Abstract
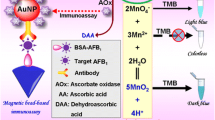
An accurate and sensitive competitive enzyme-linked immunosorbent assay (ELISA) based on persistent luminescence nanoparticles Zn2GeO4:Mn2+, Eu3+ (ZGME) was developed for detecting ochratoxin A (OTA), a powerfully toxic mycotoxin usually found in grains. As a signal output element of autofluorescence-free biosensors, ZGME can be integrated into ELISA with glucose oxidase (GOx)-binding OTA molecules due to its excellent pH-responsive persistent luminescence. In the absence of OTA, the OTA-GOx conjugate was captured by the anti-OTA monoclonal antibody (anti-OTA mAb) pre-coated on the 96-well plate. The results indicate a decrease in the pH value of the solution, which triggered the quenching of ZGME luminescence due to GOx-dependent gluconic acid production. The presence of OTA inhibited the binding of OTA-GOx on the plate, thus decreasing the production of gluconic acid and increasing the persistent luminous intensity of ZGME. Under the optimized concentrations of anti-OTA mAb and OTA-GOx, quantitative determination of OTA was achieved by plotting the increase or decrease in persistent luminescence intensity of ZGME at 535 nm. In this study, the linear range was from 0.1 μg L−1 to 63 μg L−1, and the limit of detection (LOD) was as low as 0.045 μg L−1. In five food samples (corn grit, brown rice, soybean, rice, and wheat), the results exhibited good stability and repeatability, with a recovery range from 81.3% to 94.4% and a relative standard deviation (RSD) of less than 4.2%. Hence, the established method provides a sensitive, accurate, and autofluorescence-free approach for the determination of OTA in different grain samples.






Similar content being viewed by others
References
Samuel MS, Jeyaram K, Datta S, Chandrasekar N, Balaji R, Selvarajan E. Detection, Contamination, Toxicity, and prevention methods of ochratoxins: An update review. J Agric Food Chem. 2021;69:13974–89. https://doi.org/10.1021/acs.jafc.1c05994.
Liu WC, Pushparaj K, Meyyazhagan A, Arumugam VA, Pappuswamy M, Bhotla HK, Baskaran R, Issara U, Balasubramanian B, Mousavi KA. Ochratoxin A as an alarming health threat for livestock and human: A review on molecular interactions, mechanism of toxicity, detection, detoxification, and dietary prophylaxis. Toxicon. 2022;213:59–75. https://doi.org/10.1016/j.toxicon.2022.04.012.
Ferrara M, Perrone G, Gallo A. Recent advances in biosynthesis and regulatory mechanisms of principal mycotoxins. Curr Opin Food Sci. 2022;48:100923. https://doi.org/10.1016/j.cofs.2022.100923.
Wei D, Pan A, Zhang C, Guo M, Lou C, Zhang J, Wu H, Wang X. Fast extraction of aflatoxins, ochratoxins and enniatins from maize with magnetic covalent organic framework prior to HPLC-MS/MS detection. Food Chem. 2023;404:134464. https://doi.org/10.1016/j.foodchem.2022.134464.
Zhang T, Xing B, Han Q, Lei Y, Wu D, Ren X, Wei Q. Electrochemical immunosensor for ochratoxin A detection based on Au octahedron plasmonic colloidosomes. Anal Chim Acta. 2018;1032:114–21. https://doi.org/10.1016/j.aca.2018.05.035.
Goud KY, Reddy KK, Satyanarayana M, Kummari S, Gobi KV. A review on recent developments in optical and electrochemical aptamer-based assays for mycotoxins using advanced nanomaterials. Mikrochim Acta. 2019;187(1):29. https://doi.org/10.1007/s00604-019-4034-0.
Tong WP, Fang H, Xiong HP, Wei DX, Leng YK, Hu XY, Huang XL, Xiong YH. Eco-friendly fluorescent ELISA based on bifunctional phage for ultrasensitive detection of ochratoxin A in corn. Foods. 2021;10:2429–41. https://doi.org/10.3390/foods10102429.
Liang Y, Huang XL, Yu RJ, Zhou YF, Xiong YH. Fluorescence ELISA for sensitive detection of ochratoxin A based on glucose oxidase-mediated fluorescence quenching of CdTe QDs. Anal Chim Acta. 2016;936:195–201. https://doi.org/10.1016/j.aca.2016.06.018.
Xing KY, Peng J, Shan S, Liu DF, Huang YN, Lai WH. Green enzyme-linked immunosorbent assay based on the single-stranded binding protein-assisted aptamer for the detection of mycotoxin. Anal Chem. 2020;92:8422–6. https://doi.org/10.1021/acs.analchem.0c01073.
Qiao MX, Liu Y, Wei M. Dual-signal output fluorescent aptasensor based on DNA programmability and gold nanoflowers for multiple mycotoxins detection. Anal Bioanal Chem. 2023;415:277–88. https://doi.org/10.1007/s00216-022-04403-x.
Qu CL, Zhao LY, He X, Yu SC, Wei M. Magnetic beads–assisted fluorescence aptasensing approach based on dual DNA tweezers for detection of ochratoxin A and fumonisin B1 in wine and corn. Anal Bioanal Chem. 2021;413:6677–85. https://doi.org/10.1007/s00216-021-03635-7.
Zhang H, Wang YL, Lin YT, Chu WJ, Luo Z, Zhao MQ, Hu JD, Miao XM, He F. A catalytic hairpin assembly–based Förster resonance energy transfer sensor for ratiometric detection of ochratoxin A in food samples. Anal Bioanal Chem. 2022. https://doi.org/10.1007/s00216-022-04479-5.
Tian JY, Wei WQ, Wang JW, Ji SJ, Chen GC, Lu JS. Fluorescence resonance energy transfer aptasensor between nanoceria and graphene quantum dots for the determination of ochratoxin A. Anal Chim Acta. 2018;1000:265–72. https://doi.org/10.1016/j.aca.2017.08.018.
Hou YJ, Long N, Jia BY, Liao XF, Yang MH, Fu LZ, Zhou LD, Sheng P, Kong WJ. Development of a label-free electrochemical aptasensor for ultrasensitive detection of ochratoxin A. Food Control. 2022;135:108833. https://doi.org/10.1016/j.foodcont.2022.108833.
Chen RP, Li S, Sun YF, Huo BY, Xia YT, Qin YK, Li SN, Shi BS, He DF, Liang J, Gao ZX. Surface-enhanced Raman spectroscopy aptasensor for simultaneous determination of ochratoxin A and zearalenone using Au@Ag core-shell nanoparticles and gold nanorods. Mikrochim Acta. 2021;188:281–90. https://doi.org/10.1007/s00604-021-04919-6.
Chen XJ, Gao D, Sun FX, Li ZZ, Wang Y, Qiu CX, He KF, Wang J. Nanomaterial-based aptamer biosensors for ochratoxin A detection: a review. Anal Bioanal Chem. 2022;414:2953–69. https://doi.org/10.1007/s00216-022-03960-5.
Li QB, Cui YL, Liao M, Feng T, Tan GY, Wang BM, Liu SZ. A monoclonal antibody-based indirect competitive enzymelinked immunosorbent assay for flubendiamide detection. Sci Rep. 2019;9:2131–9. https://doi.org/10.1038/s41598-019-38649-w.
Dong YQ, Zhang J, Xing Y, Song ZR, Wang YF, Meng M, Deng C, Tong ZS, Yin YM, Xi RM. Quantification of ponceau 4R in foods by indirect competitive enzyme-linked immunosorbent assay (icELISA). J Agric Food Chem. 2015;63:6338–45. https://doi.org/10.1021/acs.jafc.5b02129.
Liang L, Chen N, Jia YY, Ma QQ, Wang J, Yuan Q, Tan WH. Recent progress in engineering near-infrared persistent luminescence nanoprobes for time-resolved biosensing/bioimaging. Nano Res. 2019;12:1279–92. https://doi.org/10.1007/s12274-019-2343-6.
Lin QS, Li ZH, Ji CH, Yuan Q. Electronic structure engineering and biomedical applications of low energy-excited persistent luminescence nanoparticles. Nanoscale Adv. 2020;2:1380–94. https://doi.org/10.1039/c9na00817a.
Zhao X, Chen LJ, Zhao KC, Liu YS, Liu J, Yan XP. Autofluorescence-free chemo/biosensing in complex matrixes based on persistent luminescence nanoparticles. TrAC-Trends in Anal Chem. 2019;118:65–72. https://doi.org/10.1016/j.trac.2019.05.025.
Feng Y, Su YY, Liu R, Lv Y. Engineering activatable nanoprobes based on time-resolved luminescence for chemo/biosensing. TrAC-Trends in Anal Chem. 2021;140:116283. https://doi.org/10.1016/j.trac.2021.116283.
Wang BB, Zhao X, Chen LJ, Yang C, Yan XP. Functionalized persistent luminescencenanoparticle-based aptasensor for autofluorescence-free determination of kanamycin in food samples. Anal Chem. 2021;93:2589–95. https://doi.org/10.1021/acs.analchem.0c04648.
Li J, Yang C, Wang WL, Yan XP. Functionalized gold and persistent luminescence nanoparticle-based ratiometric absorption and TR-FRET nanoplatform for high-throughput sequential detection of l-cysteine and insulin. Nanoscale. 2018;10:14931–7. https://doi.org/10.1039/c8nr04414g.
Zhang F, Hu D, Su X, Hong Z, Feng W, Xu M, Li F. Two birds with one stone: Amine-functionalized MSNs@Eu(OH)CO3 nanoprobe for efficient dissolution-enhanced afterglow bioassay. Nano Res. 2022;15:8360–6. https://doi.org/10.1007/s12274-022-4464-6.
Li J, Huang X, Zhao X, Chen LJ, Yan XP. pH-Responsive torpedo-like persistent luminescence nanoparticles for autofluorescence-free biosensing and high-level information encryption. Angew Chem Int Ed Engl. 2021;60:2398–405. https://doi.org/10.1002/anie.202011553.
Yin Z, Zhu L, Lv Z, Li M, Tang D. Persistent luminescence nanorods-based autofluorescence-free biosensor for prostate-specific antigen detection. Talanta. 2021;233:122563. https://doi.org/10.1016/j.talanta.2021.122563.
Song CM, Liu QT, Zhi AM, Yang JF, Zhi YB, Li QM, Hu XF, Deng RG, Casas J, Tang L, Zhang GP. Development of a lateral flow colloidal gold immunoassay strip for the rapid detection of olaquindox residues. J Agric Food Chem. 2011;59:9319–26. https://doi.org/10.1021/jf202213m.
Yao HZ, Zhang YC, Yan X. Dopant concentration-dependent morphological evolution of Zn2GeO4:Mn2+/Eu3+ phosphor and optical temperature sensing performance. J Alloy Compd. 2019;770:149–57. https://doi.org/10.1016/j.jallcom.2018.08.105.
Lv X, Zhang Y, Liu G, Du L, Wang S. Aptamer-based fluorescent detection of ochratoxin A by quenching of gold nanoparticles. RSC Adv. 2017;7:16290–4. https://doi.org/10.1039/c7ra01474k.
Zhu X, Li W, Lin L, Huang X, Xu H, Yang G, Lin Z. Target-responsive ratiometric fluorescent aptasensor for OTA based on energy transfer between [Ru(bpy)3]2+ and silica quantum dots. Mikrochim Acta. 2020;187:270–8. https://doi.org/10.1007/s00604-020-04245-3.
Becheva ZR, Atanasova MK, Ivanov YL, Godjevargova TI. Magnetic nanoparticle-based fluorescence immunoassay for determination of ochratoxin A in milk. Food Anal Methods. 2020;13:2238–48. https://doi.org/10.1007/s12161-020-01848-7.
Jiang H, Li X, Xiong Y, Pei K, Nie L, Xiong Y. Silver nanoparticle-based fluorescence-quenching lateral flow immunoassay for sensitive detection of ochratoxin A in grape juice and wine. Toxins. 2017;9:83–94. https://doi.org/10.3390/toxins9030083.
Zhao H, Xiong D, Yan Y, Ma C. Amplified fluorescent aptasensor for ochratoxin A assay based on graphene oxide and RecJf exonuclease. Toxins. 2020;12:670–81. https://doi.org/10.3390/toxins12110670.
Liu Y, Yan H, Shangguan JF, Yang X, Wang M, Liu W. A fluorometric aptamer-based assay for ochratoxin A using magnetic separation and a cationic conjugated fluorescent polymer. Mikrochim Acta. 2018;185:427–34. https://doi.org/10.1007/s00604-018-2962-8.
Acknowledgements
We are thankful for the financial support from the National Natural Science Foundation of China (No. 21966024 and 21768003), the Natural Science Foundation of Ningxia (No. 2022AAC03045), and the Key research and development projects in Xingtai, Hebei, Special project in the field of social development (No. 2022zz049).
Author information
Authors and Affiliations
Contributions
Yinghui Wang: Conceptualization, Methodology, Investigation, Data curation, Writing—original draft. Ling Yu: Software, Data curation, Formal analysis. Hui Zhang: Formal analysis. Runzhi Zhu: Formal analysis. Zhe Meng: Writing—review & editing.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Yu, L., Zhang, H. et al. Competitive ELISA based on pH-responsive persistent luminescence nanoparticles for autofluorescence-free biosensor determination of ochratoxin A in cereals. Anal Bioanal Chem 415, 1877–1887 (2023). https://doi.org/10.1007/s00216-023-04591-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-04591-0