Abstract
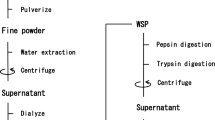
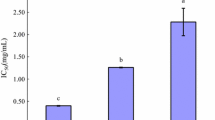
Angiotensin I-converting enzyme (ACE) inhibitors have been widely used as antihypertensive agents. However, most synthetic ACE inhibitors ineluctably have severe side effects. Researchers have focused on various ACE-inhibitory peptides derived from dietary food. In the present study, we reported peptides produced from porcine blood, an important food in Asian countries. Through enzymatic hydrolysis, we found that peptides from this animal compound have ACE-inhibitory effects. Porcine hemoglobin was hydrolyzed using ordinary proteases, including alcalase, trypsin, neutral, papain, protamex, and pepsin. Results showed that pepsin was the most efficient protease in producing active peptides, and the pepsin hydrolysate of porcine hemoglobin showed the highest activity (IC50 = 1.53 ± 0.03 mg/mL). Combining DA 201-C macroporous resin chromatography, Sephadex LH-20 gel chromatography, and reversed-phase high-performance liquid chromatography, the fraction 2-IV was purified from pepsin hydrolysis of porcine hemoglobin; this compound exhibited the highest ACE-inhibitory activity (IC50 = 0.02 ± 0.01 mg/mL). Through Edman degradation, we also found that the exact amino acid sequence of fraction 2-IV was Gln–Glu–Leu–Pro–Gly. The results indicated that porcine hemoglobin peptides possessed significant ACE-inhibitory effect in vitro, which is an important complement of the previous work.





Similar content being viewed by others
Abbreviations
- ACE:
-
Angiotensin I-converting enzyme
- Ang I:
-
Angiotensin I
- Ang II:
-
Angiotensin II
- DDW:
-
Double-distilled water
- DH:
-
Degree of hydrolysis
- HPLC:
-
High-performance liquid chromatography
- IC50 :
-
50 % ACE inhibition
- KKS:
-
Kallikrein–kinin system
- RAS:
-
Rennin–angiotensin–aldosterone system
- RP-HPLC:
-
Reversed-phase high-performance liquid chromatography
References
Lopez-Fandino R, Otte J, van Camp J (2006) Physiological, chemical and technological aspects of milk-protein-derived peptides with antihypertensive and ACE-inhibitory activity. Int Dairy J 16(11):1277–1293. doi:10.1016/j.idairyj.2006.06.004
Silva ACSE, Flynn JT (2012) The renin-angiotensin-aldosterone system in 2011: role in hypertension and chronic kidney disease. Pediatr Nephrol 27(10):1835–1845. doi:10.1007/s00467-011-2002-y
Murray BA, FitzGerald RJ (2007) Angiotensin converting enzyme inhibitory peptides derived from food proteins: biochemistry, bioactivity and production. Curr Pharm Design 13(8):773–791. doi:10.2174/138161207780363068
Atkinson AB, Robertson JI (1979) Captopril in the treatment of clinical hypertension and cardiac failure. Lancet 2(8147):836–839. doi:10.1016/S0140-6736(79)92186-X
FitzGerald RJ, Murray BA, Walsh DJ (2004) Hypotensive peptides from milk proteins. J Nutr 134(4):980s–988s
Liu LL, Liu LY, Lu BY, Xia DZ, Zhang Y (2012) Evaluation of antihypertensive and antihyperlipidemic effects of bamboo shoot angiotensin converting enzyme inhibitory peptide in vivo. J Agric Food Chem 60(45):11351–11358. doi:10.1021/Jf303471f
Ferreira SH, e Silva MR (1965) Potentiation of bradykinin and eledoisin by BPF (bradykinin potentiating factor) from Bothrops jararaca venom. Experientia 21(6):347–349
Hyun CK, Shin HK (2000) Utilization of bovine blood plasma proteins for the production of angiotensin I converting enzyme inhibitory peptides. Process Biochem 36:6
Zhao Q, Le Coeur C, Piot JM (1997) Analysis of peptides from bovine hemoglobin and tuna myoglobin enzymatic hydrolysate: use of HPLC with on-line second-order derivative spectroscopy for the characterization of biologically active peptides. Analytica Chimica Acta 352:20
Zhao Q, Garreau I, Sannier F, Piot JM (1997) Opioid peptides derived from hemoglobin: hemorphins. Biopolymers 43(2):75–98. doi:10.1002/(SICI)1097-0282(1997)43:2<75:AID-BIP2>3.0.CO;2-X
Wu T, Coeur-Tourneur C, Dhont G, Cassez A, Fertein E, He XD, Chen WD (2014) Simultaneous monitoring of temporal profiles of NO3, NO2 and O-3 by incoherent broadband cavity enhanced absorption spectroscopy for atmospheric applications. J Quant Spectrosc Ra 133:199–205. doi:10.1016/j.jqsrt.2013.08.002
Wu KC, Lin ZY, Chiang SH, Chang CY (2004) Antioxidant properties of porcine blood protein before and after enzymatic hydrolysis. J Biomass Ener Soc China 23:6
Miyaguchi Y, Sakai K, Yonekura M, Tsutsumi M (1989) Some properties of succinglated globin. Nippon Shokuhin Kogyo Gakkaishi 36:6
Chen C, Chi YJ, Zhao MY, Xu W (2012) Influence of degree of hydrolysis on functional properties, antioxidant and ACE inhibitory activities of egg white protein hydrolysate. Food Sci Biotechnol 21(1):27–34. doi:10.1007/s10068-012-0004-6
Cushman DW, Cheung HS (1971) Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem Pharmacol 20(7):1637–1648
Lee JK, Jeon JK, Byun HG (2011) Effect of angiotensin I converting enzyme inhibitory peptide purified from skate skin hydrolysate. Food Chem 125(2):495–499. doi:10.1016/j.foodchem.2010.09.039
Knecht R, Seemuller U, Liersch M, Fritz H, Braun DG, Chang JY (1983) Sequence determination of eglin C using combined microtechniques of amino acid analysis, peptide isolation, and automatic Edman degradation. Anal Biochem 130(1):65–71
Chang CY, Wu KC, Chiang SH (2007) Antioxidant properties and protein compositions of porcine haemoglobin hydrolysates. Food Chem 100(4):1537–1543. doi:10.1016/j.foodchem.2005.12.019
Vossenberg P, Beeftink HH, Nuijens T, Quaedflieg PJLM, Stuart MAC, Tramper J (2013) Dipeptide synthesis in near-anhydrous organic media: long-term stability and reusability of immobilized Alcalase. J Mol Catal B-Enzym 93:23–27. doi:10.1016/j.molcatb.2013.03.014
Zhao YH, Li BF, Liu ZY, Dong SY, Zhao X, Zeng MY (2007) Antihypertensive effect and purification of an ACE inhibitory peptide from sea cucumber gelatin hydrolysate. Process Biochem 42(12):1586–1591. doi:10.1016/j.procbio.2007.08.011
Zhong F, Liu JM, Ma JG, Shoemaker CF (2007) Preparation of hypocholesterol peptides from soy protein and their hypocholesterolemic effect in mice. Food Res Int 40(6):661–667. doi:10.1016/j.foodres.2006.11.011
Zhang FX, Wang Z, Xu SY (2009) Macroporous resin purification of grass carp fish (Ctenopharyngodon idella) scale peptides with in vitro angiotensin-I converting enzyme (ACE) inhibitory ability. Food Chem 117(3):387–392. doi:10.1016/j.foodchem.2009.04.015
Suetsuna K, Ukeda H, Ochi H (2000) Isolation and characterization of free radical scavenging activities peptides derived from casein. J Nutr Biochem 11(3):128–131. doi:10.1016/S0955-2863(99)00083-2
Suetsuna K, Yamagami M, Kuwata K (1988) Inhibitory activity against angiotensin I-converting enzyme of peptides originating from fish and shellfish muscle. Nippon Suisan Gakkaishi 54(10):5
Pihlanto-Leppala A, Koskinen P, Piilola K, Tupasela T, Korhonen H (2000) Angiotensin I-converting enzyme inhibitory properties of whey protein digests: concentration and characterization of active peptides. J Dairy Res 67(1):53–64
Haque E, Chand R (2008) Antihypertensive and antimicrobial bioactive peptides from milk proteins. Eur Food Res Technol 227(1):7–15. doi:10.1007/s00217-007-0689-6
Chen QH, Xuan GD, Fu ML, He GQ, Wang W, Zhang HB, Ruan H (2007) Effect of angiotensin I-converting enzyme inhibitory peptide from rice dregs protein on antihypertensive activity in spontaneously hypertensive rats. Asia Pac J Clin Nutr 16:281–285
Jia J, Zhou Y, Lu J, Chen A, Li Y, Zheng G (2010) Enzymatic hydrolysis of Alaska pollack (Theragra chalcogramma) skin and antioxidant activity of the resulting hydrolysate. J Sci Food Agric 90(4):635–640. doi:10.1002/jsfa.3861
Ruiz JAG, Ramos M, Recio I (2004) Angiotensin converting enzyme-inhibitory activity of peptides isolated from Manchego cheese. Stability under simulated gastrointestinal digestion. Int Dairy J 14(12):1075–1080. doi:10.1016/j.idairyj.2004.04.007
Saito T, Nakamura T, Kitazawa H, Kawai Y, Itoh T (2000) Isolation and structural analysis of antihypertensive peptides that exist naturally in Gouda cheese. J Dairy Sci 83(7):1434–1440. doi:10.3168/jds.S0022-0302(00)75013-2
Yu Y, Hu J, Miyaguchi Y, Bai X, Du Y, Lin B (2006) Isolation and characterization of angiotensin I-converting enzyme inhibitory peptides derived from porcine hemoglobin. Peptides 27(11):2950–2956. doi:10.1016/j.peptides.2006.05.025
Acknowledgments
The authors wish to thank Dr. Zhaohui Zhang from the Agricultural Biological Detection Laboratory, Chinese Academy of Agricultural Sciences, for the analysis of peptide sequence. This research was supported by a Grant (XDJK2009C055) from the Southwest University’s basic scientific research business expenses special, and the Laboratory of Quality and Safety Risk Assessment for Agro-products on Storage and Preservation (Chongqing), Ministry of Agriculture, China.
Conflict of interest
The authors declare no conflict of interests.
Compliance with Ethics Requirements
All procedures on animals were in strict accordance with the guide for the care and using of laboratory animals published by the US national Institutes of Health and was approved by the Institutional Animal Care Committee at the Southwest University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Deng, H., Zheng, J., Zhang, F. et al. Isolation of angiotensin I-converting enzyme inhibitor from pepsin hydrolysate of porcine hemoglobin. Eur Food Res Technol 239, 933–940 (2014). https://doi.org/10.1007/s00217-014-2290-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-014-2290-0