103–111
Lan G, Li C, He L, Zeng X et al (2020) Effects of different strains and fermentation method on nattokinase activity, biogenic amines, and sensory characteristics of natto. J Food Sci Tech MYS 57:4414–4423
Larsson C, Pahlman IL, Ansell R et al (1998) The importance of the glycerol 3-phosphate shuttle during aerobic growth of Saccharomyces cerevisiae. Yeast 14:347–357
Li YD, Wang JH, Wang T et al (2022) Differences between Kazak Cheeses fermented by single and mixed strains using untargeted metabolomics. Foods 11:966
Luo WH, Sun DW, Zhu ZW, Wang QJ (2017) Improving freeze tolerance of yeast and dough properties for enhancing frozen dough quality - a review of effective methods. Trends Food Sci Tech 72:25–33
Ma S, Wang Z, Guo XF et al (2021) Sourdough improves the quality of whole-wheat flour products: mechanisms and challenges—a review. Food Chem 360:130038
Mizuno M, Matsuzaki T, Ozeki N et al (2022) Cell membrane fluidity and ROS resistance define DMSO tolerance of cryopreserved synovial MSCs and HUVECs. Stem Cell Res Ther 13:177
Morita Y, Nakamori S, Takagi H (2002) Effect of proline and arginine metabolism on freezing stress of Saccharomyces cerevisiae. J Biosci Bioeng 94:390–394
Pallapati AR, Sirigiri SD, Jain S et al (2021) Lysine245 plays a crucial role in stability and function of glycerol 3-phosphate dehydrogenase (Gpd1) in Saccharomyces cerevisiae. J Cell Biochem 122:1726–1736
Pathway T, Park JI, Grant CM et al (1997) The freeze-thaw stress response of the yeast Saccharomyces cerevisiae is growth phase specific and is controlled by nutritional state via the RAS-cyclic AMP signal transduction pathway. Appl Environ Microbiol 63(10):3818–3824
Pico J, Bernal J, Gomez M (2015) Wheat bread aroma compounds in crumb and crust: a review. Food Res Int 75:200–215
Rashidi A, Hadinezhad M, Rajabzadeh N et al (2016) Frozen baguette bread dough I. Rheological behavior during storage. J Cereal Sci 72:24–29
Rodríguez-Vargas S, Sánchez-García A, Martínez-Rivas J et al (2007) Fluidization of membrane lipids enhances the tolerance of Saccharomyces cerevisiae to freezing and salt stress. Appl Environ Microbiol 73:110–116
Salim-ur R, Paterson A, Piggott JR (2006) Flavour in sourdough breads: a review. Trends Food Sci Tech 17:557–566
Shima J, Takagi H (2009) Stress-tolerance of baker’s-yeast (Saccharomyces cerevisiae) cells: stress-protective molecules and genes involved in stress tolerance. Biotechnol Appl Biochem 53:155–164
Takagi H, Iwamoto F, Nakamori S (1997) Isolation of freeze-tolerant laboratory strains of Saccharomyces cerevisiae from proline-analogue-resistant mutants. Appl Microbiol Biotechnol 47:405–411
Tan H, Dong J, Wang G, Xu H et al (2014) Enhanced freeze tolerance of baker’s yeast by overexpressed trehalose-6-phosphate synthase gene (TPS1) and deleted trehalase genes in frozen dough. J Ind Microbiol Biot 41:1275–1285
Tanghe A, Dijck PV, Thevelein JM (2003) Determinants of freeze tolerance in microorganisms, physiological importance, and biotechnological applications. Adv Appl Microbiol 53:129–176
Villarreal P, Carrasco M, Barahona S et al (2018) Antarctic yeasts: analysis of their freeze-thaw tolerance and production of antifreeze proteins, fatty acids and ergosterol. BMC Microbiol 18:66
Wallace LR, Lou WC, Camill DV et al (1987) Frozen bread dough. US Patent application: EP83300314.8
Wang P, Jin Z, Xu X (2015) Physicochemical alterations of wheat gluten proteins upon dough formation and frozen storage - a review from gluten, glutenin and gliadin perspectives. Trends Food Sci Technol 46:189–198
Wang X, Pei DD, Teng YF (2018) Effects of enzymes to improve sensory quality of frozen dough bread and analysis on its mechanism. J Food Sci Technol 55:389–398
Wang ZX, Zhuge J, Fang HY et al (2001) Glycerol production by microbial fermentation: a review. Biotechnol Adv 19:201–223
Xue MC, Wang LP, Hao YL et al (2018) Screening of freeze-tolerant yeasts and their bread dough fermentative properties. Food Machin, China 4:6–11
Zhang GH, Wu T, Sadiq FA et al (2016) A study revealing the key aroma compounds of steamed bread made by Chinese traditional sourdough. J Zhejiang Univ-SC B 17:787–797
Zhu X, Yuan P, Zhang T et al (2022) Effect of carboxymethyl chitosan on the storage stability of frozen dough: state of water, protein structures and quality attributes. Food Res Int 151:110863
Acknowledgements
This research was supported by the Scientific Research Project of the Key Research Base of Humanities and Social Sciences for Universities in Sichuan Province, Sichuan Provincial Department of Education, China (CC17Z19).
Author information
Authors and Affiliations
Contributions
Formal analysis, writing–original draft: WH. Methodology, validation: MX. Resources, supervision: HY. Methodology, validation: XZ. Resources: YL. Resources: YC. Conceptualization, visualization, supervision, writing—review and editing: LW.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, W., Xue, M., Yu, H. et al. Co-culture fermentation characteristics of antifreeze yeast and mining of related freezing-resistant genes. Eur Food Res Technol 249, 1161–1172 (2023). https://doi.org/10.1007/s00217-023-04204-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-023-04204-1